Development of Forest Tree Species Composition: Selected Results of the National Forest Inventory of Lithuania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lithuania as a Case Study
2.2. Lithuanian National Forest Inventory
2.3. Forest Vegetation and Soil Dynamics
2.4. Forest Development
| Potential Natural Forest Vegetation (Forest Type Series) * | Forest Development Phases: Successional Status of Tree Species | |||
|---|---|---|---|---|
| Stand Initiation (0–20 Years): Pioneer | Stem Exclusion and Understorey Initiation (20–60 Years): Post-Pioneer | Understorey Reinitiation (60–120 Years): Pre-Climax | Steady State (>120 Years): Climax | |
| Climatic (aeg cmh hox mox ox oxn) | Pinus sylvestris (hox mox ox) Betula pubescens (aeg cmh oxn) Betula pendula (aeg cmh hox mox ox oxn) Salix fragilis (aeg) Populus tremula (aeg cmh hox mox ox oxn) Alnus incana (aeg cmh hox oxn) Alnus glutinosa (aeg cmh oxn) | Pinus sylvestris (hox mox ox) Betula pubescens (aeg cmh oxn) Betula pendula (aeg cmh hox mox ox oxn) Salix fragilis (aeg) Populus tremula (aeg cmh hox mox ox oxn) Alnus incana (aeg cmh hox oxn) Alnus glutinosa (aeg cmh oxn) Quercus robur (aeg cmh hox mox ox oxn) Fraxinus excelsior (aeg cmh oxn) Carpinus betulus (aeg hox) Picea abies (aeg hox mox ox oxn) | Pinus sylvestris (hox mox ox) Betula pendula (aeg cmh hox mox ox oxn) Alnus glutinosa (aeg cmh oxn) Quercus robur (aeg cmh hox mox ox oxn) Fraxinus excelsior (aeg cmh oxn) Carpinus betulus (aeg hox) Picea abies (aeg hox mox ox oxn) Ulmus glabra (aeg cmh hox oxn) Ulmus laevis (aeg cmh hox) Tilia cordata (aeg cmh hox oxn) Acer platanoides (aeg cmh hox ox oxn) Fagus sylvatica (hox) | Pinus sylvestris (hox mox ox) Quercus robur (aeg cmh hox mox ox oxn) Fraxinus excelsior (aeg cmh oxn) Carpinus betulus (aeg hox) Picea abies (aeg hox mox ox oxn) Ulmus glabra (aeg cmh hox oxn) Ulmus laevis (aeg cmh hox) Tilia cordata (aeg cmh hox oxn) Acer platanoides (aeg cmh hox ox oxn) Fagus sylvatica (hox) |
| Edaphic (cal cl fil m ur v vm) | Pinus sylvestris (v cl vm m) Betula pubescens (cal fil ur) Betula pendula (cal fil m ur v vm) Salix fragilis (ur) Salix alba (ur) Populus tremula (m vm) Alnus incana (fil) Alnus glutinosa (cal fil ur) | Pinus sylvestris (v cl vm m) Betula pubescens (cal fil ur) Betula pendula (cal fil m ur v vm) Salix fragilis (ur) Salix alba (ur) Populus tremula (m vm) Alnus incana (fil) Alnus glutinosa (cal fil ur) Quercus robur (cal) Fraxinus excelsior (fil ur) Carpinus betulus (ox) Picea abies (cal fil m ur vm) | Pinus sylvestris (v cl vm m) Betula pendula (cal fil m ur v vm) Alnus glutinosa (cal fil ur) Quercus robur (cal) Fraxinus excelsior (fil ur) Carpinus betulus (ox) Picea abies (cal fil m ur vm) | Pinus sylvestris (cl m v vm) Quercus robur (cal) Fraxinus excelsior (fil ur) Carpinus betulus (ox) Picea abies (cal fil m ur vm) |
| Biotic (c cir csp lsp msp) | Pinus sylvestris (csp lsp msp) Betula pubescens (c cir csp msp) Alnus glutinosa (c cir) | Pinus sylvestris (csp lsp msp) Betula pubescens (c cir csp msp) Alnus glutinosa (c cir) Picea abies (c cir) | Pinus sylvestris (csp lsp msp) Alnus glutinosa (c cir) Picea abies (c cir) | Pinus sylvestris (csp lsp msp) Picea abies (c cir) |
3. Results
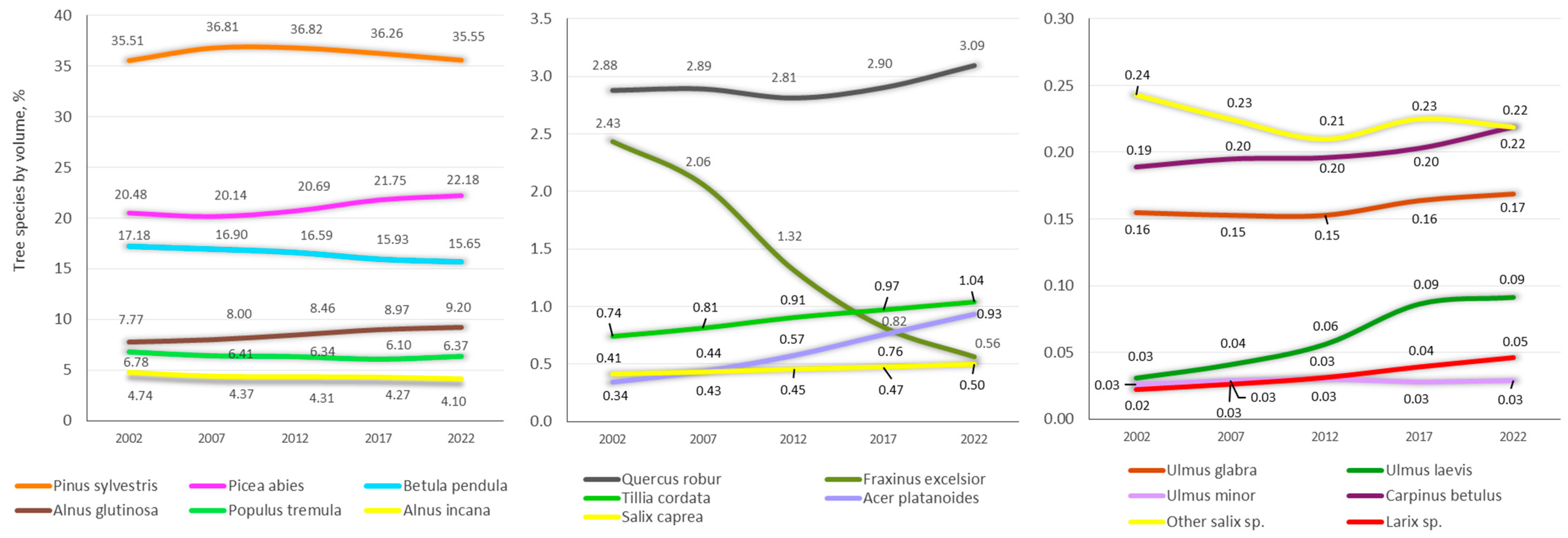
3.1. Growing Stock Volume of Lithuanian Forest Stands
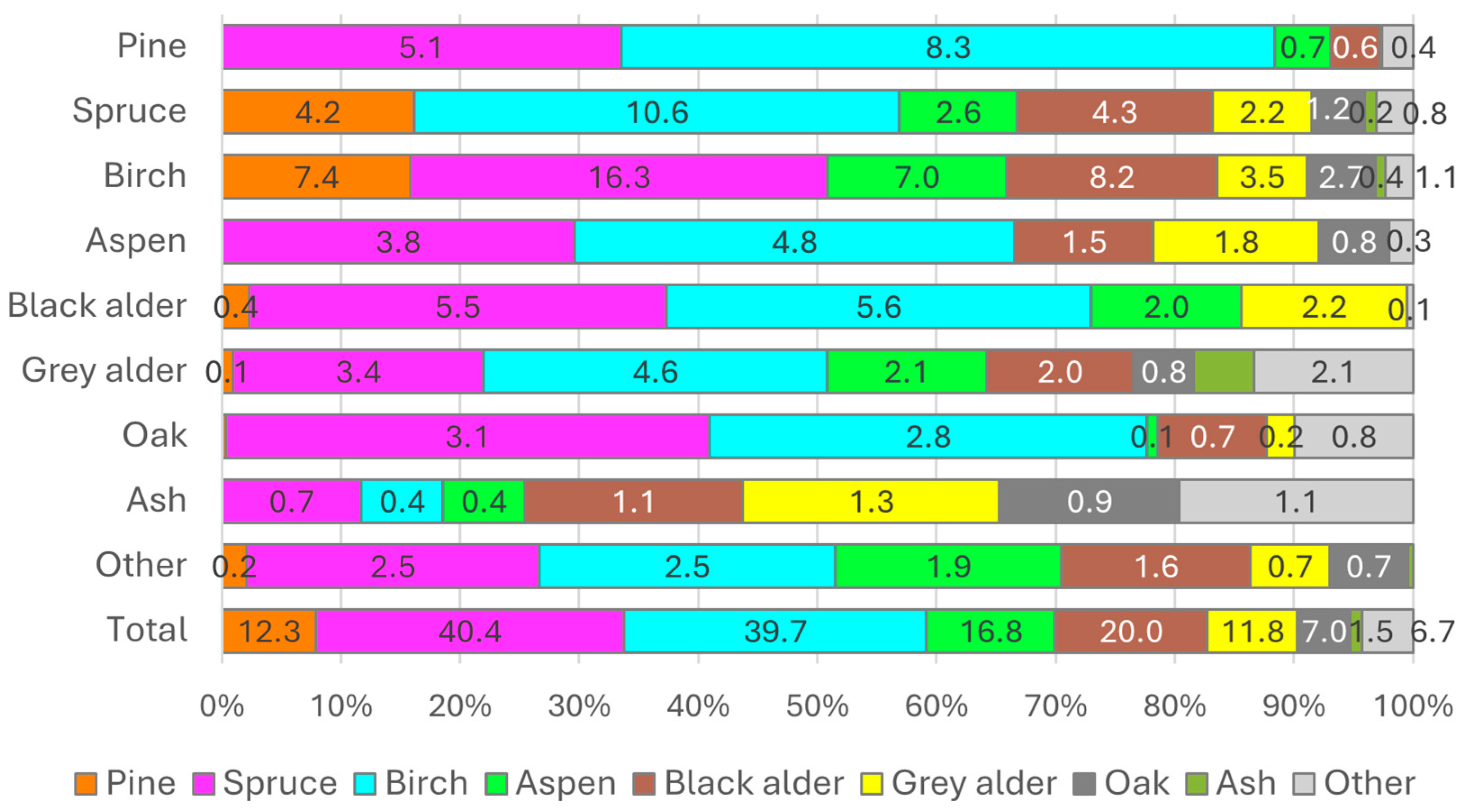
3.2. Growing Stock Composition and Structure of Lithuanian Forest Stands
3.3. Stand Regeneration
4. Discussion and Conclusions
4.1. Implications of Current Forest Management
4.2. A Case for Closer-to-Nature Forest Management
5. Final Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Larsen, J.B.; Angelstam, P.; Bauhus, J.; Carvalho, J.F.; Diaci, J.; Dobrowolska, D.; Gazda, A.; Gustafsson, L.; Krumm, F.; Knoke, T. Closer-to-Nature Forest Management. From Science to Policy 12; EFI European Forest Institute: Joensuu, Finland, 2022; Volume 12. [Google Scholar]
- Motta, R.; Larsen, J.B. Un Nuovo Paradigma per La Gestione Forestale Sostenibile: La Selvicoltura “Più” Prossima Alla Natura. For. J. Silvic. For. Ecol. 2022, 19, 52. [Google Scholar] [CrossRef]
- European Commission. Guidelines on Closer-to-Nature Forest Management; Publications Office of the European Union: Brussels, Belgium, 2023; ISBN 978-92-68-00222-3. [Google Scholar]
- Kaliszewski, A. Forest Policy Goals in Poland in Light of the Current Forestry Aims in Europe Part 2. Forest Policy Priorities in Europe. Leśne Pr. Badaw. 2018, 79, 169–179. [Google Scholar] [CrossRef]
- Carnus, J.-M.; Hengeveld, G.; Mason, B. Sustainability Impact Assessment of Forest Management Alternatives in Europe: An Introductory Background and Framework. Ecol. Soc. 2012, 17, 49. [Google Scholar] [CrossRef]
- Rosa, F.; Di Fulvio, F.; Lauri, P.; Felton, A.; Forsell, N.; Pfister, S.; Hellweg, S. Can Forest Management Practices Counteract Species Loss Arising from Increasing European Demand for Forest Biomass under Climate Mitigation Scenarios? Environ. Sci. Technol. 2023, 57, 2149–2161. [Google Scholar] [CrossRef]
- Sierota, Z.; Miścicki, S. Is It Possible to Compromise Forest Conservation with Forest Use? Earth 2022, 3, 1059–1075. [Google Scholar] [CrossRef]
- Kuusk, A.; Pisek, J.; Lang, M.; Märdla, S. Estimation of Gap Fraction and Foliage Clumping in Forest Canopies. Remote Sens. 2018, 10, 1153. [Google Scholar] [CrossRef]
- Petrokas, R.; Baliuckas, V.; Manton, M. Successional Categorization of European Hemi-Boreal Forest Tree Species. Plants 2020, 9, 1381. [Google Scholar] [CrossRef] [PubMed]
- McRoberts, R.E.; Tomppo, E.O.; Næsset, E. Advances and Emerging Issues in National Forest Inventories. Scand. J. For. Res. 2010, 25, 368–381. [Google Scholar] [CrossRef]
- Fridman, J.; Holm, S.; Nilsson, M.; Nilsson, P.; Ringvall, A.H.; Ståhl, G. Adapting National Forest Inventories to Changing Requirements—The Case of the Swedish National Forest Inventory at the Turn of the 20th Century. Silva Fenn. 2014, 48, 1095. [Google Scholar] [CrossRef]
- Berglund, H.; Kuuluvainen, T. Representative Boreal Forest Habitats in Northern Europe, and a Revised Model for Ecosystem Management and Biodiversity Conservation. Ambio 2021, 50, 1003–1017. [Google Scholar] [CrossRef]
- Manton, M.; Ruffner, C.; Kibirkštis, G.; Brazaitis, G.; Marozas, V.; Pukienė, R.; Makrickiene, E.; Angelstam, P. Fire Occurrence in Hemi-Boreal Forests: Exploring Natural and Cultural Scots Pine Fire Regimes Using Dendrochronology in Lithuania. Land 2022, 11, 260. [Google Scholar] [CrossRef]
- Petrokas, R.; Manton, M.; Kavaliauskas, D. Tree Regeneration and Ontogenetic Strategies of Northern European Hemiboreal Forests: Transitioning towards Closer-to-Nature Forest Management. PeerJ 2024, 12, e17644. [Google Scholar] [CrossRef] [PubMed]
- Gough, C.M.; Curtis, P.S.; Hardiman, B.S.; Scheuermann, C.M.; Bond-Lamberty, B. Disturbance, Complexity, and Succession of Net Ecosystem Production in North America’s Temperate Deciduous Forests. Ecosphere 2016, 7, e01375. [Google Scholar] [CrossRef]
- Šilingas, M.; Suchockas, V.; Varnagirytė-Kabašinskienė, I. Evaluation of Undergrowth under the Canopy of Deciduous Forests on Very Fertile Soils in the Lithuanian Hemiboreal Forest. Forests 2022, 13, 2172. [Google Scholar] [CrossRef]
- Petrokas, R.; Ibanga, D.-A.; Manton, M. Deep Ecology, Biodiversity and Assisted Natural Regeneration of European Hemiboreal Forests. Diversity 2022, 14, 892. [Google Scholar] [CrossRef]
- Jõgiste, K.; Korjus, H.; Stanturf, J.A.; Frelich, L.E.; Baders, E.; Donis, J.; Jansons, A.; Kangur, A.; Köster, K.; Laarmann, D.; et al. Hemiboreal Forest: Natural Disturbances and the Importance of Ecosystem Legacies to Management. Ecosphere 2017, 8, e01706. [Google Scholar] [CrossRef]
- Batcheler, M.; Smith, M.M.; Swanson, M.E.; Ostrom, M.; Carpenter-Boggs, L. Assessing Silvopasture Management as a Strategy to Reduce Fuel Loads and Mitigate Wildfire Risk. Sci. Rep. 2024, 14, 5954. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.S.I.; Barov, B.; Burfield, I.J.; Gregory, R.D.; Norris, K.; Butler, S.J. Quantifying the Detrimental Impacts of Land-Use and Management Change on European Forest Bird Populations. PLoS ONE 2013, 8, e64552. [Google Scholar] [CrossRef] [PubMed]
- Petrokas, R.; Manton, M. Adaptive Relationships in Hemi-Boreal Forests: Tree Species Responses to Competition, Stress, and Disturbance. Plants 2023, 12, 3256. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.-J.; Nabuurs, G.-J.; Hengeveld, G.; Reyer, C.; Hanewinkel, M.; Zimmermann, N.E.; Cullmann, D. Alternative Forest Management Strategies to Account for Climate Change-Induced Productivity and Species Suitability Changes in Europe. Reg. Environ. Change 2015, 15, 1581–1594. [Google Scholar] [CrossRef]
- Corona, P.; Chirici, G.; McRoberts, R.E.; Winter, S.; Barbati, A. Contribution of Large-Scale Forest Inventories to Biodiversity Assessment and Monitoring. For. Ecol. Manag. 2011, 262, 2061–2069. [Google Scholar] [CrossRef]
- Santoro, M.; Eriksson, L.E.B.; Fransson, J.E.S. Reviewing ALOS PALSAR Backscatter Observations for Stem Volume Retrieval in Swedish Forest. Remote Sens. 2015, 7, 4290–4317. [Google Scholar] [CrossRef]
- Armolaitis, K.; Varnagirytė-Kabašinskienė, I.; Žemaitis, P.; Stakėnas, V.; Beniušis, R.; Kulbokas, G.; Urbaitis, G. Evaluation of Organic Carbon Stocks in Mineral and Organic Soils in Lithuania. Soil Use Manag. 2022, 38, 355–368. [Google Scholar] [CrossRef]
- Bradshaw, C.J.A.; Warkentin, I.G. Global Estimates of Boreal Forest Carbon Stocks and Flux. Glob. Planet. Change 2015, 128, 24–30. [Google Scholar] [CrossRef]
- Pereira, P.; Mierauskas, P.; Novara, A. Stakeholders’ Perceptions about Fire Impacts on Lithuanian Protected Areas. Land Degrad. Dev. 2016, 27, 871–883. [Google Scholar] [CrossRef]
- Brukas, V. New World, Old Ideas—A Narrative of the Lithuanian Forestry Transition. J. Environ. Policy Plan. 2015, 17, 495–515. [Google Scholar] [CrossRef]
- State Forest Service Lithuanian Forestry Statistics 2021. Available online: https://amvmt.lrv.lt/lt/atviri-duomenys-1/misku-statistikos-leidiniai/misku-ukio-statistika/2021-m-1/ (accessed on 23 January 2025).
- Kuliešis, A.; Kulbokas, G.; Kasperavičius, A.; Kazanavičiūtė, V.; Kvalkauskienė, M. Lithuanian National Forest Inventory, 1998–2017. From Measurements to Decision Making; Lututė: Kaunas, Lithuania, 2021; ISBN 978-9955-37-234-9. [Google Scholar]
- Mason, W.L.; Diaci, J.; Carvalho, J.; Valkonen, S. Continuous Cover Forestry in Europe: Usage and the Knowledge Gaps and Challenges to Wider Adoption. For. Int. J. For. Res. 2022, 95, 1–12. [Google Scholar] [CrossRef]
- Babelytė, A. Assessment of the Risk of Unsustainable Production of Forest Biomass for Lithuania—SURE System; Lithuanian Biomass Energy Association: Vilnius, Lithuania, 2023; p. 52. [Google Scholar]
- Hallas, T.; Steyrer, G.; Laaha, G.; Hoch, G. Two Unprecedented Outbreaks of the European Spruce Bark Beetle, Ips Typographus L. (Col., Scolytinae) in Austria since 2015: Different Causes and Different Impacts on Forests. Cent. Eur. For. J. 2024, 70, 263–274. [Google Scholar] [CrossRef]
- Pliūra, A.; Bakys, R.; Suchockas, V.; Marčiulynienė, D.; Gustienė, A.; Verbyla, V.; Lygis, V. Ash Dieback in Lithuania: Disease History, Research on Impact and Genetic Variation in Disease Resistance, Tree Breeding and Options for Forest Management. In Dieback of European Ash (Fraxinus spp.): Consequences and Guidelines for Sustainable Management; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017; pp. 150–165. ISBN 978-91-576-8696-1. [Google Scholar]
- Marçais, B.; Kosawang, C.; Laubray, S.; Kjær, E.; Kirisits, T. Chapter 13—Ash Dieback. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 215–237. [Google Scholar]
- Persson, R.; Holmgren, P. Evolution and Prospects of Global Forest Assessments. Available online: http://www.fao.org/docrep/005/y4001e/Y4001E02.htm (accessed on 24 January 2025).
- Grantham, H.S.; Duncan, A.; Evans, T.D.; Jones, K.R.; Beyer, H.L.; Schuster, R.; Walston, J.; Ray, J.C.; Robinson, J.G.; Callow, M.; et al. Anthropogenic Modification of Forests Means Only 40% of Remaining Forests Have High Ecosystem Integrity. Nat. Commun. 2020, 11, 5978. [Google Scholar] [CrossRef] [PubMed]
- Lithuanian State Forest Service On the approval of the Forest Management Work. Approved by Order No 11-10-V of the Director of the Lithuanian State Forest Service. 14 January 2010. Available online: https://e-seimas.lrs.lt/portal/legalAct/lt/TAD/TAIS.370142/asr (accessed on 14 December 2024).
- Žemaitis, P.; Armoška, E.; Stakėnas, V.; Kulbokas, G. Wood Decay and Norway Spruce Vulnerability to Wind-Inflicted Mortality in Monospecific and Mixed Stands in Hemiboreal Forests. For. Ecol. Manag. 2024, 569, 122163. [Google Scholar] [CrossRef]
- Kuliešis, A.; Kasperavičius, A.; Kulbokas, G.; Brukas, V.; Petrauskas, E.; Mozgeris, G. Lithuania Forest Inventory-Based Projection Systems for Wood and Biomass Availability; Barreiro, S., Schelhaas, M.-J., McRoberts, R.E., Kändler, G., Eds.; Managing Forest Ecosystems; Springer International Publishing: Cham, Switzerland, 2017; Volume 29, pp. 223–239. ISBN 978-3-319-56199-8. [Google Scholar]
- Kuliešis, A.; Kulbokas, G.; Kasperavičius, A.; Kuliešis, A.A. Miškų inventorizacijos sistema ir jos tobulinimas pagal intensyvios miškininkystės reikmes. Miškininkystė 2010, 68, 61–77. [Google Scholar]
- Kuliešis, A.; Kasperavičius, A.; Kulbokas, G. Lithuania. In National Forest Inventories; Vidal, C., Alberdi, I.A., Hernández Mateo, L., Redmond, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 521–547. ISBN 978-3-319-44014-9. [Google Scholar]
- Kuliešis, A.; Kasperavičius, A.; Kulbokas, G. Brandžių medynų tūrių kaitos ir pagamintos medienos apskaitos iššūkiai. Mūsų Girios 2016, 3, 9–10. [Google Scholar]
- Madrigal-González, J.; Ruiz-Benito, P.; Ratcliffe, S.; Calatayud, J.; Kändler, G.; Lehtonen, A.; Dahlgren, J.; Wirth, C.; Zavala, M.A. Complementarity Effects on Tree Growth Are Contingent on Tree Size and Climatic Conditions across Europe. Sci. Rep. 2016, 6, 32233. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Isbell, F.; Deng, W.; Hong, P.; Dee, L.E.; Thompson, P.; Loreau, M. How Complementarity and Selection Affect the Relationship between Ecosystem Functioning and Stability. Ecology 2021, 102, e03347. [Google Scholar] [CrossRef] [PubMed]
- Smallidge, P. Forest Succession and Management—Cornell Small Farms. Available online: https://smallfarms.cornell.edu/2016/04/forest-succession-and-management/ (accessed on 16 November 2024).
- Anyomi, K.A.; Neary, B.; Chen, J.; Mayor, S.J. A Critical Review of Successional Dynamics in Boreal Forests of North America. Environ. Rev. 2022, 30, 563–594. [Google Scholar] [CrossRef]
- Samec, P.; Volánek, J.; Kučera, M.; Cudlín, P. Effect of Soil Diversity on Forest Plant Species Abundance: A Case Study from Central-European Highlands. Forests 2021, 12, 534. [Google Scholar] [CrossRef]
- Sellan, G.; Thompson, J.; Majalap, N.; Brearley, F.Q. Soil Characteristics Influence Species Composition and Forest Structure Differentially among Tree Size Classes in a Bornean Heath Forest. Plant Soil 2019, 438, 173–185. [Google Scholar] [CrossRef]
- Reczyńska, K. Diversity and Ecology of Oak Forests in SW Poland (Sudetes Mts.). Phytocoenologia 2015, 45, 85–106. [Google Scholar] [CrossRef]
- Li, Y.; Henrion, M.; Moore, A.; Lambot, S.; Opfergelt, S.; Vanacker, V.; Jonard, F.; Van Oost, K. Factors Controlling Peat Soil Thickness and Carbon Storage in Temperate Peatlands Based on UAV High-Resolution Remote Sensing. Geoderma 2024, 449, 117009. [Google Scholar] [CrossRef]
- Stutz, K.P.; Lang, F. Forest Ecosystems Create Pedogenic Patchworks through Woody Debris, Trees, and Disturbance. Geoderma 2023, 429, 116246. [Google Scholar] [CrossRef]
- Krotiuk, A.; Bedernichek, T. Concept of Forest Development Phases: Identification and Classification Issues. Environ. Sci. Proc. 2020, 3, 9. [Google Scholar] [CrossRef]
- Spathelf, P.; Bolte, A. Continuous-Cover Forestry—The Appropriate Concept for Climate Change Adaptation of Forests in Germany? Ciência Florest. 2025, 35, e90715. [Google Scholar] [CrossRef]
- Buivydaitė, V. Classification of Soils of Lithuania Based on FAO-Unesco Soil Classification System and WRB. In Proceedings of the 17 World Congress of Soil Science, Bangkok, Thailand, 14–20 August 2002; pp. 2189-1–2189-13. [Google Scholar]
- Anjos, L.; Gaistardo, C.C.; Deckers, J.; Dondeyne, S.; Eberhardt, E.; Gerasimova, M.; Harms, B.; Jones, A.; Krasilnikov, P.; Reinsch, T.; et al. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; FAO: Rome, Italy, 2015. [Google Scholar]
- Vaičys, M.; Mažvila, J. The Influence of Soil Characteristics on Plant Productivity and Ecological Stability. Ekologija 2009, 55, 99–106. [Google Scholar] [CrossRef]
- Brazaitis, G.; Marozas, V.; Augutis, D.; Preikša, Ž.; Šaudytė-Manton, S. Lithuanian Forest Habitat Management Recommendations—“Guidelines for the Management of Natural Forest Habitat Types of EC Importance”; Naturalit: Vilnius, Lietuva, 2021. [Google Scholar]
- Petrokas, R.; Kavaliauskas, D. Concept for Genetic Monitoring of Hemiboreal Tree Dynamics in Lithuania. Land 2022, 11, 1249. [Google Scholar] [CrossRef]
- Vaičys, M. Miško Augaviečių Tipai; Ozolinčius, R., Ed.; Lututė: Kaunas, Lietuva, 2006; ISBN 978-9955-692-41-6. [Google Scholar]
- Buivydaitė, V.; Vaičys, M.; Juodis, J.; Motuzas, A.-J. Lietuvos Dirvožemių Klasifikacija. Kn. 34; Lietuvos Mokslas: Vilnius, Lietuva, 2001; ISBN 9986795118. [Google Scholar]
- Vester, H.F. Forest development as a basis for management: Tree architecture and tree temperaments. In Ecology and Management of Tropical Secondary Forest: Science, People, and Policy, Proceedings of a Conference Held at CATIE, Turrialba, Costa Rica, 10–12 November 1997; Guariguata, M.R., Finegan, B., Eds.; Serie Técnica: Reuniones Técnicas; Centro Agronómico Tropical de Investigación y Enseñanza: Turrialba, Costa Rica, 1998; Volume 4, pp. 35–48. ISBN 9977-57-318-2. [Google Scholar]
- Oliver, C.D.; Larson, B.C. Brief Notice: Forest Stand Dynamics (Update Edition). For. Sci. 1996, 42, 397. [Google Scholar] [CrossRef]
- Gray, A.N.; Pelz, K.; Hayward, G.D.; Schuler, T.; Salverson, W.; Palmer, M.; Schumacher, C.; Woodall, C.W. Perspectives: The Wicked Problem of Defining and Inventorying Mature and Old-Growth Forests. For. Ecol. Manag. 2023, 546, 121350. [Google Scholar] [CrossRef]
- Kimmins, J.P. Forest Ecosystem Management: An Environmental Necessity, but Is It a Practical Reality or Simply an Ecotopian Ideal. In Proceedings of the XII World Forestry Congress, Québec, QC, Canada, 21–28 September 2003. [Google Scholar]
- Taki, H.; Yamaura, Y.; Okochi, I.; Inoue, T.; Okabe, K.; Makino, S. Effects of Reforestation Age on Moth Assemblages in Plantations and Naturally Regenerated Forests. Insect Conserv. Divers. 2010, 3, 257–265. [Google Scholar] [CrossRef]
- Fang, Z.; Bao, W.; Yan, X.; Liu, X. Understory Structure and Vascular Plant Diversity in Naturally Regenerated Deciduous Forests and Spruce Plantations on Similar Clear-Cuts: Implications for Forest Regeneration Strategy Selection. Forests 2014, 5, 715–743. [Google Scholar] [CrossRef]
- Bremer, L.L.; Farley, K.A. Does Plantation Forestry Restore Biodiversity or Create Green Deserts? A Synthesis of the Effects of Land-Use Transitions on Plant Species Richness. Biodivers. Conserv. 2010, 19, 3893–3915. [Google Scholar] [CrossRef]
- Dobrowski, S.Z.; Aghai, M.M.; Chichilnisky du Lac, A.; Downer, R.; Fargione, J.; Haase, D.L.; Hoecker, T.; Kildisheva, O.A.; Murdoch, A.; Newman, S.; et al. ‘Mind the Gap’—Reforestation Needs vs. Reforestation Capacity in the Western United States. Front. For. Glob. Change 2024, 7, 1402124. [Google Scholar] [CrossRef]
- Song, S.; Ding, Y.; Li, W.; Meng, Y.; Zhou, J.; Gou, R.; Zhang, C.; Ye, S.; Saintilan, N.; Krauss, K.W.; et al. Mangrove Reforestation Provides Greater Blue Carbon Benefit than Afforestation for Mitigating Global Climate Change. Nat. Commun. 2023, 14, 756. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, G.J.; Storck-Tonon, D.; Dáttilo, W.; Izzo, T.J. Is Being Green What Matters? Functional Diversity of Cavity-Nesting Bees and Wasps and Their Interaction Networks with Parasites in Different Reforestation Types in Amazonia. Insect Conserv. Divers. 2021, 14, 620–634. [Google Scholar] [CrossRef]
- Shorohova, E.; Kuuluvainen, T.; Kangur, A.; Jõgiste, K. Natural Stand Structures, Disturbance Regimes and Successional Dynamics in the Eurasian Boreal Forests: A Review with Special Reference to Russian Studies. Ann. For. Sci. 2009, 66, 201. [Google Scholar] [CrossRef]
- Lansing, J.S.; Thurner, S.; Chung, N.N.; Coudurier-Curveur, A.; Karakaş, Ç.; Fesenmyer, K.A.; Chew, L.Y. Adaptive Self-Organization of Bali’s Ancient Rice Terraces. Proc. Natl. Acad. Sci. USA 2017, 114, 6504–6509. [Google Scholar] [CrossRef] [PubMed]
- Šilingas, M.; Šilingienė, G. The Lower Storeys of Main Tree Species in Deciduous Pioneer Tree Stands of Fertile Sites: Case of Lithuania. Balt. For. 2022, 28, 208–216. [Google Scholar] [CrossRef]
- Smith, C.; Thwaites, R. ForesTIM: Evaluating Plantation Forest Land Management by Identifying Unsustainable Practices. Aust. For. 2013, 61, 89–102. [Google Scholar] [CrossRef]
- Karazija, S. Forest Types of Lithuania; Mokslas: Vilnius, Lietuva, 1988; ISBN 978-5-420-00421-0. [Google Scholar]
- Kuuluvainen, T.; Gauthier, S. Young and Old Forest in the Boreal: Critical Stages of Ecosystem Dynamics and Management under Global Change. For. Ecosyst. 2018, 5, 26. [Google Scholar] [CrossRef]
- Brassard, B.W.; Chen, H.Y.H. Stand Structural Dynamics of North American Boreal Forests. Crit. Rev. Plant Sci. 2006, 25, 115–137. [Google Scholar] [CrossRef]
- Zeller, L.; Pretzsch, H. Effect of Forest Structure on Stand Productivity in Central European Forests Depends on Developmental Stage and Tree Species Diversity. For. Ecol. Manag. 2019, 434, 193–204. [Google Scholar] [CrossRef]
- Makrickas, E.; Manton, M.; Angelstam, P.; Grygoruk, M. Trading Wood for Water and Carbon in Peatland Forests? Rewetting Is Worth More than Wood Production. J. Environ. Manag. 2023, 341, 117952. [Google Scholar] [CrossRef] [PubMed]
- Esseen, P.-A.; Ehnström, B.; Ericson, L.; Sjöberg, K. Boreal Forests. Ecol. Bull. 1997, 46, 16–47. [Google Scholar]
- Pautasso, M.; Aas, G.; Queloz, V.; Holdenrieder, O. European Ash (Fraxinus Excelsior) Dieback–A Conservation Biology Challenge. Biol. Conserv. 2013, 158, 37–49. [Google Scholar] [CrossRef]
- Barredo, J.I.; Brailescu, C.; Teller, A.; Sabatini, F.M.; Mauri, A.; Janouskova, K. Mapping and Assessment of Primary and Old-Growth Forests in Europe; Amt Fur Veroffentlichungen der EU; Publications Office of the European Union: Luxemburg, 2021. [Google Scholar]
- Hämäläinen, A.; Runnel, K.; Ranius, T.; Strengbom, J. Diversity of Forest Structures Important for Biodiversity Is Determined by the Combined Effects of Productivity, Stand Age, and Management. Ambio 2024, 53, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Kuliešis, A.; Kasperavičius, A.; Kulbokas, G.; Kuliešis, A.A.; Pivoriūnas, A.; Aleinikovas, M.; Šilinskas, B.; Škėma, M.; Beniušienė, L. Using Continuous Forest Inventory Data for Control of Wood Production and Use in Large Areas: A Case Study in Lithuania. Forests 2020, 11, 1039. [Google Scholar] [CrossRef]
- Mozgeris, G.; Mörtberg, U.; Pang, X.-L.; Trubins, R.; Treinys, R. Future Projection for Forest Management Suggests a Decrease in the Availability of Nesting Habitats for a Mature-Forest-Nesting Raptor. For. Ecol. Manag. 2021, 491, 119168. [Google Scholar] [CrossRef]
- Survila, M. Protecting Old-Growth Forests as an Environmental Investment—Nordic Investment Bank. Available online: https://www.nib.int/cases/protecting-old-growth-forests-as-an-environmental-investment/ (accessed on 21 November 2024).
- Duncker, P.; Barreiro, S.; Hengeveld, G.; Lind, T.; Mason, W.; Ambrozy, S.; Spiecker, H. Classification of Forest Management Approaches: A New Conceptual Framework and Its Applicability to European Forestry. Ecol. Soc. 2012, 17, 51. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sarvašová, Z.; Cienciala, E.; Beranová, J.; Vančo, M.; Ficko, A.; Pardos, M. Analysis of Governance Systems Applied in Multifunctional Forest Management in Selected European Mountain Regions/Analýza Systémov Governancie Využívaných Pri Multifunkčnom Manažmente Lesov vo Vybraných Európskych Horských Oblastiach. Cent. Eur. For. J. 2014, 60, 159–167. [Google Scholar] [CrossRef]
- Nagel, T.A.; Rodríguez-Recio, M.; Aakala, T.; Angelstam, P.; Avdagić, A.; Borowski, Z.; Bravo-Oviedo, A.; Brazaitis, G.; Campagnaro, T.; Ciach, M.; et al. Can Triad Forestry Reconcile Europe’s Biodiversity and Forestry Strategies? A Critical Evaluation of Forest Zoning. Ambio 2024, 12, 1–10. [Google Scholar] [CrossRef]
- Felton, A.; Löfroth, T.; Angelstam, P.; Gustafsson, L.; Hjältén, J.; Felton, A.M.; Simonsson, P.; Dahlberg, A.; Lindbladh, M.; Svensson, J.; et al. Keeping Pace with Forestry: Multi-Scale Conservation in a Changing Production Forest Matrix. Ambio 2020, 49, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Křenová, Z.; Janík, T.; Romportl, D. One Park, Two Owners—Inconsistencies in Forest Stewardship. Conserv. Sci. Pract. 2022, 4, e12834. [Google Scholar] [CrossRef]
- Böttcher, H.; Verkerk, P.J.; Gusti, M.; HavlÍk, P.; Grassi, G. Projection of the Future EU Forest CO2 Sink as Affected by Recent Bioenergy Policies Using Two Advanced Forest Management Models. GCB Bioenergy 2012, 4, 773–783. [Google Scholar] [CrossRef]
- European Commission. New EU Forest Strategy for 2030. COM (2021) 572 Final. In Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; Publications Office of the European Union: Brussels, Belgium, 2021. [Google Scholar]
- PEFC. What Is Sustainable Forest Management? Available online: https://www.pefc.org/what-we-do/our-approach/what-is-sustainable-forest-management (accessed on 27 November 2024).
- Bauhus, J.; Forrester, D.I.; Gardiner, B.; Jactel, H.; Vallejo, R.; Pretzsch, H. Ecological Stability of Mixed-Species Forests. In Mixed-Species Forests; Pretzsch, H., Forrester, D.I., Bauhus, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 337–382. ISBN 978-3-662-54551-5. [Google Scholar]
- Vasseur, L. Restoration of Deciduous Forests. Available online: https://www.nature.com/scitable/knowledge/library/restoration-of-deciduous-forests-96642239/ (accessed on 20 December 2024).
- Spathelf, P.; Stanturf, J.; Kleine, M.; Jandl, R.; Chiatante, D.; Bolte, A. Adaptive Measures: Integrating Adaptive Forest Management and Forest Landscape Restoration. Ann. For. Sci. 2018, 75, 55. [Google Scholar] [CrossRef]
- Ding, J.; Delgado-Baquerizo, M.; Wang, J.-T.; Eldridge, D.J. Ecosystem Functions Are Related to Tree Diversity in Forests but Soil Biodiversity in Open Woodlands and Shrublands. J. Ecol. 2021, 109, 4158–4170. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.; et al. The Exceptional Value of Intact Forest Ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef]
- Bergeron, Y.; Irulappa Pillai Vijayakumar, D.B.; Ouzennou, H.; Raulier, F.; Leduc, A.; Gauthier, S. Projections of Future Forest Age Class Structure under the Influence of Fire and Harvesting: Implications for Forest Management in the Boreal Forest of Eastern Canada. For. Int. J. For. Res. 2017, 90, 485–495. [Google Scholar] [CrossRef]
- Pasques, O.; Munné-Bosch, S. Ancient Trees Are Essential Elements for High-Mountain Forest Conservation: Linking the Longevity of Trees to Their Ecological Function. Proc. Natl. Acad. Sci. USA 2024, 121, e2317866121. [Google Scholar] [CrossRef] [PubMed]
- Vangi, E.; Dalmonech, D.; Cioccolo, E.; Marano, G.; Bianchini, L.; Puchi, P.F.; Grieco, E.; Cescatti, A.; Colantoni, A.; Chirici, G.; et al. Stand Age Diversity (and More than Climate Change) Affects Forests’ Resilience and Stability, Although Unevenly. J. Environ. Manag. 2024, 366, 121822. [Google Scholar] [CrossRef] [PubMed]
- Durante, S.; Augusto, L.; Achat, D.L.; Legout, A.; Brédoire, F.; Ranger, J.; Seynave, I.; Jabiol, B.; Pousse, N. Diagnosis of Forest Soil Sensitivity to Harvesting Residues Removal–A Transfer Study of Soil Science Knowledge to Forestry Practitioners. Ecol. Indic. 2019, 104, 512–523. [Google Scholar] [CrossRef]
- Pare, D.; Manka, F.; Barrette, J.; Augustin, F.; Beguin, J. Indicators of Site Sensitivity to the Removal of Forest Harvest Residues at the Sub-Continental Scale: Mapping, Comparisons, and Challenges. Ecol. Indic. 2021, 125, 107516. [Google Scholar] [CrossRef]
- Hekkala, A.-M.; Jönsson, M.; Kärvemo, S.; Strengbom, J.; Sjögren, J. Habitat Heterogeneity Is a Good Predictor of Boreal Forest Biodiversity. Ecol. Indic. 2023, 148, 110069. [Google Scholar] [CrossRef]
- Nabuurs, G.J.; Begemann, A.; Linser, S.; Paillet, Y.; Pettenella, D.; Ermgassen, S. Sustainable Finance and Forest Biodiversity Criteria; From Science to Policy; European Forest Institute: Joensuu, Finland, 2024; Volume 16, ISBN 978-952-7426-83-8. [Google Scholar]
- Pardos, M.; del Río, M.; Pretzsch, H.; Jactel, H.; Bielak, K.; Bravo, F.; Brazaitis, G.; Defossez, E.; Engel, M.; Godvod, K.; et al. The Greater Resilience of Mixed Forests to Drought Mainly Depends on Their Composition: Analysis along a Climate Gradient across Europe. For. Ecol. Manag. 2021, 481, 118687. [Google Scholar] [CrossRef]
- Murray, C.; Marmorek, D.R. Adaptive Management: A Science-Based Approach to Managing Ecosystems in the Face of Uncertainty. In Proceedings of the Making Ecosystem-Based Management Work: Proceedings of the Fifth International Conference on Science and Management of Protected Areas, Victoria, BC, Canada, 11–16 May 2003. [Google Scholar]


| Forest Habitats (NATURA 2000) | Forest Type Series * | Site Types ** | Soil Types **** |
|---|---|---|---|
| Scots pine forests (9010 9060 91D0 91T0) | Cladoniosa (cl) | Šae, Nae | AR, RG |
| Vacciniosa (v) | Šal, Nal | AR, PZ, RG | |
| Vaccinio-myrtillosa (vm) | Šbl, Šbp, Nbl, Nbp | AR, PZ | |
| Myrtillosa (m) | Lal, Lbl, Lbp | AR, PL, PZ | |
| Myrtillo-sphagnosa (msp msps) | Ual, Ubl, Ubp, Ma, Mb *** | GL, PZ | |
| Carico-sphagnosa (csp csps) | Pb, Mb | HSf-s | |
| Ledo-sphagnosa (lsp lsps) | Pa, Ma | HSf | |
| Mixed Norway spruce forests (9050 9160 9180 9190 9070) | Oxalidosa (ox) | Šcl, Šcp, Šcs, Ncl, Ncp, Ncs | AR, LV, RT, PL, CM, FL |
| Myrtillo-oxalidosa (mox) | Lcl, Lcp, Lcs | AR, LV, RT, PL, CM | |
| Hepatico-oxalidosa (hox) | Šdl, Šdp, Šds, Ndl, Ndp, Nds | AR, LV, RT, PL, CM, FL | |
| Oxalido-nemorosa (oxn) | Ldl, Ldp, Lds | LV, CM, FL | |
| Mixed broadleaved forests (9020 9080 91F0 91E0) | Aegopodiosa (aeg) | Šfp, Šfs, Nfp, Nfs | LV, CM |
| Carico-mixtoherbosa (cmh) | Lfl, Lfp, Lfs | LV, CM | |
| Calamagrostidosa (cal cals) | Ucl, Ucp, Ucs, Mc *** | GL | |
| Filipendulo-mixtoherbosa (fil fils) | Udl, Udp, Uds, Md *** | GL, FL | |
| Urticosa (ur) | Ufl, Ufp, Ufs | GL | |
| Carico-iridosa (cir cirs) | Pd, Md | HSs | |
| Caricosa (c cs) | Pc, Mc | HSs |
| Stand Type * | NFI 2022 | NFI 2017 | NFI 2012 | NFI 2007 | NFI 2002 |
|---|---|---|---|---|---|
| Scots pine (p) | 82p 10e 5b (31.5) | 83p 10e 5b (32.0) | 83p 10e 5b (32.9) | 84p 9e 5b (33.5) | 84p 9e 5b (34.1) |
| Norway spruce (e) | 74e 8b 6p (18.6) | 74e 8b 7p (17.8) | 73e 9b 7p (17.5) | 72e 9b 7p (18.2) | 71e 10b 7p (17.8) |
| Silver and downy birch (b) | 62b 15e 5j (19.9) | 62b 15e 6j (20.3) | 62b 15e 6j (20.5) | 61b 14e 6j (20.3) | 61b 14e 6j (19.6) |
| Eurasian aspen (d) | 62d 12e 10b (7.3) | 62d 12e 11b (6.9) | 61d 13e 11b (6.7) | 60d 13e 12b (6.4) | 60d 13e 12b (6.3) |
| Black alder (j) | 72j 11b 7e (11.3) | 71j 12b 8e (11.1) | 70j 13b 8e (10.2) | 69j 13b 8e (9.3) | 67j 13b 8e (9.1) |
| Grey alder (bt) | 70bt 8b 5e (6.3) | 71bt 8b 6e (6.4) | 73bt 8b 5e (6.6) | 74bt 8b 4e (6.4) | 73bt 9b 5e (6.9) |
| English oak (a) | 57a 14e 8b (2.6) | 56a 14e 8b (2.5) | 55a 16e 7b (2.4) | 55a 16e 7b (2.2) | 56a 14e 8b (2.4) |
| European ash (u) | 44u 16k 9e (0.5) | 51u 10k 10e (0.7) | 58u 7e 6d (1.2) | 59u 7e 7d (2.1) | 61u 7e 7d (2.4) |
| Other species | 26l 17k 7bl (2.1) | 27l 14k 7b (2.3) | 28l 13k 8bl (2.0) | 27l 12k 10a (1.5) | 22l 11k 10bl (1.5) |
| All stands | 36p 22e 16b (100) | 36p 22e 16b (100) | 37p 21e 17b (100) | 37p 20e 17b (100) | 36p 20e 17b (100) |
| Stand Type * | Forest Development Phases/Stand Age (Years) ** | Forest Type Series *** | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤20 | 21–40 | 41–60 | 61–80 | 81–100 | 101–120 | ≥121 | ||
| Scots pine (p) | 88p | 85p | 86p | 84p | 80p | 79p | 80p | cal cals cl cmh csp csps hox lsp lsps m mox msp msps ox oxn v vm |
| 4b | 7b | 6b | 8e | 14e | 14e | 15e | ||
| 4e | 5e | 5e | 5b | 4b | 4b | 3b | ||
| Norway spruce (e) | 47e | 77e | 81e | 73e | 71e | 66e | 64e | c cs cal cals cir cirs cmh csp csps fil fils hox m mox msp msps ox oxn vm |
| 13a | 8b | 8b | 8b | 8p | 11p | 13p | ||
| 12p | 4a | 3p | 8p | 8b | 9b | 7b | ||
| Silver and downy birch (b) | 60b | 69b | 67b | 59b | 54b | 49b | - | aeg c cs cal cals cir cirs cmh csp csps fil fils hox lsp lsps m mox msp msps ox oxn v vm |
| 9e | 10e | 11e | 19e | 21e | 29e | |||
| 9p | 5j | 5j | 6d | 7j | 7j | |||
| Eurasian aspen (d) | 65d | 64d | 60d | 62d | 60d | - | - | aeg c cs cal cals cir cirs cmh csp csps fil fils hox mox ox oxn ur |
| 11b | 10b | 13b | 16e | 20e | ||||
| 9e | 7e | 12e | 9b | 10b | ||||
| Black alder (j) | 71j | 74j | 78j | 69j | 57j | 60j | 62j | c cs cal cals cir cirs cmh csp csps fil fils hox mox ox oxn ur urs |
| 10b | 13b | 10b | 13b | 19e | 27e | 36e | ||
| 8e | 4e | 4e | 9e | 11b | 10b | 2a | ||
| Grey alder (bt) | 62bt | 72bt | 71bt | 69bt | - | - | - | aeg c cs cal cals cir cirs cmh fil fils hox mox ox oxn |
| 14b | 8b | 8b | 14e | |||||
| 5j | 5e | 5j | 5j | |||||
| English oak (a) | 48a | 43a | 57a | 58a | 56a | 58a | 56a | aeg c cs cmh fil fils hox mox ox oxn |
| 18b | 28b | 17e | 17e | 13e | 9e | 16e | ||
| 17l | 11e | 11b | 11b | 10b | 8l | 8l | ||
| European ash (u) | 48u | 47gl | 46k | 60u | 48u | 46u | 44e | aeg c cs cir cirs fil fils hox mox ox oxn |
| 24e | 35u | 33u | 13k | 20l | 38a | 32u | ||
| 10bt | 8bt | 8a | 10a | 13b | 13b | 14b | ||
| Other species | 18a | 20bl | 22l | 35l | 54l | 30k | 75l | aeg c cs cal cals cmh fil fils hox mox ox oxn ur urs |
| 16gl | 12gl | 13k | 23k | 14k | 27l | 25e | ||
| 13bl | 10k | 10gl | 8a | 7u | 14sb | |||
| Forest Type Series * | Forest Development Phases/Stand Age (Years) ** | NFI 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| ≤20 | 21–40 | 41–60 | 61–80 | 81–100 | 101–120 | ≥121 | ||
| Cladoniosa (cl) | NED | NED | NED | NED | NED | NED | NED | NED |
| Vacciniosa (v) | 100p | 78p | 97p | 98p | 98p | 99p | 99p | 97p |
| 17b | 3b | 2b | 1b | 1pk | 1b | 2b | ||
| 2pb | 1pk | |||||||
| Vaccinio-myrtillosa (vm) | 77p | 66p | 78p | 87p | 85p | 84p | 87p | 84p |
| 12b | 18e | 12e | 8e | 12e | 13e | 10e | 11e | |
| 9e | 14b | 9b | 4b | 3b | 3b | 2b | 5b | |
| Myrtillosa (m) | 45p | 53e | 49e | 56p | 59p | 69p | 66p | 55p |
| 30e | 24b | 31p | 30e | 31e | 25e | 27e | 32e | |
| 18b | 21p | 9b | 12b | 9b | 5b | 5b | 10b | |
| Myrtillo-sphagnosa (msp msps) | 37p | 44b | 39p | 65p | 62p | 53p | 64p | 54p |
| 35b | 35e | 33b | 27e | 18e | 37e | 29e | 27e | |
| 7a | 17p | 28e | 7b | 15b | 9b | 6b | 17b | |
| Carico-sphagnosa (csp csps) | 71b | 55b | 43b | 50p | 65p | 64p | 69p | 53p |
| 10p | 16d | 25p | 23b | 19e | 25e | 21e | 22b | |
| 8e | 13p | 19e | 20e | 14b | 10b | 7b | 19e | |
| Ledo-sphagnosa (lsp lsps) | 57p | 67b | 80p | 88p | 94p | 91p | 95p | 82p |
| 43b | 27p | 17b | 12b | 4b | 5e | 4b | 15b | |
| 4j | 3e | 1d | 4b | 2e | ||||
| Oxalidosa (ox) | 28b | 31e | 43p | 51p | 47p | 56p | 59p | 44p |
| 20e | 21b | 21e | 22e | 32e | 23e | 22e | 24e | |
| 14d | 19p | 17b | 13b | 9b | 8b | 11a | 14b | |
| Myrtillo-oxalidosa (mox) | 28b | 39e | 39e | 41e | 54e | 47e | 44e | 42e |
| 22e | 23b | 23b | 22b | 16p | 22p | 36p | 20b | |
| 15d | 10bt | 10j | 14p | 12b | 14b | 13a | 11p | |
| Hepatico-oxalidosa (hox) | 23b | 26d | 19e | 17b | 30e | 29e | 29e | 21e |
| 18a | 22bt | 14bt | 16e | 19b | 24a | 24a | 14b | |
| 18bt | 18e | 13b | 15p | 15a | 13l | 23p | 13d | |
| Oxalido-nemorosa (oxn) | 26d | 25e | 30e | 27b | 35b | 33e | 40a | 26e |
| 17b | 20bt | 19bt | 25e | 23e | 19b | 25e | 22b | |
| 14bt | 18b | 17b | 20d | 13d | 12a | 8d | 16d | |
| Aegopodiosa (aeg) | 100a *** | 66bt | 36bt | 34u | 38d | 47b | - | 25bt |
| 11bl | 25e | 24b | 32a | 32a | 15u | |||
| 8b | 17u | 23bt | 14k | 10e | 14b | |||
| Carico-mixtoherbosa (cmh) | 23b | 26bt | 32b | 42b | 22e | 36l | - | 25b |
| 17e | 24e | 23bt | 26d | 17a | 35b | 17bt | ||
| 17bt | 19d | 15j | 11j | 13d | 21a | 17d | ||
| Calamagrostidosa (cal cals) | 53j | 36b | 37j | 36j | 30b | 31e | 62e | 35j |
| 25b | 32j | 31b | 30e | 21e | 26b | 18b | 28b | |
| 8e | 15e | 20e | 19b | 21j | 25j | 15d | 22e | |
| Filipendulo-mixtoherbosa (fil fils) | 60j | 38j | 56j | 51j | 37j | 37e | 53e | 48j |
| 14b | 19b | 22b | 20b | 27b | 28b | 30u | 21b | |
| 11bt | 13bt | 8bt | 11e | 22e | 26j | 10b | 11e | |
| Urticosa (ur) | 68j | 32gl | 29bt | 82j | 41j | - | - | 53j |
| 22u | 29j | 27d | 8b | 40b | 13b | |||
| 10b | 19b | 16j | 5bt | 8u | 10bt | |||
| Carico-iridosa (cir cirs) | 90j | 37j | 89j | 62j | 45j | 76j | - | 71j |
| 9b | 24b | 7b | 27b | 32e | 22e | 15b | ||
| 1a | 17e | 3e | 10e | 24b | 3b | 10e | ||
| Caricosa (c cs) | 60j | 50j | 51j | 33j | 30b | 39e | - | 42j |
| 18b | 35b | 31b | 31b | 28e | 22j | 31b | ||
| 7p | 5e | 8e | 20e | 27j | 19b | 14e | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrokas, R.; Manton, M.; Kulbokas, G.; Muraškienė, M. Development of Forest Tree Species Composition: Selected Results of the National Forest Inventory of Lithuania. Plants 2025, 14, 667. https://doi.org/10.3390/plants14050667
Petrokas R, Manton M, Kulbokas G, Muraškienė M. Development of Forest Tree Species Composition: Selected Results of the National Forest Inventory of Lithuania. Plants. 2025; 14(5):667. https://doi.org/10.3390/plants14050667
Chicago/Turabian StylePetrokas, Raimundas, Michael Manton, Gintaras Kulbokas, and Milda Muraškienė. 2025. "Development of Forest Tree Species Composition: Selected Results of the National Forest Inventory of Lithuania" Plants 14, no. 5: 667. https://doi.org/10.3390/plants14050667
APA StylePetrokas, R., Manton, M., Kulbokas, G., & Muraškienė, M. (2025). Development of Forest Tree Species Composition: Selected Results of the National Forest Inventory of Lithuania. Plants, 14(5), 667. https://doi.org/10.3390/plants14050667









