Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the EOs
2.2. Enantioselective Analyses of the EOs
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Distillation of the EOs
4.3. Qualitative Analyses of the EOs (GC-MS)
4.4. Quantitative Analyses of the EOs (GC-FID)
4.5. Enantioselective Analyses of the EOs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Megadiverse Countries, UNEP-WCMC. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 1 January 2025).
- Malagón, O.; Ramírez, J.; Andrade, J.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, G.; Chiriboga, X.; Finzi, P.V.; Vidari, G. New 3,4-Secocycloartane and 3,4-Secodammarane Triterpenes from the Ecuadorian Plant Coussarea macrophylla. Chem. Biodivers. 2015, 12, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant Lepechinia mutica Benth (Lamiaceae). Chem. Biodivers. 2017, 14, e1700292. [Google Scholar] [CrossRef]
- Malagón, O.; Cartuche, P.; Montaño, A.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of the Endemic Andean Species Gynoxys miniphylla Cuatrec. (Asteraceae): Chemical and Enantioselective Analyses. Plants 2022, 11, 398. [Google Scholar] [CrossRef]
- Gilardoni, G.; Lara, L.R.; Cumbicus, N.; Malagón, O. A New Leaf Essential Oil from Endemic Gynoxys laurifolia (Kunth) Cass. of Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 2878. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of Gynoxys rugulosa Muschl. (Asteraceae) Growing in Southern Ecuador: Chemical and Enantioselective Analyses. Plants 2023, 12, 849. [Google Scholar] [CrossRef]
- Cumbicus, C.; Malagón, O.; Cumbicus, N.; Gilardoni, G. The Leaf Essential Oil of Gynoxys buxifolia (Kunth) Cass. (Asteraceae): A Good Source of Furanoeremophilane and Bakkenolide A. Plants 2023, 12, 1323. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Betancourt, E.A.; León, E.S.; Malagón, O.; Cumbicus, N.; Gilardoni, G. New Essential Oils from Ecuadorian Gynoxys cuicochensis Cuatrec. and Gynoxys sancti-antonii Cuatrec. Chemical Compositions and Enantioselective Analyses. ACS Omega 2024, 9, 25902–25913. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Leaf Essential Oil from the Andean Species Gynoxys szyszylowiczii Hieron. of Southern Ecuador: Chemical and Enantioselective Analyses. Sci. Rep. 2024, 14, 16360. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Rodríguez, M.d.C.; Calvopiña, K.; Malagón, O.; Cumbicus, N.; Gilardoni, G. Gynoxys reinaldii Cuatrec. and Gynoxys pulchella (Kunth) Cass.: Chemical and Enantioselective Analyses of Two Unprecedented Essential Oils from Ecuador. Plants 2024, 13, 3543. [Google Scholar] [CrossRef] [PubMed]
- Tropicos.org. Missouri Botanical Garden. Available online: https://www.tropicos.org (accessed on 1 January 2025).
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 286–288. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-193263321. [Google Scholar]
- Ding, Q.; Deng, Y.; Sun, Y.; Huagn, A.; Sun, Y. Analysis of volatile components in ox feces by capillary gas chromatography. Beijing Daxue Xuebao Ziran Kexueban 1998, 34, 720–725. [Google Scholar]
- Fanciullino, A.-L.; Gancel, A.-L.; Froelicher, Y.; Luro, F.; Ollitrault, P.; Brillouet, J.-M. Effects of Nucleo-Cytoplasmic Interactions on Leaf Volatile Compounds from Citrus Somatic Diploid Hybrids. J. Agric. Food Chem. 2005, 53, 4517–4523. [Google Scholar] [CrossRef]
- Kollmannsberger, H.; Nitz, S.; Drawert, F. Uber die Aromastoffzusammensetzung von Hochdruckextrakten. I. Pfeffer (Piper nigrum, Var. muntok). Z. Lebensm. Unters. Forsch. 1992, 194, 545–551. [Google Scholar] [CrossRef]
- Pala-Paul, J.; Brophy, J.J.; Perez-Alonso, M.J.; Usano, J.; Soria, S.C. Essential Oil Composition of the Different Parts of Eryngium corniculatum Lam. (Apiaceae) from Spain. J. Chromatogr. A 2007, 1175, 289–293. [Google Scholar] [CrossRef]
- Beck, J.J.; Higbee, B.S.; Marrill, G.B.; Roitman, J.N. Comparison of Volatile emissions from undamaged and mechanically damaged almonds. J. Sci. Food Argic. 2008, 88, 1363–1368. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007, 39, 563–572. [Google Scholar]
- Ruiz Perez-Cacho, P.; Mahattanatawee, K.; Smoot, J.M.; Rouseff, R. Identification of Sulfur Volatiles in Canned Orange Juices Lacking Orange Flavor. J. Agric. Food Chem. 2007, 55, 5761–5767. [Google Scholar] [CrossRef]
- Fernandez-Segovia, I.; Escriche, I.; Gomez-Sintes, M.; Fuentes, A.; Serra, J.A. Influence of different preservation treatments on the volatile fraction of desalted cod. Food Chem. 2006, 98, 473–482. [Google Scholar] [CrossRef]
- Bendahou, M.; Muselli, A.; Grignon-Dubois, M.; Benyoucef, M.; Desjobert, J.-M.; Bernardini, A.-F.; Costa, J. Antimicrobial activity and chemical composition of Origanum glandulosum Desf. essential oil and extract obtained by microwave extraction: Comparison with hydrodistillation. Food Chem. 2008, 106, 132–139. [Google Scholar]
- Chung, H.Y.; Fung, P.K.; Kim, J.-S. Aroma impact components in commercial plain sufu. J. Agric. Food Chem. 2005, 53, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.-L.; Ollitrault, P.; Froelicher, Y.; Tomi, F.; Jacquemond, C.; Luro, F.; Brillouet, J.-M. Leaf volatile compounds of seven citrus somatic tetraploid hybrids sharing willow leaf mandarin (Citrus deliciosa Ten.) as their common parent. J. Agric. Food Chem. 2003, 51, 6006–6013. [Google Scholar] [CrossRef] [PubMed]
- Zeppa, G.; Giordano, M.; Bertolino, M.; Gerbi, V. Application of artificial neural network on mono- and sesquiterpenes compounds determined by headspace solid-phase microextraction-gas chromatography-mass spectrometry for the Piedmont ricotta cheese traceability. J. Chromatogr. A 2005, 1071, 247–253. [Google Scholar]
- Cardeal, Z.L.; da Silva, M.D.R.G.; Marriott, P.J. Comprehensive two-dimensional gas chromatography/mass spectrometric analysis of pepper volatiles. Rapid Commun. Mass Spectrom. 2006, 20, 2823–2836. [Google Scholar] [CrossRef]
- Buttery, R.G.; Parker, F.D.; Teranishi, R.; Mon, T.R.; Ling, L.C. Volatile components of alfalfa leaf-cutter bee cells. J. Agric. Food Chem. 1981, 29, 955–958. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica × Fortunella sp. × Citrus aurantifolia). J. Essent. Oil Res. 2004, 16, 328–333. [Google Scholar] [CrossRef]
- Bendimerad, N.; Bendiab, S.A.T. Composition and antibacterial activity of Pseudocytisus integrifolius (Salisb.) essential oil from Algeria. J. Agric. Food Chem. 2005, 53, 2947–2952. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Ranarivelo, L.; Ratsimbason, M.; Bernardini, A.-F.; Casanova, J. Constituents of the essential oil of six Helichrysum species from Madagascar. Flavour Fragr. J. 2001, 16, 253–256. [Google Scholar] [CrossRef]
- Miyazawa, M.; Kawauchi, Y.; Utsumi, Y.; Takahashi, T. Character impact odorants of wild edible plant—Cacalia hastata L. var. orientalis—Used in Japanese traditional food. J. Oleo Sci. 2010, 59, 527–533. [Google Scholar]
- Viña, A.; Murillo, E. Essential oil composition from twelve varieties of basil (Ocimum spp.) grown in Columbia. J. Braz. Chem. Soc. 2003, 14, 744–749. [Google Scholar] [CrossRef]
- Petersen, M.A.; Poll, L.; Larsen, L.M. Comparison of volatiles in raw and boiled potatoes using a mild extraction technique combined with GC odour profiling and GC-MS. Food Chem. 1998, 61, 461–466. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Identifizierung von Aromastoffen aus der Kruste von Roggenbrot. Z. Lebensm. Unters. Forsch. 1983, 177, 173–180. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Jell, J.A.; Cass, D.M., Jr. Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography-olfactometry. Flavour Fragr. J. 2003, 18, 275–281. [Google Scholar] [CrossRef]
- Kawakami, M.; Sachs, R.M.; Shibamoto, T. Volatile constituents of essential oils obtained from newly developed tea tree (Melaleuca alternifolia) clones. J. Agric. Food Chem. 1990, 38, 1657–1661. [Google Scholar] [CrossRef]
- Petka, J.; Ferreira, V.; González-Viñas, M.A.; Cacho, J. Sensory and Chemical Characterization of the Aroma of a White Wine Made with Devín Grapes. J. Agric. Food Chem. 2006, 54, 909–915. [Google Scholar] [CrossRef]
- Selli, S.; Rannou, C.; Prost, C.; Robin, J.; Serot, T. Characterization of Aroma-Active Compounds in Rainbow Trout (Oncorhynchus mykiss) Eliciting an Off-Odor. J. Agric. Food Chem. 2006, 54, 9496–9502. [Google Scholar] [CrossRef]
- Polatoglu, K.; Demirci, F.; Demirci, B.; Goren, N.; Baser, K.H.C. Antimicrobial activity and essential oil composition of a new T. argyrophyllum (C. Koch) Tvzel var. argyrophyllium chemotype. J. Oleo Sci. 2010, 59, 307–313. [Google Scholar] [CrossRef]
- Lee, G.-H.; Suriyaphan, O.; Cadwallader, K.R. Aroma components of cooked tail meat of American lobster (Homarus americanus). J. Agric. Food Chem. 2001, 49, 4324–4332. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Demirci, B.; Kürkcüoglu, M.; Aytac, Z.; Duman, H. Composition of the essential oils of Zosima absinthifolia (Vent.) Link and Ferula elaeochytris Korovin from Turkey. Flavour Fragr. J. 2000, 15, 371–372. [Google Scholar] [CrossRef]
- Salgueiro, L.R.; Pinto, E.; Goncalves, M.J.; Costa, I.; Palmeira, A.; Cavaleiro, C.; Pina-Vaz, C.; Rodrigues, A.G.; Martinez-De-Oliveira, J. Antifungal activity of the essential oil of Thymus capitellatus against Candida, Aspergillus and dermatophyte strains. Flavour Fragr. J. 2006, 21, 749–753. [Google Scholar] [CrossRef]
- Jerkovic, I.; Mastelic, J.; Milos, M.; Juteau, F.; Masotti, V.; Viano, J. Chemical variability of Artemisia vulgaris L. essential oils originated from the Mediterranean area of France and Croatia. Flavour Fragr. J. 2003, 18, 436–440. [Google Scholar] [CrossRef]
- Gauvin, A.; Ravaomanarivo, H.; Smadja, J. Comparative analysis by gas chromatography-mass spectrometry of the essential oils from bark and leaves of Cedrelopsis grevei Baill, an aromatic and medicinal plant from Madagascar. J. Chromatogr. A 2004, 1029, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Pala-Paul, J.; Copeland, L.M.; Brophy, J.J.; Goldsack, R.J. Essential oil composition of two variants of Prostanthera lasianthos Labill. from Australia. Biochem. Syst. Ecol. 2006, 34, 48–55. [Google Scholar] [CrossRef]
- Watcharananun, W.; Cadwallader, K.R.; Huangrak, K.; Kim, H.; Lorjaroenphon, Y. Identification of predominant odorants in Thai desserts flavored by smoking with Tian Op, a traditional Thai scented candle. J. Agric. Food Chem. 2009, 57, 996–1005. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; O’Sullivan, W. Chemistry of the Australian gymnosperms. Part VII. The leaf oils of the genus Actinostrobus. Biochem. Syst. Ecol. 2004, 32, 867–873. [Google Scholar] [CrossRef]
- Smadja, J.; Rondeau, P.; Sing, A.S.C. Volatile constituents of five Citrus Petitgrain essential oils from Reunion. Flavour Fragr. J. 2005, 20, 399–402. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. HRGC/FID/MSD Analysis of Secondary Metabolites Isolated from Xilopia aromatica by Different Extraction Techniques. In Proceedings of the 25th International Symposium on Capillary Chromatography, Riva Del Garda, Italy, 13–17 May 2002. [Google Scholar]
- Guillot, S.; Peytavi, L.; Bureau, S.; Boulanger, R.; Lepoutre, J.-P.; Crouzet, J.; Schorr-Galindo, S. Aroma characterization of various apricot varieties using headspace-solid phase microextraction combined with gas chromatography-mass spectrometry and gas chromatography-olfactometry. Food Chem. 2006, 96, 147–155. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef]
- Riu-Aumatell, M.; Lopez-Tamames, E.; Buxaderas, S. Assessment of the Volatile Composition of Juices of Apricot, Peach, and Pear According to Two Pectolytic Treatments. J. Agric. Food Chem. 2005, 53, 7837–7843. [Google Scholar] [CrossRef]
- Noorizadeh, H.; Farmany, A.; Noorizadeh, M. Quantitative structure-retention relationships analysis of retention index of essential oils. Quim. Nova 2011, 34, 242–249. [Google Scholar] [CrossRef]
- Paolini, J.; Costa, J.; Bernardini, A.F. Analysis of the essential oil from the roots of Eupatorium cannabinum subsp. corsicum (L.) by GC, GC-MS and C13-NMR. Phytochem. Anal. 2007, 18, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Mahattanatawee, K.; Rouseff, R.; Filomena Valim, M.; Naim, M. Identification and aroma impact of norisoprenoids in orange juice. J. Agric. Food Chem. 2005, 53, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Pintore, G.; Chessa, M.; Manconi, P.; Zanetti, S.; Deriu, A.; Trillini, B. Chemical composition and antimicrobial activities of essential oil of Stachys glutinosa L. from Sardinia. Nat. Prod. Commun. 2006, 12, 1133–1136. [Google Scholar]
- Bassole, I.H.N.; Ouattara, A.S.; Nebie, R.; Ouattara, C.A.T.; Kabore, Z.I.; Traore, S.A. Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry 2003, 62, 209–212. [Google Scholar] [CrossRef]
- Mondello, L.; Dugo, P.; Basile, A.; Dugo, G. Interactive use of linear retention indices, on polar and apolar columns, with a MS-library for reliable identification of complex mixtures. J. Microcolumn Sep. 1995, 7, 581–591. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.-P.; Schorr-Galindo, S. Impact of ecological post-harvest processing of coffee aroma: II Roasted coffee. J. Food Compos. Anal. 2007, 20, 297–307. [Google Scholar] [CrossRef]
- Pozo-Bayon, M.A.; Ruiz-Rodriguez, A.; Pernin, K.; Cayot, N. Influence of eggs on the aroma composition of a sponge cake and on the aroma release in model studies on flavored sponge cakes. J. Agric. Food Chem. 2007, 55, 1418–1426. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A multivariate statistical approach to Centaurea classification using essential oil composition data of some species from Turkey. Plant Syst. Evol. 2006, 261, 217–228. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C. Corn leaf volatiles: Identification using Tenax trapping for possible insect attractants. J. Agric. Food Chem. 1984, 32, 1104–1106. [Google Scholar] [CrossRef]
- Muselli, A.; Rossi, P.-G.; Desjobert, J.-M.; Bernardini, A.-F.; Berti, L.; Costa, J. Chemical composition and antibacterial activity of Otanthus maritimus (L.) Hoffmanns. Link essential oils from Corsica. Flavour Fragr. J. 2007, 22, 217–223. [Google Scholar] [CrossRef]
- Waggott, A.; Davies, I.W. Identification of Organic Pollutants Using Linear Temperature Programmed Retention Indices (LTPRIs)—Part II. 1984. Available online: https://dwi-content.s3.eu-west-2.amazonaws.com/wp-content/uploads/2020/10/27110228/dwi0382.pdf (accessed on 1 January 2025).
- Brat, P.; Rega, B.; Alter, P.; Reynes, M.; Brillouet, J.-M. Distribution of volatile compounds in the pulp, cloud, and serum of freshly squeezed orange juice. J. Agric. Food Chem. 2003, 51, 3442–3447. [Google Scholar] [CrossRef] [PubMed]
- Verzera, A.; Trozzi, A.; Cotroneo, A.; Lorenzo, D.; Dellacassa, E. Uruguayan essential oil. 12. Composition of Nova and Satsuma mandarin oils. J. Agric. Food Chem. 2000, 48, 2903–2909. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Menichini, F.; Noccioli, C.; Morelli, I.; Pistelli, L. Volatile constituents of different organs of Psoralea bituminosa L. Flavour Fragr. J. 2004, 19, 166–171. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Mizota, Y.; Kubota, T.; Nishimura, O.; Masuda, H.; Sotoyama, K.; Tomita, M. Aroma extract dilution analysis. Evluation of aroma of pasteurized and UHT processed milk by aroma extract dilution analysis. Nippon Shokuhin Kagaku Kogaku Kaishi 1999, 46, 587–597. [Google Scholar] [CrossRef]
- Museli, A.; Pau, M.; Desjobert, J.-M.; Foddai, M.; Usai, M.; Costa, J. Volatile constituents of Achillea ligustica All. by HS-SPME/GC/GC-MS. Comparison with essential oils obtained by hydrodistillation from Corsica and Sardinia. Chromatographia 2009, 69, 575–585. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shafi, P.M.; Rosamma, M.K.; Geissler, M. Analysis of the composition and aroma of the essential leaf oil of Syzygium travancoricum from South India by GC-FID, GC-MS, and olfactometry. Seasonal changes of composition. Chromatogr. Sup. 2001, 53 (Suppl. S1), s372–s374. [Google Scholar]
- Gancel, A.-L.; Ollé, D.; Ollitrault, P.; Luro, F.; Brillouet, J.-M. Leaf and peel volatile compounds of an interspecific citrus somatic hybrid [Citrus aurantifolia (Christm.) Swing. + Citrus paradisi Macfayden]. Flavour Fragr. J. 2002, 17, 416–424. [Google Scholar] [CrossRef]
- Limberger, R.P.; Scopel, M.; Sobral, M.; Henriques, A.T. Comparative analysis of volatiles from Drimys brasiliensis Miers and D. angustifolia Miers (Winteraceae) from Southern Brazil. Biochem. Syst. Ecol. 2007, 35, 130–137. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Tigrine-Kordjani, N.; Chemat, S.; Meklati, B.Y.; Chemat, F. Rapid Extraction of Volatile Compounds Using a New Simultaneous Microwave Distillation: Solvent Extraction Device. Chromatographia 2007, 65, 217–222. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Martin, M.-A.C.; Bercion, S.R.; Legault, J. Chemical composition of leaf essential oil of Hedyosmum arborescens and evaluation of its anticancer activity. Nat. Prod. Commun. 2007, 2, 1269–1272. [Google Scholar] [CrossRef]
- Suleimenov, Y.M.; Atazhanova, G.A.; Ozek, T.; Demirci, B.; Kulyyasov, A.T.; Adekenov, S.M.; Baser, K.H.C. Essential oil composition of three species of Achillea from Kazakhstan. Chem. Nat. Compd. 2006, 37, 447–450. [Google Scholar] [CrossRef]
- Takeoka, G.; Butter, R.G. Volatile constituents of pineapple (Ananas comosus [L.] Merr.). In Flavor Chemistry. Trends and Developments; Teranishi, R., Buttery, R.G., Shahidi, F., Eds.; American Chemical Society: Washington, DC, USA, 1989; pp. 223–237. [Google Scholar]
- Shellie, R.; Marriott, P.; Zappia, G.; Mondello, L.; Dugo, G. Interactive use of linear retention indices on polar and apolar columns with an MS-Library for reliable characterization of Australian tea tree and other Melaleuca sp. Oils. J. Essent. Oil Res. 2003, 15, 305–312. [Google Scholar] [CrossRef]
- Mau, J.-L.; Ko, P.T.; Chyau, C.-C. Aroma characterization and antioxidant activity of supercritical carbon dioxide extracts from Terminalia catappa leaves. Food Res. Int. 2003, 36, 97–104. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Berno, P.; Pizzale, L.; Conte, L.S. Sesquiterpene, alkene, and alkane hydrocarbons in virgin olive oils of different varieties and geographical origins. J. Agric. Food Chem. 2001, 49, 3278–3283. [Google Scholar] [CrossRef]
- Yu, E.J.; Kim, T.H.; Kim, K.H.; Lee, H.J. Aroma-active compounds of Pinus densiflora (red pine) needles. Flavour Fragr. J. 2004, 19, 532–537. [Google Scholar] [CrossRef]
- Ka, M.-H.; Choi, E.H.; Chun, H.-S.; Lee, K.-G. Antioxidative activity of volatile extracts isolated from Angelica tenuissima roots, peppermint leaves, pine needles, and sweet flag leaves. J. Agric. Food Chem. 2005, 53, 4124–4129. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Tomi, F.; Bernardini, A.-F.; Casanova, J. Chemical variability of the essential oil of Helichrysum faradifani Sc. Ell. from Madagascar. Flavour Fragr. J. 2006, 21, 111–114. [Google Scholar] [CrossRef]
- Zheng, C.H.; Kim, K.H.; Kim, T.H.; Lee, H.J. Analysis and characterization of aroma-active compounds of Schizandra chinensis (omija) leaves. J. Sci. Food Agric. 2005, 85, 161–166. [Google Scholar] [CrossRef]
- Boti, J.B.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Chemical variability of Juniperus oxycedrus ssp. oxycedrus berry and leaf oils from Corsica, analysed by combination of GC, GC-MS and 13C-NMR. Flavour Fragr. J. 2006, 21, 268–273. [Google Scholar] [CrossRef]
- Martinez, J.; Rosa, P.T.V.; Menut, C.; Leydet, A.; Brat, P.; Pallet, D.; Meireles, M.A.A. Valorization of Brazilian vetiver (Vetiveria zizanioides (L.) Nash ex Small) oil. J. Agric. Food Chem. 2004, 52, 6578–6584. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Thuy, N.T.; Shin, J.H.; Baek, H.H.; Lee, H.J. Aroma-active compounds of miniature beefsteakplant (Mosla dianthera Maxim.). J. Agric. Food Chem. 2000, 48, 2877–2881. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, B.A. Production, Composition, Properties and Application of Essential Oils. 2004. Available online: http://viness.narod.ru (accessed on 7 January 2025).
- Orav, A.; Kann, J. Determination of peppermint and orange aroma compounds in food and beverages. Proc. Est. Acad. Sci. Chem. 2001, 50, 217–225. [Google Scholar]
- Le Quere, J.-L.; Latrasse, A. Composition of the Essential Oils of Blackcurrant Buds (Ribes nigrum L.). J. Agric. Food Chem. 1990, 38, 3–10. [Google Scholar] [CrossRef]
- Cozzani, S.; Muselli, A.; Desjobert, J.-M.; Bernardini, A.-F.; Tomi, F.; Casanova, J. Chemical composition of essential oil of Teucrium polium subsp. capitatum (L.) from Corsica. Flavour Fragr. J. 2005, 20, 436–441. [Google Scholar] [CrossRef]
- Cavalli, J.-F.; Tomi, F.; Bernardini, A.-F.; Casanova, J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Peppard, T.L.; Ramus, S.A. Use of Kovats’ gas chromatographic retention indices in beer flavor studies. Am. Soc. Brew. Chem. Proc. 1988, 46, 26–30. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.-F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed analysis of the essential oil from Cistus albidus L. by combination of GC/RI, GC/MS and 13C-NMR spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [CrossRef]
- Molleken, U.; Sinnwell, V.; Kubeczka, K.H. The essential oil composition of fruits from Smyrnium perfoliatum. Phytochemistry 1998, 47, 1079–1083. [Google Scholar] [CrossRef]
- Quijano, C.E.; Linares, D.; Pino, J.A. Changes in volatile compounds of fermented cereza agria [Phyllanthus acidus (L.) Skeels] fruit. Flavour Fragr. J. 2007, 22, 392–394. [Google Scholar] [CrossRef]
- Choi, H.-S. Character impact odorants of Citrus hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Pennarun, A.-L.; Prost, C.; Haure, J.; Demaimay, M. Comparison of two microalgal diets. 2. Influence on odorant composition and organoleptic qualities of raw oysters (Crassostrea gigas). J. Agric. Food Chem. 2003, 51, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajima, Y. Off-flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994, 42, 1323–1327. [Google Scholar] [CrossRef]
- Bendiabdellah, A.; El Amine Dib, M.; Djabou, N.; Allali, H.; Tabti, B.; Costa, J.; Myseli, A. Biological activities and volatile constituents of Daucus muricatus L. from Algeria. Chem. Centr. J. 2012, 6, 48. [Google Scholar] [CrossRef]
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajima, Y. Volatile flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994, 42, 984–988. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vázquez, C. Characterization of volatiles in strawberry guava (Psidium cattleianum Sabine) fruit. J. Agric. Food Chem. 2001, 49, 5883–5887. [Google Scholar] [CrossRef]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Trozzi, A.; Ragusa, S. The leaf volatile constituents of Isatis tinctoria by solid phase microextraction and gas chromatography/mass spectrometry. Planta Medica 2006, 72, 924–928. [Google Scholar] [CrossRef]
- Duman, H.; Kartal, M.; Altun, L.; Demirci, B.; Baser, K.H.C. The essential oil of Stachys laetivirens Kotschy Boiss. ex Rech. fil., endemic in Turkey. Flavour Fragr. J. 2005, 20, 48–50. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Borisov, R.S. Chromatographic-mass spectrometric analysis of Fishcer-Tropsch synthesis products. J. Anal. Chem. USSR 2002, 57, 544–551. [Google Scholar] [CrossRef]
- Kamariah, A.S.; Lim, L.B.L.; Baser, K.H.C.; Ozek, T.; Demirci, B. Composition of the essential oil of Plumeria obtusa L. Flavour Fragr. J. 1999, 14, 237–240. [Google Scholar] [CrossRef]
- Tkachev, A.V.; Dobrotvorsky, A.K.; Vjalkov, A.I.; Morozov, S.V. Chemical composition of lipophylic compounds from the body surface of unfed adult Ixodes persulcatus ticks (Acari: Ixodidae). Exp. Appl. Acarol. 2000, 24, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kaya, A.; Baser, K.H.C.; Demirci, B.; Koca, F. The essential oil of Acinos alpinus (L.) Moench growing in Turkey. Flavour Fragr. J. 1999, 14, 55–59. [Google Scholar] [CrossRef]
- Radulovic, N.; Blagojevic, P.; Palic, R. Comparative study of the leaf volatiles of Arctostaphylos uva-ursi (L.) Spreng. and Vaccinium vitis-idaea L. (Ericaceae). Molecules 2010, 15, 6168–6185. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products. A Biosynthetic Approach, 3rd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2009; ISBN 9780470741672. [Google Scholar]
- Allenspach, M.; Steuer, C. α-Pinene: A Never-Ending Story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Mozuraitis, R.; Stranden, M.; Ramirez, M.I.; Borg-Karlson, A.K.; Mustaparta, H. (-)-Germacrene D Increases Attraction and Oviposition by the Tobacco Budworm Moth Heliothis virescens. Chem. Senses 2002, 27, 505–509. [Google Scholar] [CrossRef]
- Stranden, M.; Liblikas, I.; Koenig, W.A.; Almaas, T.J.; Borg-Karlson, A.K.; Mustaparta, H. (–)-GermacreneD Receptor Neurones in Three Species of Heliothine Moths: Structure-activity Relationships. J. Comp. Physiol. A 2003, 189, 563–577. [Google Scholar] [CrossRef]
- Albuquerque, B.N.D.L.; Da Silva, M.F.R.; Da Silva, P.C.B.; De Lira Pimentel, C.S.; Lino Da Rocha, S.K.; De Aguiar, J.C.R.O.F.; Neto, A.C.A.; Paiva, P.M.G.; Gomes, M.G.M.; Da Silva-Júnior, E.F.; et al. Oviposition Deterrence, Larvicidal Activity and Docking of β-Germacrene-D-4-ol Obtained from Leaves of Piper corcovadensis (Piperaceae) against Aedes aegypti. Ind. Crops Prod. 2022, 182, 114830. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; Vieira, M.d.C.; Tasca, G.R.A.M.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, Anti-inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Gilardoni, G.; Matute, Y.; Ramírez, J. Chemical and Enantioselective Analysis of the Leaf Essential Oil from Piper coruscans Kunth (Piperaceae), a Costal and Amazonian Native Species of Ecuador. Plants 2020, 9, 791. [Google Scholar] [CrossRef]
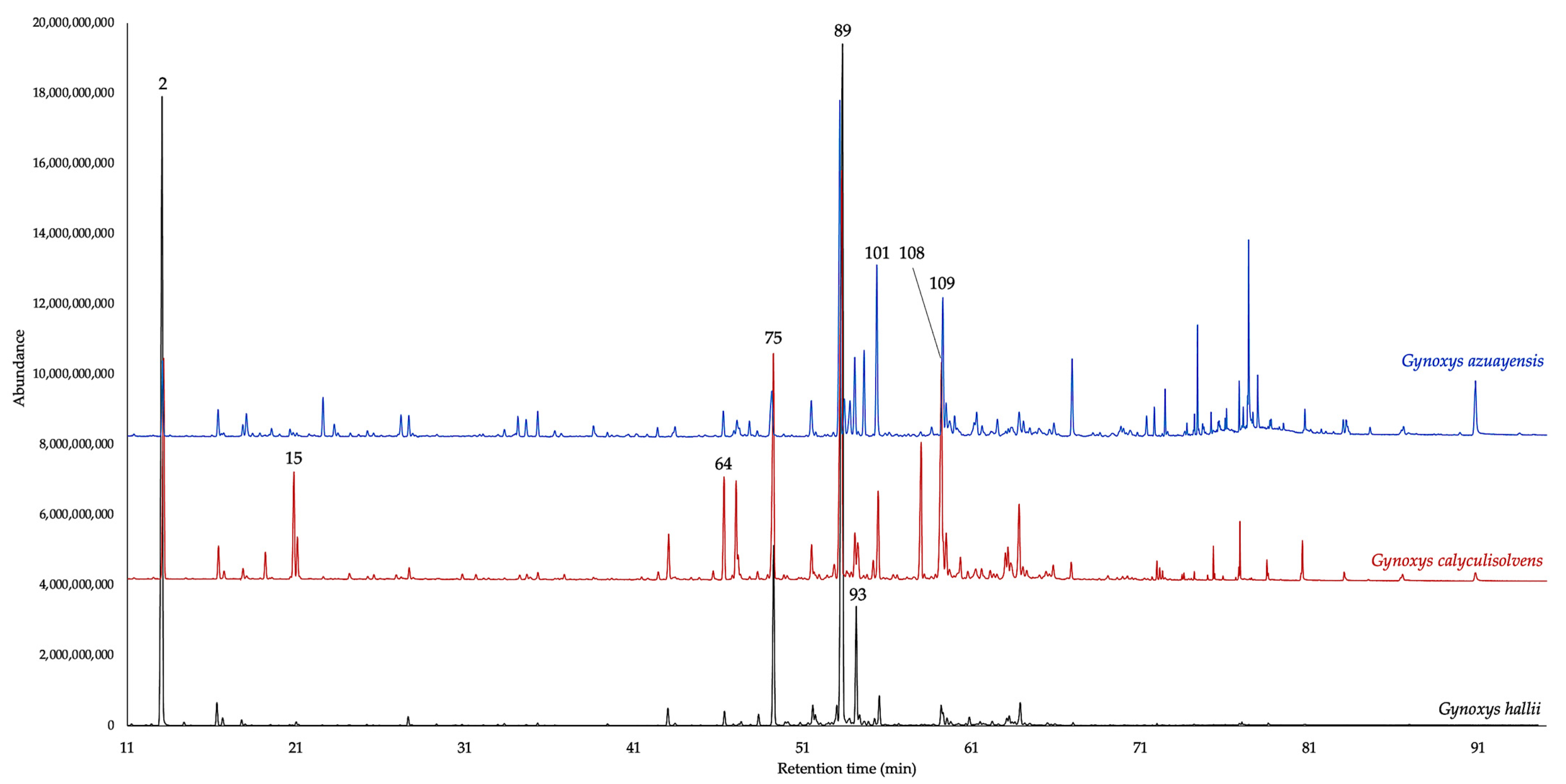
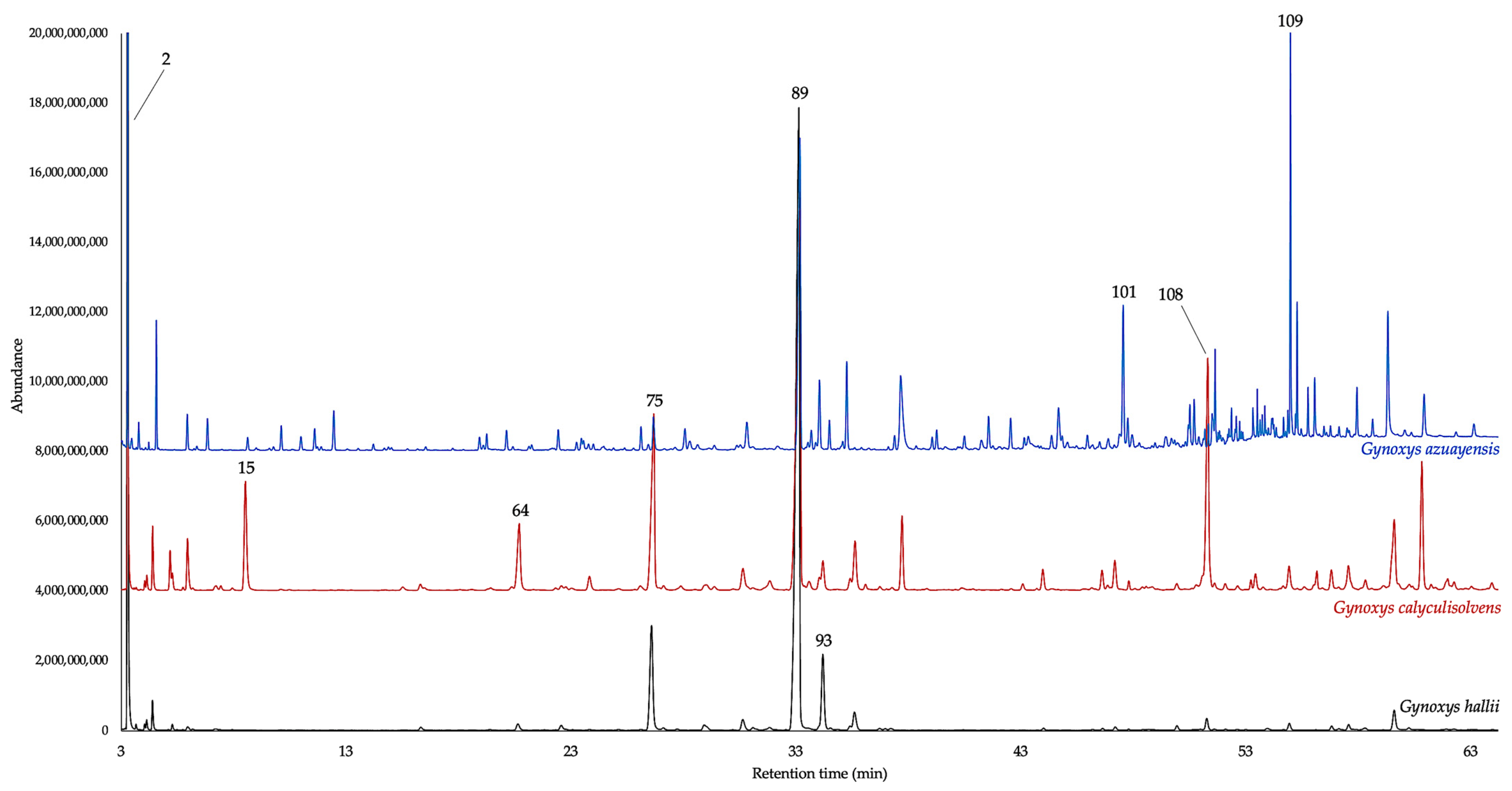

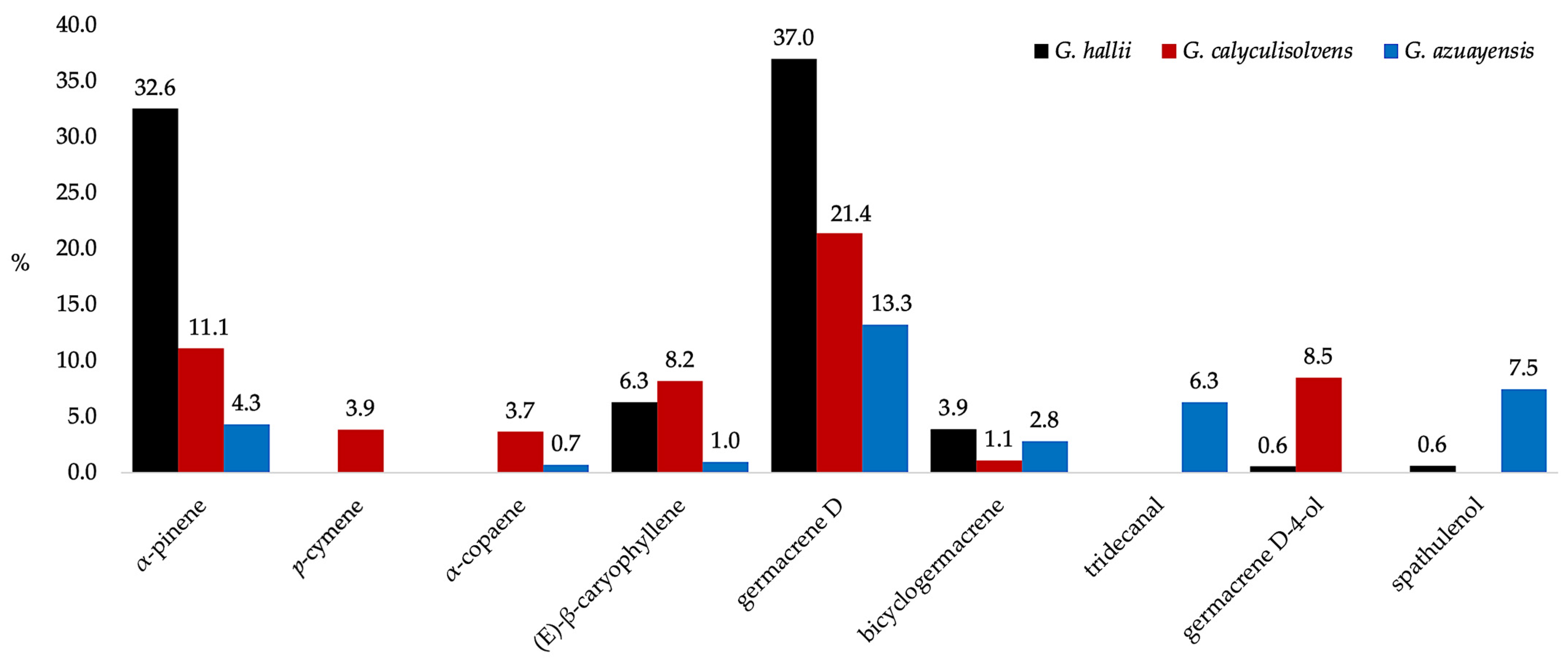

| N. | Compounds | 5% Phenyl Methyl Polysiloxane | Polyethylene Glycol | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LRI | G. hallii | G. calyculisolvens | G. azuayensis | Lit. | LRI | G. hallii | G. calyculisolvens | G. azuayensis | Lit. | ||||||||||
| Calc. | Ref. | % | σ | % | σ | % | σ | Calc. | Ref. | % | σ | % | σ | % | σ | ||||
| 1 | heptanal | 910 | 901 | 0.1 | 0.02 | - | - | - | - | [15] | 1158 | 1158 | trace | - | - | - | - | - | [16] |
| 2 | α-pinene | 935 | 932 | 33.6 | 2.39 | 11.2 | 2.59 | 4.5 | 1.42 | [15] | 1015 | 1015 | 31.5 | 2.38 | 11.0 | 2.41 | 4.1 | 1.14 | [17] |
| 3 | α-fenchene | 948 | 945 | 0.1 | 0.01 | - | - | - | - | [15] | 1074 | 1073 | 0.3 | 0.04 | - | - | - | - | [18] |
| 4 | thuja-2,4(10)-diene | 953 | 953 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 5 | sabinene | 976 | 969 | 0.7 | 0.05 | 1.2 | 0.10 | 1.2 | 0.32 | [15] | 1113 | 1113 | 0.7 | 0.05 | 1.1 | 0.07 | 1.2 | 0.26 | [19] |
| 6 | benzaldehyde | 978 | 978 | - | - | - | - | 0.1 | 0.04 | [15] | 1514 | 1516 | - | - | - | - | trace | - | [20] |
| 7 | β-pinene | 980 | 979 | 0.3 | 0.02 | 0.3 | 0.02 | 0.2 | 0.11 | [15] | 1102 | 1102 | o.t.p. 3 | - | 0.3 | 0.02 | 0.1 | 0.08 | [21] |
| 8 | myrcene | 994 | 988 | 0.2 | 0.02 | 0.4 | 0.03 | 0.6 | 0.10 | [15] | 1159 | 1159 | 0.2 | 0.01 | 0.5 | 0.02 | 0.5 | 0.06 | [22] |
| 9 | 2-pentyl furan | 997 | 984 | 0.1 | 0.02 | 0.2 | 0.05 | 1.4 | 0.34 | [15] | 1229 | 1229 | 0.2 | 0.03 | 0.3 | 0.04 | 1.4 | 0.10 | [23] |
| 10 | meta-mentha-1(7),8-diene | 1003 | 1000 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 11 | α-phellandrene | 1007 | 1002 | - | - | 0.9 | 0.08 | - | - | [15] | 1165 | 1164 | - | - | 0.7 | 0.02 | - | - | [24] |
| 12 | (2E,4E)-heptadienal | 1013 | 1005 | trace | - | - | - | 0.6 | 0.10 | [15] | 1463 | 1463 | trace | - | - | - | o.t.p. 64 | - | [25] |
| 13 | n-octanal | 1015 | 998 | - | - | - | - | [15] | 1285 | 1286 | - | - | - | - | 0.2 | 0.07 | [23] | ||
| 14 | α-terpinene | 1021 | 1014 | - | - | - | - | 0.1 | 0.03 | [15] | 1168 | 1167 | - | - | - | - | 0.1 | 0.01 | [26] |
| 15 | p-cymene | 1027 | 1020 | 0.1 | 0.01 | - | - | - | - | [15] | 1237 | 1237 | trace | - | - | - | - | - | [27] |
| 16 | o-cymene | 1028 | 1022 | - | - | 4.0 | 0.56 | - | - | [15] | 1238 | 1234 | - | - | 3.7 | 0.42 | - | - | [28] |
| 17 | (2E,4Z)-heptadienal | 1028 | 1013 | - | - | - | - | 0.6 | 0.09 | [15] | 1488 | 1480 | - | - | - | - | 0.6 | 0.18 | [29] |
| 18 | limonene | 1033 | 1024 | trace | - | 1.4 | 0.06 | 0.4 | 0.04 | [15] | 1187 | 1186 | 0.1 | 0.01 | 1.3 | 0.04 | 0.2 | 0.02 | [30] |
| 19 | β-phellandrene | 1031 | 1025 | trace | - | - | - | - | - | [15] | 1162 | 1161 | trace | - | - | - | - | - | [31] |
| 20 | (Z)-β-ocimene | 1042 | 1032 | - | - | - | - | 0.1 | 0.02 | [15] | 1232 | 1232 | - | - | - | - | 0.2 | 0.18 | [32] |
| 21 | (E)-β-ocimene | 1052 | 1044 | trace | - | 0.1 | 0.01 | 1.4 | 0.35 | [15] | 1247 | 1247 | trace | - | 0.1 | 0.01 | 1.4 | 0.18 | [33] |
| 22 | γ-terpinene | 1060 | 1054 | trace | - | - | - | - | - | [15] | 1221 | 1221 | trace | - | - | - | - | - | [34] |
| 23 | benzene acetaldehyde | 1061 | 1062 | - | - | - | - | 0.9 | 0.09 | [15] | 1639 | 1639 | - | - | - | - | 1.0 | 0.18 | [35] |
| 24 | (2E)-octen-1-al | 1072 | 1049 | - | - | - | - | 0.2 | 0.05 | [15] | 1421 | 1421 | - | - | - | - | 0.1 | 0.05 | [36] |
| 25 | cis-linalool oxide (furanoid) | 1078 | 1067 | - | - | - | - | 0.4 | 0.09 | [15] | 1442 | 1445 | - | - | - | - | 0.5 | 0.02 | [37] |
| 26 | terpinolene | 1086 | 1086 | trace | - | - | - | - | - | [15] | 1242 | 1239 | trace | - | - | - | - | - | [38] |
| 27 | 6-camphenone | 1104 | 1095 | - | - | 0.20 | 0.01 | - | - | [15] | 1416 | - | - | - | 0.10 | 0.02 | - | - | § |
| 28 | linalool | 1110 | 1095 | - | - | trace | - | 1.3 | 0.06 | [15] | 1558 | 1560 | - | - | trace | - | 1.1 | 0.07 | [39] |
| 29 | n-nonanal | 1116 | 1100 | 0.3 | 0.02 | 0.4 | 0.03 | 0.8 | 0.27 | [15] | 1389 | 1389 | 0.2 | 0.01 | 0.3 | 0.04 | 0.4 | 0.08 | [40] |
| 30 | γ-campholene aldehyde | 1134 | 1122 | 0.1 | 0.02 | - | - | - | - | [15] | 1435 | 1439 | trace | - | - | - | - | - | [41] |
| 31 | trans-pinocarveol | 1147 | 1135 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 32 | (E)-epoxy-ocimene | 1148 | 1137 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 33 | trans-verbenol | 1154 | 1140 | 0.1 | 0.02 | 0.2 | 0.03 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 34 | eucarvone | 1166 | 1146 | trace | - | 0.2 | 0.02 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 35 | (2E)-nonen-1-al | 1173 | 1157 | - | - | trace | - | 0.2 | 0.03 | [15] | 1528 | 1528 | - | - | trace | - | 0.2 | 0.05 | [42] |
| 36 | safranal | 1176 | 1197 | - | - | trace | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 37 | p-mentha-1,5-dien-8-ol | 1185 | 1166 | - | - | - | - | 0.1 | 0.03 | [15] | 1731 | 1738 | - | - | - | - | 0.2 | 0.02 | [43] |
| 38 | terpinen-4-ol | 1190 | 1174 | 0.1 | 0.02 | 0.2 | 0.02 | 0.4 | 0.02 | [15] | 1599 | 1599 | 0.5 | 0.02 | 0.5 | 0.14 | 0.4 | 0.11 | [44] |
| 39 | n-dodecane | 1200 | 1200 | 0.1 | 0.04 | - | - | 0.8 | 0.12 | - | 1200 | 1200 | - | - | - | - | 0.9 | 0.20 | - |
| 40 | myrtenol | 1204 | 1194 | 0.1 | 0.11 | - | - | - | - | [15] | 1740 | 1747 | trace | - | - | - | - | - | [45] |
| 41 | myrtenal | 1206 | 1195 | - | - | 0.3 | 0.05 | - | - | [15] | 1601 | 1601 | - | - | 0.3 | 0.02 | - | - | [46] |
| 42 | α-terpineol | 1207 | 1207 | - | - | - | - | 0.8 | 0.10 | [15] | 1698 | 1700 | - | - | - | - | o.t.p. 93 | - | [47] |
| 43 | verbenone | 1209 | 1204 | trace | - | 0.3 | 0.06 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 44 | n-decanal | 1217 | 1201 | 0.1 | 0.03 | 0.3 | 0.02 | 1.1 | 0.27 | [15] | 1493 | 1495 | trace | - | 0.7 | 0.06 | 0.9 | 0.08 | [48] |
| 45 | nerol | 1237 | 1227 | - | - | - | - | 0.1 | 0.01 | [15] | 1805 | 1808 | - | - | - | - | o.t.p. 101 | - | [49] |
| 46 | thymol, methyl ether | 1238 | 1232 | - | - | 0.2 | 0.2 | - | - | [15] | 1543 | 1555 | - | - | 0.3 | 0.05 | - | - | [50] |
| 47 | cumin aldehyde | 1256 | 1238 | - | - | trace | - | - | - | [15] | 1726 | 1738 | - | - | trace | - | - | - | [51] |
| 48 | geranial | 1262 | 1264 | - | - | 0.2 | 0.02 | - | - | [15] | 1720 | 1718 | - | - | trace | - | - | - | [52] |
| 49 | geraniol | 1263 | 1249 | - | - | - | - | 1.1 | 0.06 | [15] | 1856 | 1856 | - | - | - | - | 1.1 | 0.12 | [53] |
| 50 | (2E)-decanal | 1275 | 1260 | 0.1 | 0.01 | 0.5 | 0.02 | 0.1 | 0.04 | [15] | 1635 | 1638 | 0.4 | 0.02 | 0.7 | 0.03 | 0.1 | 0.02 | [54] |
| 51 | nonanoic acid | 1293 | 1267 | - | - | - | - | 0.1 | 0.01 | [15] | 2189 | 2190 | - | - | - | - | 0.2 | 0.11 | [55] |
| 52 | n-tridecane | 1300 | 1300 | - | - | - | - | 0.1 | 0.02 | - | 1300 | 1300 | - | - | - | - | 0.1 | 0.04 | - |
| 53 | trans-pinocarvyl acetate | 1303 | 1298 | - | - | 0.1 | 0.01 | - | - | [15] | 1638 | 1641 | - | - | 0.1 | 0.03 | - | - | [56] |
| 54 | (2E,4Z)-decadienal | 1309 | 1292 | trace | - | - | - | 0.1 | 0.02 | [15] | 1760 | 1759 | trace | - | - | - | 0.2 | 0.05 | [57] |
| 55 | carvacrol | 1317 | 1315 | - | - | 2.0 | 0.03 | - | - | [58] | 2186 | 2186 | - | - | 2.7 | 0.18 | - | - | [59] |
| 56 | undecanal | 1318 | 1305 | - | - | - | - | 0.4 | 0.07 | [15] | 1602 | 1598 | - | - | - | - | 0.5 | 0.09 | [60] |
| 57 | p-vinylguaiacol | 1323 | 1309 | 1.0 | 0.32 | 2.2 | 0.35 | - | - | [15] | 2182 | 2182 | 1.3 | 0.25 | 1.9 | 0.31 | - | - | [61] |
| 58 | δ-elemene | 1330 | 1335 | 0.1 | 0.02 | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 59 | unidentified (MW = 150) | 1332 | - | - | - | - | - | 0.9 | 0.04 | - | 1801 | - | - | - | - | - | 0.7 | 0.11 | - |
| 60 | (2E,4E)-decadienal | 1334 | 1334 | - | - | - | - | [15] | 1801 | 1800 | - | - | - | - | [62] | ||||
| 61 | α-cubebene | 1347 | 1348 | trace | - | 0.1 | 0.01 | - | - | [15] | 1416 | 1420 | 0.5 | 0.03 | trace | - | - | - | [63] |
| 62 | neryl acetate | 1367 | 1359 | - | - | 0.3 | 0.02 | - | - | [15] | 1682 | 1680 | - | - | 0.1 | 0.01 | - | - | [49] |
| 63 | α-ylangene | 1374 | 1373 | 0.5 | 0.02 | - | - | - | - | [15] | 1447 | 1450 | 0.5 | 0.05 | - | - | - | - | [64] |
| 64 | α-copaene | 1377 | 1374 | - | - | 3.6 | 0.43 | 0.7 | 0.17 | [15] | 1469 | 1471 | - | - | 3.7 | 0.42 | 0.7 | 0.17 | [65] |
| 65 | β-bourbonene | 1384 | 1387 | - | - | 0.2 | 0.01 | - | - | [15] | 1497 | 1490 | - | - | 0.2 | 0.02 | - | - | [66] |
| 66 | α-isocomene | 1386 | 1387 | - | - | - | - | 0.1 | 0.06 | [15] | 1500 | 1510 | - | - | - | - | 0.2 | 0.03 | [65] |
| 67 | geranyl acetate | 1386 | 1379 | - | - | 2.8 | 0.29 | - | - | [15] | 1716 | 1713 | - | - | 2.9 | 0.25 | - | - | [50] |
| 68 | β-cubebene | 1386 | 1387 | 0.4 | 0.02 | 1.0 | 0.04 | - | - | [15] | 1510 | 1508 | trace | - | 0.8 | 0.07 | - | - | [67] |
| 69 | β-elemene | 1389 | 1389 | 0.2 | 0.01 | - | - | [15] | 1554 | 1559 | trace | - | - | - | - | - | [68] | ||
| 70 | (E)-β-damascenone | 1389 | 1383 | - | - | - | - | 1.7 | 0.31 | [15] | 1808 | 1803 | - | - | - | - | o.t.p. 101 | - | [69] |
| 71 | decanoic acid | 1391 | 1364 | - | - | - | - | [15] | 2243 | 2244 | - | - | - | - | 1.6 | 0.56 | [70] | ||
| 72 | n-tetradecane | 1400 | 1400 | trace | - | 0.1 | 0.01 | 0.6 | 0.02 | - | 1400 | 1400 | - | - | 0.1 | 0.01 | 0.5 | 0.19 | - |
| 73 | α-gurjunene | 1408 | 1409 | 0.4 | 0.02 | 0.3 | 0.04 | 0.2 | 0.04 | [15] | 1506 | 1507 | - | - | 0.3 | 0.02 | 0.1 | 0.01 | [71] |
| 74 | methyl eugenol | 1416 | 1403 | - | - | 0.6 | 0.05 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 75 | (E)-β-caryophyllene | 1418 | 1417 | 6.2 | 0.27 | 8.1 | 0.33 | 1.2 | 0.25 | [15] | 1551 | 1550 | 6.4 | 0.20 | 8.3 | 0.46 | 0.7 | 0.17 | [72] |
| 76 | dodecanal | 1420 | 1408 | - | - | - | - | [15] | 1700 | 1698 | - | - | - | - | o.t.p. 93 | - | [73] | ||
| 77 | unidentified (MW = 190) | 1420 | - | - | - | - | - | - | 2115 | - | - | - | - | - | 0.6 | 0.10 | - | ||
| 78 | β-duprezianene | 1421 | 1421 | - | - | - | - | [15] | 2211 | - | - | - | - | - | o.t.p. 126 | - | § | ||
| 79 | β-copaene | 1433 | 1430 | - | - | 0.2 | 0.03 | 0.1 | 0.01 | [15] | 1549 | 1550 | - | - | trace | - | 0.2 | 0.04 | [64] |
| 80 | allo-aromadendrene | 1446 | 1437 | 0.1 | 0.01 | 0.1 | 0.02 | - | - | [15] | - | - | 0.3 | 0.02 | - | - | - | - | [74] |
| 81 | α-humulene | 1452 | 1452 | 0.7 | 0.03 | 1.1 | 0.07 | 1.1 | 0.18 | [15] | 1643 | 1645 | 0.8 | 0.06 | 1.1 | 0.01 | 1.2 | 0.18 | [75] |
| 82 | α-guaiene | 1455 | 1453 | 0.9 | 0.19 | - | - | - | - | [15] | 1586 | 1583 | 1.1 | 0.05 | - | - | - | - | [76] |
| 83 | 9-epi-(E)-caryophyllene | 1463 | 1464 | - | - | - | - | 0.2 | 0.03 | [15] | 1618 | 1630 | - | - | - | - | trace | - | [77] |
| 84 | cis-cadina-1(6),4-diene | 1465 | 1461 | - | - | 0.2 | 0.07 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 85 | 4,5-di-epi-aristolochene | 1466 | 1471 | 0.2 | 0.05 | - | - | - | - | [15] | - | - | o.t.p. 82 | - | - | - | - | - | - |
| 86 | β-acoradiene | 1474 | 1469 | 0.7 | 0.16 | - | - | - | - | [15] | 1691 | 1693 | 0.2 | 0.02 | - | - | - | - | [78] |
| 87 | trans-cadina-1(6),4-diene | 1479 | 1475 | - | - | 0.3 | 0.17 | - | - | [15] | 1613 | - | - | - | 0.5 | 0.12 | - | - | § |
| 88 | γ-muurolene | 1480 | 1478 | - | - | - | - | 0.2 | 0.07 | [15] | 1665 | 1665 | - | - | - | - | 0.1 | 0.09 | [79] |
| 89 | germacrene D | 1486 | 1480 | 35.7 | 1.02 | 20.8 | 0.65 | 14.1 | 2.35 | [15] | 1684 | 1684 | 38.3 | 1.63 | 22.0 | 0.23 | 12.4 | 1.69 | [80] |
| 90 | (E)-β-ionone | 1490 | 1487 | - | - | - | - | 1.9 | 0.84 | [15] | 1925 | 1926 | - | - | - | - | 1.6 | 0.18 | [81] |
| 91 | valencene | 1491 | 1496 | - | - | 0.2 | 0.02 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 92 | α-zingiberene | 1495 | 1493 | - | - | - | - | 0.7 | 0.14 | [15] | 1720 | 1721 | - | - | - | - | 0.6 | 0.22 | [82] |
| 93 | bicyclogermacrene | 1500 | 1500 | 3.8 | 0.11 | 1.2 | 0.05 | 2.6 | 0.54 | [15] | 1708 | 1711 | 4.0 | 0.17 | 1 | 0.03 | 3.0 | 0.66 | [83] |
| 94 | α-muurolene | 1501 | 1500 | 0.2 | 0.03 | 1.2 | 0.05 | - | - | [15] | 1705 | 1700 | 0.2 | 0.01 | 1.1 | 0.02 | - | - | [84] |
| 95 | β-himachalene | 1504 | 1500 | trace | - | - | - | - | - | [15] | 1704 | 1704 | - | - | - | - | [85] | ||
| 96 | (E,E)-α-farnesene | 1509 | 1505 | - | - | trace | - | 2.4 | 1.03 | [15] | 1742 | 1745 | - | - | - | - | 2.5 | 1.01 | [49] |
| 97 | germacrene A | 1511 | 1508 | - | - | trace | - | - | - | [15] | 1737 | 1738 | - | - | trace | - | - | - | [86] |
| 98 | γ-cadinene | 1515 | 1513 | 0.3 | 0.07 | 0.5 | 0.04 | - | - | [15] | 1666 | 1666 | 0.1 | 0.02 | 0.5 | 0.04 | - | - | [87] |
| 99 | δ-amorphene | 1519 | 1511 | 1.2 | 0.12 | - | - | - | - | [15] | 1707 | 1710 | 1.3 | 0.12 | - | - | - | - | [88] |
| 100 | δ-cadinene | 1522 | 1522 | - | - | 2.4 | 0.16 | - | - | [15] | 1713 | 1708 | - | - | 2.0 | 0.16 | - | - | [89] |
| 101 | tridecanal | 1522 | 1509 | - | - | - | - | 6.4 | 0.22 | [15] | 1810 | 1811 | - | - | - | - | 6.2 | 0.24 | [60] |
| 102 | trans-cadina-1,4-diene | 1535 | 1533 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 103 | cis-muurol-5-en-4-β-ol | 1554 | 1550 | - | - | 0.1 | 0.03 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 104 | (E)-nerolidol | 1565 | 1561 | 0.1 | 0.02 | 0.2 | 0.08 | - | - | [15] | 2003 | 2005 | 0.1 | 0.02 | 0.2 | 0.02 | - | - | [73] |
| 105 | elemicin | 1565 | 1555 | - | - | 1.9 | 1.74 | - | - | [15] | 2212 | 2214 | - | - | 1.5 | 0.09 | - | - | [90] |
| 106 | unidentified (MW = 220) | 1577 | - | - | - | - | - | 0.4 | 0.05 | - | 1891 | - | - | - | - | - | 0.2 | 0.03 | - |
| 107 | β-copaen-4-α-ol | 1580 | 1590 | - | - | 0.2 | 0.02 | - | - | [15] | 2226 | - | - | - | 0.1 | 0.01 | - | - | § |
| 108 | germacrene D-4-ol | 1581 | 1574 | 0.5 | 0.18 | 8.4 | 0.49 | - | - | [15] | 2037 | 2038 | 0.6 | 0.07 | 8.6 | 0.43 | - | - | [91] |
| 109 | spathulenol | 1583 | 1577 | 0.6 | 0.14 | - | - | 7.8 | 1.26 | [15] | 2121 | 2121 | - | - | - | - | 7.1 | 1.08 | [92] |
| 110 | unidentified (mw: 220) | 1591 | - | - | - | 0.9 | 0.28 | - | - | - | 2074 | - | - | - | 1.1 | 0.11 | - | - | - |
| 111 | caryophyllene oxide | 1591 | 1582 | 0.5 | 0.15 | 1.8 | 0.26 | 3.1 | 0.16 | [15] | 1955 | 1955 | 0.2 | 0.07 | 1.8 | 0.22 | 1.9 | 0.16 | [89] |
| 112 | unidentified (MW = 220) | 1595 | - | - | - | - | - | - | 1984 | - | - | - | - | - | 0.3 | 0.09 | - | ||
| 113 | unidentified (MW = 220) | 1596 | - | - | - | - | - | - | 2247 | - | - | - | - | - | 0.5 | 0.16 | - | ||
| 114 | n-hexadecane | 1600 | 1600 | - | - | - | - | o.t.p. 111 | - | - | 1600 | 1600 | - | - | - | - | 0.2 | 0.04 | - |
| 115 | guaiol | 1605 | 1600 | - | - | 0.6 | 0.06 | - | - | [15] | 2054 | 2064 | - | - | 0.4 | 0.01 | - | - | [93] |
| 116 | ledol | 1610 | 1602 | 0.4 | 0.04 | - | - | - | - | [15] | 2018 | 2016 | 0.3 | 0.03 | - | - | - | - | [94] |
| 117 | humulene epoxide II | 1621 | 1608 | - | - | - | - | 1.1 | 0.07 | [15] | 2012 | 2024 | - | - | - | - | 0.6 | 0.10 | [95] |
| 118 | tetradecanal | 1623 | 1611 | - | - | - | - | [15] | 1915 | 1919 | - | - | - | - | 0.5 | 0.04 | [96] | ||
| 119 | junenol | 1627 | 1618 | trace | - | - | - | 0.5 | 0.13 | [15] | 2028 | 2028 | trace | - | - | - | 0.2 | 0.06 | [95] |
| 120 | unidentified (MW = 220) | 1629 | - | - | - | - | - | 1.1 | 0.17 | - | 2241 | - | - | - | - | - | o.t.p. 71 | - | - |
| 121 | himachalol | 1641 | 1652 | - | - | 0.10 | 0.04 | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 122 | allo-aromadendrene epoxide | 1641 | 1639 | 0.1 | 0.02 | 0.1 | 0.03 | - | - | [15] | 2092 | 2095 | 0.1 | 0.02 | 0.1 | 0.01 | - | - | [97] |
| 123 | epi-α-cadinol | 1650 | 1638 | 0.3 | 0.05 | 0.7 | 0.11 | - | - | [15] | 2126 | 2126 | 0.3 | 0.04 | 0.7 | 0.08 | - | - | [38] |
| 124 | epi-α-muurolol | 1652 | 1640 | 0.5 | 0.09 | 0.7 | 0.12 | 0.4 | 0.06 | [15] | 2181 | 2182 | 0.9 | 0.06 | 0.8 | 0.10 | 0.5 | 0.14 | [98] |
| 125 | α-muurolol | 1654 | 1644 | 0.1 | 0.08 | 0.4 | 0.13 | 0.5 | 0.12 | [15] | 2166 | 2165 | 0.3 | 0.05 | 0.4 | 0.03 | 0.5 | 0.14 | [99] |
| 126 | α-cadinol | 1664 | 1652 | 0.5 | 0.18 | 1.9 | 0.23 | 1.2 | 0.07 | [15] | 2213 | 2211 | o.t.p. 124 | - | 1.8 | 0.29 | 2.0 | 0.13 | [100] |
| 127 | unidentified (MW = 220) | 1673 | - | - | - | - | - | 0.8 | 0.17 | - | 2067 | - | - | - | - | - | 0.6 | 0.07 | - |
| 128 | epi-zizanone | 1674 | 1668 | 0.1 | 0.02 | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 129 | 14-hydroxy-9-epi-(E)-caryophyllene | 1684 | 1668 | 0.5 | - | - | - | - | - | [15] | 2066 | - | 0.6 | 0.18 | - | - | - | - | § |
| 130 | khusinol | 1692 | 1679 | 0.1 | 0.02 | 0.4 | 0.04 | - | - | [15] | 2237 | - | - | - | 0.4 | 0.02 | - | - | § |
| 131 | n-heptadecane | 1700 | 1700 | - | - | - | - | 0.2 | 0.08 | - | 1700 | 1700 | - | - | - | - | 0.1 | 0.07 | - |
| 132 | amorpha-4,9-dien-2-ol | 1701 | 1700 | 0.1 | 0.04 | - | - | 0.9 | 0.27 | [15] | 2263 | - | 0.1 | 0.03 | - | - | 1.2 | 0.16 | § |
| 133 | n-pentadecanal | 1725 | 1724 | - | - | 0.5 | 0.02 | 2.7 | 0.07 | [15] | 2023 | 2020 | - | - | 0.5 | 0.04 | 2.9 | 0.16 | [31] |
| 134 | β-acoradienol | 1742 | 1762 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 135 | unidentified (MW = 220) | 1781 | - | - | - | - | - | 0.8 | 0.12 | - | 2231 | - | - | - | - | - | 0.6 | 0.19 | - |
| 136 | unidentified (MW = 220) | 1784 | - | - | - | - | - | 0.5 | 0.03 | - | 2268 | - | - | - | - | - | 0.2 | 0.05 | - |
| 137 | avocadynofuran | 1784 | 1780 | - | - | 0.3 | 0.09 | [15] | - | - | - | - | - | - | - | - | - | ||
| 138 | 1-octadecene | 1795 | 1789 | - | - | 0.1 | 0.01 | - | - | [15] | 1835 | 1823 | - | - | 0.4 | 0.04 | - | - | [101] |
| 139 | n-octadecane | 1800 | 1800 | trace | - | trace | - | 0.4 | 0.11 | - | 1800 | 1800 | 0.1 | 0.02 | trace | - | 0.2 | 0.11 | - |
| 140 | 14-hydroxy-δ-cadinene | 1805 | 1803 | trace | - | - | - | - | - | [15] | - | - | - | - | - | - | - | - | - |
| 141 | n-hexadecanal | 1834 | 1830 | 0.1 | 0.02 | 0.1 | 0.02 | 0.8 | 0.22 | [15] | 2134 | 2137 | - | - | - | - | 1.1 | 0.04 | [102] |
| 142 | 6,10,14-trimethyl-2-pentadecanone | 1856 | 1855 | - | - | - | - | 0.9 | 0.08 | [15] | 2127 | 2125 | - | - | - | - | 0.6 | 0.06 | [103] |
| 143 | 1-nonadecene | 1893 | 1895 | - | - | 0.1 | 0.01 | - | - | [15] | 1936 | 1938 | - | - | 0.1 | 0.02 | - | - | [104] |
| 144 | n-nonadecane | 1900 | 1900 | trace | - | 0.5 | 0.01 | 0.2 | 0.03 | - | 1900 | 1900 | trace | - | 0.8 | 0.02 | trace | - | - |
| 145 | (5E,9E)-farnesyl acetone | 1925 | 1913 | - | - | - | - | 0.9 | 0.06 | [15] | 2244 | - | - | - | - | - | 1.1 | 0.22 | § |
| 146 | n-heptadecanal | 1935 | 1930 | trace | - | 0.1 | 0.01 | 1.5 | 0.10 | [15] | 2219 | - | - | - | - | - | 1.6 | 0.42 | § |
| 147 | methyl hexadecanoate | 1938 | 1921 | - | - | - | - | 0.2 | 0.02 | [15] | 2188 | 2191 | - | - | - | - | 0.4 | 0.17 | [105] |
| 148 | unidentified (MW = 262) | 1978 | - | - | - | - | - | 0.6 | 0.07 | - | 2235 | - | - | - | - | - | trace | - | - |
| 149 | 1-eicosene | 1996 | 1987 | - | - | 0.2 | 0.01 | - | - | [15] | 2037 | 2047 | - | - | 0.4 | 0.01 | - | - | [106] |
| 150 | n-eicosane | 2001 | 2000 | - | - | trace | - | 0.1 | 0.02 | - | 2000 | 2000 | - | - | - | - | 0.1 | 0.06 | - |
| 151 | n-octadecanal | 2034 | 2033 | - | - | - | - | 0.2 | 0.02 | [15] | 2359 | 2357 | - | - | - | - | 0.2 | 0.04 | [107] |
| 152 | unidentified (MW = 220) | 2085 | - | - | - | - | - | 0.6 | 0.10 | - | 2294 | - | - | - | - | - | 0.5 | 0.30 | - |
| 153 | 1-heneicosene | 2093 | 2098 | - | - | 0.1 | 0.01 | - | - | [108] | 2137 | 2127 | - | - | 0.2 | 0.02 | - | - | [109] |
| 154 | unidentified (MW = 294) | 2096 | - | - | - | - | - | 1.1 | 0.20 | - | 2376 | - | - | - | - | - | 1.7 | 0.15 | - |
| 155 | n-heneicosane | 2101 | 2100 | 0.1 | 0.07 | 0.4 | 0.06 | 0.4 | 0.01 | - | 2100 | 2100 | trace | - | 0.3 | 0.02 | 0.5 | 0.06 | - |
| 156 | (Z)-phytol | 2120 | 2114 | - | - | - | - | 2.5 | 0.53 | [53] | 2362 | - | - | - | - | - | 2.9 | 0.90 | § |
| 157 | unidentified (MW = 355) | 2152 | - | - | - | - | - | 0.4 | 0.05 | - | 2323 | - | - | - | - | - | 0.2 | 0.10 | - |
| 158 | 1-docosene | 2196 | 2189 | - | - | 0.2 | 0.03 | 0.1 | 0.01 | [15] | 2289 | - | - | - | - | - | 0.7 | 0.02 | § |
| 159 | n-docosane | 2200 | 2200 | - | - | trace | - | 0.1 | 0.01 | - | 2200 | 2200 | - | - | 0.2 | 0.02 | 0.1 | 0.06 | - |
| 160 | n-eicosanal | 2237 | 2229 | - | - | - | - | 0.1 | 0.01 | [110] | 2575 | 2571 | - | - | - | - | 0.2 | 0.02 | [111] |
| 161 | 1-tricosene | 2295 | 2296 | trace | - | 0.1 | 0.03 | trace | - | [108] | 2346 | - | trace | - | - | - | 0.5 | 0.05 | § |
| 162 | n-tricosane | 2300 | 2300 | - | - | 0.5 | 0.06 | 0.4 | 0.03 | - | 2300 | 2300 | - | - | 0.3 | 0.06 | 0.1 | 0.01 | - |
| 163 | 1-tetracosene | 2396 | 2396 | - | - | 0.1 | 0.04 | 1.5 | 0.15 | [108] | 2467 | - | - | - | 0.2 | 0.02 | 0.4 | 0.15 | § |
| 164 | n-tetracosane | 2400 | 2400 | - | - | 0.1 | 0.03 | - | 2400 | 2400 | - | - | trace | - | 1.1 | 0.17 | - | ||
| 165 | n-docosanal | 2440 | 2434 | - | - | - | - | 0.5 | 0.25 | [110] | 2425 | - | - | - | - | - | 0.4 | 0.05 | § |
| 166 | 1-pentacosene | 2496 | 2496 | - | - | 0.2 | 0.01 | 1.0 | 0.07 | [108] | 2476 | 2488 | - | - | 0.2 | 0.02 | 1.1 | 0.27 | [63] |
| 167 | n-pentacosane | 2500 | 2500 | - | - | 0.2 | 0.01 | 1.0 | 0.10 | - | 2500 | 2500 | - | - | 0.3 | 0.07 | 1.1 | 0.19 | - |
| 168 | 1-hexacosene | 2596 | 2596 | - | - | 0.1 | 0.07 | 1.4 | 0.21 | [112] | 2551 | - | - | - | 0.5 | 0.06 | 1.6 | 0.29 | § |
| 169 | n-hexacosane | 2600 | 2600 | - | - | 0.1 | 0.07 | - | - | - | 2600 | 2600 | - | - | 0.3 | 0.04 | - | - | - |
| monoterpenes | 35.0 | 19.5 | 8.5 | 32.8 | 18.7 | 7.8 | |||||||||||||
| oxygenated monoterpenoids | 0.4 | 7.0 | 4.2 | 0.5 | 7.0 | 3.3 | |||||||||||||
| sesquiterpenes | 52.4 | 43.9 | 25.3 | 55.0 | 43.4 | 21.7 | |||||||||||||
| oxygenated sesquiterpenoids | 4.4 | 16.8 | 20.6 | 3.5 | 16.4 | 18.0 | |||||||||||||
| others | 1.0 | 7.7 | 35.6 | 0.9 | 8.3 | 38.5 | |||||||||||||
| total | 93.2 | 94.9 | 94.2 | 92.7 | 93.8 | 89.3 | |||||||||||||
| Chiral Selector | Enantiomers | LRI | G. hallii | G. calyculisolvens | G. azuayensis | |||
|---|---|---|---|---|---|---|---|---|
| Distribution (%) | ee (%) | Distribution (%) | ee (%) | Distribution (%) | ee (%) | |||
| DAC | (1S,5S)-(–)-α-pinene | 926 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| DAC | (1R,5R)-(+)-α-pinene | 928 | - | - | - | |||
| DET | (1R,5R)-(+)-β-pinene | 949 | 9.1 | 81.9 | 9.0 | 82.0 | 69.9 | 39.7 |
| DET | (1S,5S)-(–)-β-pinene | 960 | 90.9 | 91.0 | 30.1 | |||
| DET | (1R,5R)-(+)-sabinene | 977 | 21.6 | 56.7 | 47.5 | 5.1 | 92.9 | 85.9 |
| DET | (1S,5S)-(–)-sabinene | 992 | 78.4 | 52.5 | 7.1 | |||
| DET | (R)-(–)-α-phellandrene | 1022 | - | - | 100.0 | 100.0 | - | - |
| DET | (S)-(+)-α-phellandrene | 1025 | - | - | - | |||
| DET | (R)-(–)-β-phellandrene | 1052 | 100.0 | 100.0 | - | - | - | - |
| DET | (S)-(+)-β-phellandrene | 1063 | - | - | - | |||
| DET | (S)-(–)-limonene | 1059 | 60.8 | 21.6 | 94.1 | 88.2 | - | - |
| DET | (R)-(+)-limonene | 1075 | 39.2 | 5.9 | - | |||
| DET | (R)-(–) cis-linalool oxide (furanoid) | 1099 | - | - | - | - | 30.8 | 38.4 |
| DET | (S)-(+) cis-linalool oxide (furanoid) | 1104 | - | - | 69.2 | |||
| DET | (R)-(–)-linalool | 1181 | - | - | - | - | 48.4 | 3.3 |
| DET | (S)-(+)-linalool | 1194 | - | - | 51.6 | |||
| DET | (S)-(−)-α-terpineol | 1300 | - | - | - | - | 39.4 | 21.2 |
| DET | (R)-(+)-α-terpineol | 1313 | - | - | 60.6 | |||
| DET | (1R,2S,6S,7S,8S)-(–)-α-copaene | 1321 | - | - | - | 100.0 | - | 100.0 |
| DET | (1S,2R,6R,7R,8R)-(+)-α-copaene | 1323 | - | 100.0 | 100.0 | |||
| DAC | (R)-(–)-terpinen-4-ol | 1338 | 66.7 | 33.4 | 43.5 | 14.0 | 100.0 | - |
| DAC | (S)-(+)-terpinen-4-ol | 1375 | 33.3 | 57.5 | - | |||
| DET | (R)-(+)-germacrene D | 1459 | - | 100.0 | 3.1 | 93.8 | 1.1 | 97.8 |
| DET | (S)-(–)-germacrene D | 1467 | 100.0 | 96.9 | 98.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maldonado, Y.E.; Rodríguez, M.d.C.; Bustamante, M.E.; Cuenca, S.; Malagón, O.; Cumbicus, N.; Gilardoni, G. Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity. Plants 2025, 14, 659. https://doi.org/10.3390/plants14050659
Maldonado YE, Rodríguez MdC, Bustamante ME, Cuenca S, Malagón O, Cumbicus N, Gilardoni G. Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity. Plants. 2025; 14(5):659. https://doi.org/10.3390/plants14050659
Chicago/Turabian StyleMaldonado, Yessenia E., María del Carmen Rodríguez, María Emilia Bustamante, Stefanny Cuenca, Omar Malagón, Nixon Cumbicus, and Gianluca Gilardoni. 2025. "Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity" Plants 14, no. 5: 659. https://doi.org/10.3390/plants14050659
APA StyleMaldonado, Y. E., Rodríguez, M. d. C., Bustamante, M. E., Cuenca, S., Malagón, O., Cumbicus, N., & Gilardoni, G. (2025). Gynoxys hallii Hieron., Gynoxys calyculisolvens Hieron., and Gynoxys azuayensis Cuatrec. Essential Oils—Chemical and Enantioselective Analyses of Three Unprecedented Volatile Fractions from the Ecuadorian Biodiversity. Plants, 14(5), 659. https://doi.org/10.3390/plants14050659








