Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests
Abstract
1. Introduction
2. Results
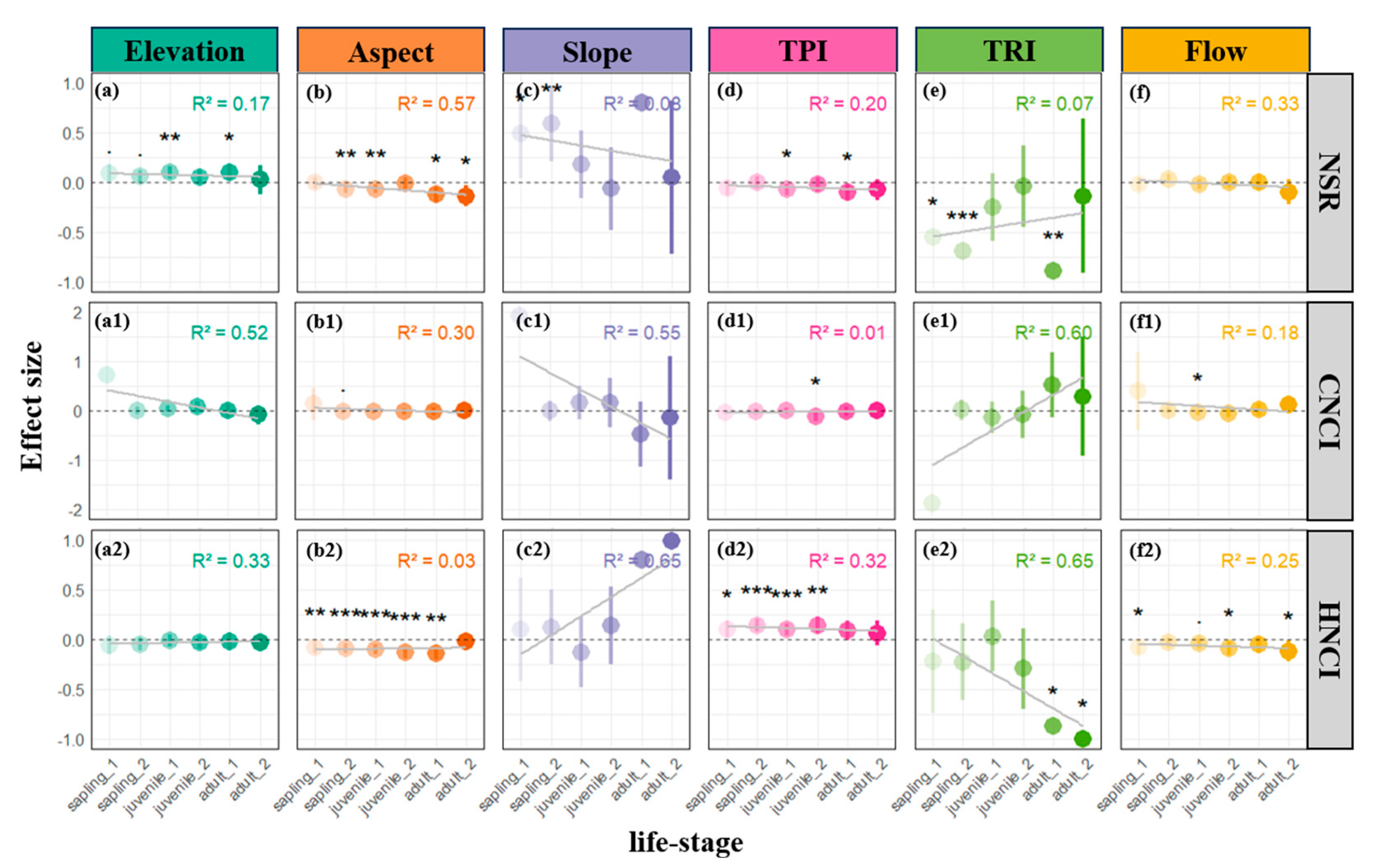
2.1. Effects of Microtopographic Factors on Plant Density, Size, and Competitive Interactions
2.2. Effects Across Life History Stages
3. Discussion
4. Materials and Methods
4.1. Study Area and Censuses
4.2. Microtopographic Factors and Neighborhood Effects
4.3. Microtopographic Effect on Neighborhood Factor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Swenson, N.G.; Brown, C.; Zhang, C.; Yang, J.; Ci, X.; Li, J.; Sha, L.; Cao, M.; Lin, L. How does habitat filtering affect the detection of conspecific and phylogenetic density dependence? Ecology 2016, 97, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Born, J.; Pluess, A.R.; Burslem, D.F.R.P.; Nilus, R.; Maycock, C.R.; Ghazoul, J. Differing life history characteristics support coexistence of tree soil generalist and specialist species in tropical rain forests. Biotropica 2014, 46, 58–68. [Google Scholar] [CrossRef]
- Givnish, T.J. On the causes of gradients in tropical tree diversity. J. Ecol. 1999, 87, 193–210. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H.; Arita, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Lv, Y.; Ni, Y.; Xu, B.; Han, X.; Cao, X.; Yang, Q.; Xu, W.; Qian, Z. How Topography and Neighbor Shape the Fate of Trees in Subtropical Forest Restoration: Environmental Filtering and Resource Competition Drive Natural Regeneration. For. Ecosyst. 2024, 11, 100169. [Google Scholar] [CrossRef]
- Russo, S.E.; Brown, P.; Tan, S.; Davies, S.J. Interspecific demographic trade-offs and soil-related habitat associations of tree species along resource gradients. J. Ecol. 2008, 96, 192–203. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Quesada, C.A.; Phillips, O.L.; Schwarz, M.; Czimczik, C.I.; Baker, T.R.; Patiño, S.; Fyllas, N.M.; Hodnett, M.G.; Herrera, R.; Almeida, S.; et al. Basin-wide variations in Amazon forest structure and function are mediated by both soils and climate. Biogeosciences 2012, 9, 2203–2246. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Talbot, J.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Begne, S.K.; Chave, J.; Cuni-Sanchez, A.; Hubau, W.; Lopez-Gonzalez, G.; et al. Diversity and carbon storage across the tropical forest biome. Sci. Rep. 2017, 7, 39102. [Google Scholar] [CrossRef]
- Zhu, Y.; Mi, X.; Ren, H.; Ma, K. Density dependence is prevalent in a heterogeneous subtropical forest. Oikos 2010, 119, 109–119. [Google Scholar] [CrossRef]
- Zhu, Y.; Comita, L.S.; Hubbell, S.P.; Ma, K. Conspecific and phylogenetic density-dependent survival differs across life stages in a tropical forest. J. Ecol. 2015, 103, 957–966. [Google Scholar] [CrossRef]
- Zhang, H.N.; Chen, S.; Xia, X.; Ge, X.; Zhou, D.; Wang, Z. The competitive mechanism between post-abandonment Chinese fir plantations and rehabilitated natural secondary forest species under an in situ conservation policy. For. Ecol. Manag. 2021, 502, 119725. [Google Scholar] [CrossRef]
- Punchi-Manage, R.; Wiegand, T.; Wiegand, K.; Getzin, S.; Huth, A.; Gunatilleke, C.S.; Gunatilleke, I.N. Neighborhood diversity of large trees shows independent species patterns in a mixed dipterocarp forest in Sri Lanka. Ecology 2015, 96, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Punchi-Manage, R.; Getzin, S.; Wiegand, T.; Kanagaraj, R.; Gunatilleke, C.V.S.; Gunatilleke, I.A.U.N.; Wiegand, K.; Huth, A. Effects of topography on structuring local species assemblages in a Sri Lankan mixed dipterocarp forest. J. Ecol. 2013, 101, 149–160. [Google Scholar] [CrossRef]
- Gibbons, J.M.; Newbery, D.M. Drought avoidance and the effect of local topography on trees in the understorey of Bornean lowland rain forest. Plant Ecol. 2003, 164, 1–18. [Google Scholar] [CrossRef]
- Jucker, T.; Bongalov, B.; Burslem, D.F.R.P.; Nilus, R.; Dalponte, M.; Lewis, S.L.; Phillips, O.L.; Qie, L.; Coomes, D.A. Topography shapes the structure, composition and function of tropical forest landscapes. Ecol. Lett. 2018, 21, 989–1000. [Google Scholar] [CrossRef]
- Muscarella, R.; Kolyaie, S.; Morton, D.C.; Zimmerman, J.K.; Uriarte, M. Effects of topography on tropical forest structure depend on climate context. J. Ecol. 2020, 108, 145–159. [Google Scholar] [CrossRef]
- Heineman, K.D.; Jensen, E.; Shapland, A.; Bogenrief, B.; Tan, S.; Rebarber, R.; Russo, S.E. The effects of belowground resources on aboveground allometric growth in Bornean tree species. For. Ecol. Manag. 2011, 261, 1820–1832. [Google Scholar] [CrossRef]
- Holdaway, R.J.; Richardson, S.J.; Dickie, I.A.; Peltzer, D.A.; Coomes, D.A. Species- and community-level patterns in fine root traits along a 120000-year soil chronosequence in temperate rain forest. J. Ecol. 2011, 99, 954–963. [Google Scholar] [CrossRef]
- Fichtner, A.; Härdtle, W.; Li, Y.; Bruelheide, H.; Kunz, M.; von Oheimb, G. From competition to facilitation: How tree species respond to neighbourhood diversity. Ecol. Lett. 2017, 20, 892–900. [Google Scholar] [CrossRef]
- Banin, L.; Feldpausch, T.R.; Phillips, O.L.; Baker, T.R.; Lloyd, J.; Affum-Baffoe, K.; Arets, E.J.; Berry, N.J.; Bradford, M.; Brienen, R.J.; et al. What controls tropical forest architecture? Testing environmental, structural and floristic drivers. Glob. Ecol. Biogeogr. 2012, 21, 1179–1190. [Google Scholar] [CrossRef]
- Paoli, G.D. Divergent leaf traits among congeneric tropical trees with contrasting habitat associations on Borneo. J. Trop. Ecol. 2006, 22, 397–408. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Escudero, A. Topography in Tropical Forests Enhances Growth and Survival Differences within and among Species via Water Availability and Biotic Interactions. Funct. Ecol. 2022, 36, 686–698. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, X.; Wu, Y.; Xu, B.; Cui, P.; Zhou, X.; Fang, Y.; Xie, L.; Ding, H. Impact of Microtopography and Neighborhood Effects on Individual Survival Across Life History Stages. Plants 2024, 13, 3216. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ning, B.; Jiao, J.; Gasanova, Z.U.; Stepanova, N.Y.; Konyushkova, M.V. Temporal change in plant communities and its relationship to soil salinity and microtopography on the Caspian Sea coast. Sci. Rep. 2020, 12, 18082. [Google Scholar] [CrossRef]
- Werner, F.A.; Homeier, J. Is tropical montane forest heterogeneity promoted by a resource-driven feedback cycle? Evidence from nutrient relations, herbivory and litter decomposition along a topographical gradient. Funct. Ecol. 2015, 29, 430–440. [Google Scholar] [CrossRef]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kéfi, S.; et al. Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef]
- Ran, F.; Liang, Y.; Yang, Y.; Yang, Y.; Wang, G. Spatial-temporal dynamics of an Abies fabri population near the alpine treeline in the Yajiageng area of Gongga Mountain, China. Acta Ecol. Sin. 2014, 34, 6872–6878. [Google Scholar]
- Montgomery, R.; Chazdon, R. Light gradient partitioning by tropical tree seedlings in the absence of canopy gaps. Oecologia 2002, 131, 165–174. [Google Scholar] [CrossRef]
- Baltzer, J.L.; Davies, S.J.; Bunyavejchewin, S.; Noor, N.S.M. The role of desiccation tolerance in determining tree species distributions along the tropical–temperate gradient. Ecology 2008, 89, 1747–1759. [Google Scholar] [CrossRef]
- Silver, W.L.; Lugo, A.E.; Keller, M. Soil oxygen availability and biogeochemistry along rainfall and topographic gradients in upland wet tropical forest soils. Biogeochemistry 1999, 44, 301–328. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Convergent elevation trends in canopy chemical traits of tropical forests. Glob. Change Biol. 2016, 22, 2216–2227. [Google Scholar] [CrossRef] [PubMed]
- Baldeck, C.A.; Harms, K.E.; Yavitt, J.B.; John, R.; Turner, B.L.; Valencia, R.; Navarrete, H.; Davies, S.J.; Chuyong, G.B.; Kenfack, D.; et al. Soil resources and topography shape local tree community structure in tropical forests. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122532. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Toribio, M.; Ibarra-Manríquez, G.; Navarrete-Segueda, A.; Paz, H. Topographic position, but not slope aspect, drives the dominance of functional strategies of tropical dry forest trees. Environ. Res. Lett. 2017, 12, 085002. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielbörger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in Plant Communities: The Past, the Present, and the Future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Takyu, M.; Aiba, S.-I.; Kitayama, K. Effects of topography on tropical lower montane forests under different geological conditions on Mount Kinabalu, Borneo. Plant Ecol. 2002, 159, 35–49. [Google Scholar] [CrossRef]
- Zhang, H.N.; Yang, Q.; Zhou, D.; Xu, W.; Gao, J.; Wang, Z. How evergreen and deciduous trees coexist during secondary forest succession: Insights into forest restoration mechanisms in Chinese subtropical forest. Glob. Ecol. Conserv. 2021, 25, e01418. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S.; Zheng, X.; Ge, X.; Li, Y.; Fang, Y.; Cui, P.; Ding, H. Neighborhood diversity structure and neighborhood species richness effects differ across life stages in a subtropical natural secondary forest. For. Ecosyst. 2022, 9, 100075. [Google Scholar] [CrossRef]
- Ding, M.; Zheng, B.; Wang, L.; Zhang, J.M.; Zhou, S.B.; Wang, Z. Diversity and spatial distribution of herbaceous species with deciduous broad-leaved forest in the Yaoluoping National Nature Reserve. Acta Ecol. Sin. 2022, 42, 8015–8030. [Google Scholar]
- Wang, Z.-G.; Zhang, Z.-X.; Wang, W.-G.; Chu, J. Preliminary analysis of forest community structure of Yaoluoping National Nature Reserve in Yuexi County, Anhui Province, China. Chin. J. Plant Ecol. 2016, 40, 615–619. [Google Scholar] [CrossRef][Green Version]
- Wu, Z.Y. Vegetation of China; Science Press: Beijing, China, 1980; pp. 823–888, (In Chinese with English Abstract). [Google Scholar]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Amatulli, G.; McInerney, D.; Sethi, T.; Strobl, P.; Domisch, S. Geomorpho90m, empirical evaluation and accuracy assessment of global high-resolution geomorphometric layers. Sci. Data 2020, 7, 162. [Google Scholar] [CrossRef]
- Fortunel, C.; Lasky, J.R.; Uriarte, M.; Valencia, R.; Wright, S.J.; Garwood, N.C.; Kraft, N.J. Topography and neighborhood crowding can interact to shape species growth and distribution in a diverse Amazonian forest. Ecology 2018, 99, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Rayburn, A.; Wiegand, T. Individual species–area relationships and spatial patterns of species diversity in a Great Basin, semi-arid shrubland. Ecography 2012, 35, 341–347. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, F.; Zheng, X.; Xu, J.; Ni, Y.; Chen, S.; Ge, X.; Fang, Y.; Li, Y.; Peng, Y.; et al. Navigating Neighborhoods: Density, Size, and Species Diversity Influences on Tree Survival in Subtropical Secondary Forests. For. Ecol. Manag. 2024, 572, 122311. [Google Scholar] [CrossRef]
- Johnson, D.J.; Condit, R.; Hubbell, S.P.; Comita, L.S. Abiotic niche partitioning and negative density dependence drive tree seedling survival in a tropical forest. Proc. R. Soc. B 2017, 284, 20172210. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Zhang, H.; Qiao, Y.; Yuan, H.; Xu, W.; Xia, X. Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests. Plants 2025, 14, 870. https://doi.org/10.3390/plants14060870
Xu J, Zhang H, Qiao Y, Yuan H, Xu W, Xia X. Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests. Plants. 2025; 14(6):870. https://doi.org/10.3390/plants14060870
Chicago/Turabian StyleXu, Jianing, Haonan Zhang, Yajun Qiao, Huanhuan Yuan, Wanggu Xu, and Xin Xia. 2025. "Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests" Plants 14, no. 6: 870. https://doi.org/10.3390/plants14060870
APA StyleXu, J., Zhang, H., Qiao, Y., Yuan, H., Xu, W., & Xia, X. (2025). Effects of Microtopography on Neighborhood Diversity and Competition in Subtropical Forests. Plants, 14(6), 870. https://doi.org/10.3390/plants14060870




