Hypoglycemic and Hypolipidemic Effects of Triterpenoid Standardized Extract of Agave durangensis Gentry
Abstract
:1. Introduction
2. Results
2.1. Characterization of Agave durangensis Extract
2.2. In Silico Study of Agave durangensis Extract Bioactive Compounds
2.3. DPP4 Inhibitory Activity In Vitro of Agave durangensis Extract
2.4. Hypoglycemic and Hypolipidemic Effect of Agave durangensis Extract
3. Discussion
4. Materials and Methods
4.1. Collection Plant and Preparation of the Extract
4.2. Phytochemical Characterization of Agave durangensis Extract
4.2.1. Triterpene Quantification
4.2.2. Phenol Quantification
4.2.3. Flavonoid Quantification
4.2.4. Condensed Tannin Quantification
4.2.5. UPLC-MS Analysis
4.3. In Silico Study
4.3.1. Obtaining and Preparation of Proteins and Ligands
4.3.2. Molecular Docking
4.3.3. In Silico Pharmacokinetic Evaluation of the Investigated Compounds
4.4. In Vitro Study
Dipeptidyl Peptidase-4 Inhibition Assay
4.5. In Vivo Study
4.5.1. Evaluation of the Hypoglycemic Effect of EAD
4.5.2. Biochemical Study
4.5.3. Dipeptidyl Peptidase-4 Activity Assay
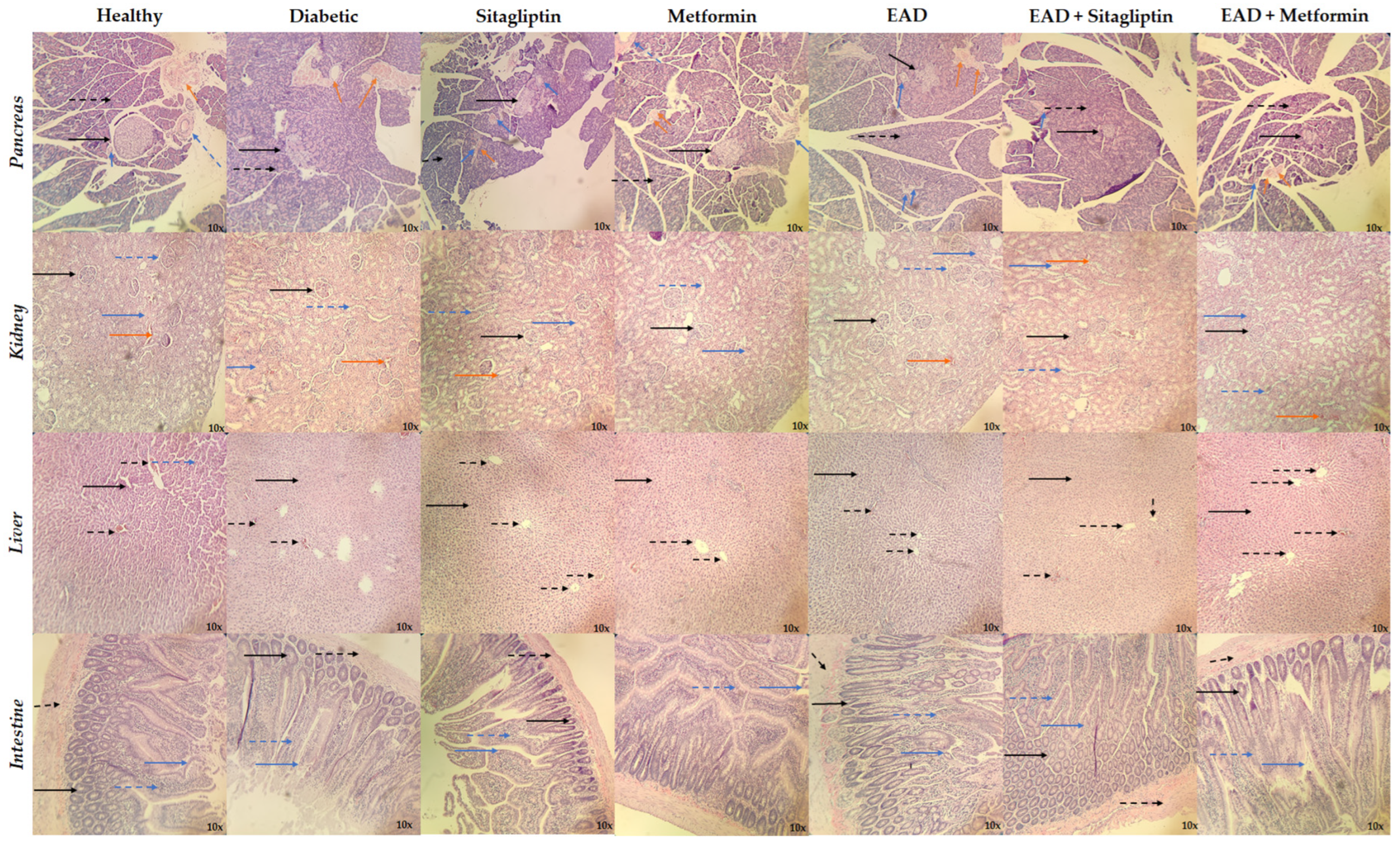
4.5.4. Histological Studies
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM2 | Diabetes mellitus type 2 |
| DPP4 | Dipeptidyl peptidase 4 |
| SGLT2 | Sodium-glucose cotransporter-2 |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Gastric inhibitory polypeptide |
| EAD | Agave durangensis Gentry extract |
| UPLC-MS | Ultra-performance liquid chromatography–mass spectrometry |
| AMPK | AMP-activated protein kinase |
| SUR1 | Sulfonylurea receptor −1 |
| PPARγ | Peroxisome-proliferator-activated receptor gamma |
| STZ | Streptozotocin |
| AST | Aspartate amine transferase |
| ALT | Alanine amine transferase |
| HDL | High-density lipoproteins |
| LDL | Low-density lipoproteins |
| VLDL | Very-low-density lipoprotein |
| HbA1c | Glycated hemoglobin |
| H&E | Hematoxylin and eosin |
References
- Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2017, 3, 17016. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, Regional and Country-Level Diabetes Prevalence Estimates for 2021 and Projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Briggs Early, K.; Bruemmer, D.; Ebekozien, O.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; et al. Pharmacologic Approaches to Glycemic Treatment: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S181–S206. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef]
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Escandón-Rivera, S.M.; Mata, R.; Andrade-Cetto, A. Molecules Isolated from Mexican Hypoglycemic Plants: A Review. Molecules 2020, 25, 4145. [Google Scholar] [CrossRef]
- García-Mendoza, A.; Galván-V, R. Riqueza de Las Familias Agavaceae y Nolinaceae En México. Bot. Sci. 1995, 24, 7–24. [Google Scholar] [CrossRef]
- García-Mendoza, A.J. Distribution of Agave (Agavaceae) in Mexico. Cactus Succul. J. 2002, 74, 177–188. [Google Scholar]
- Bermúdez-Bazán, M.; Castillo-Herrera, G.A.; Urias-Silvas, J.E.; Escobedo-Reyes, A.; Estarrón-Espinosa, M. Hunting Bioactive Molecules from the Agave Genus: An Update on Extraction and Biological Potential. Molecules 2021, 26, 6789. [Google Scholar] [CrossRef]
- Blomberg, L. Tequila, Mezcal y Pulque: Lo Auténtico Mexicano; Editorial Diana: Mexico City, Mexico, 2000; ISBN 9681332695. [Google Scholar]
- Monroy, C.; Castillo, P. Plantas Medicinales Utilizadas en el Estado de Morelos; México Centro de Investigaciones Biológicas/Universidad Autónoma del Estado Morelos: Cuernavaca, Mexico, 2007. [Google Scholar]
- Lozoya, L.L.; Lozoya, M. Flora Medicinal de México; Instituto Mexicano del Seguro Social: Ciudad de México, Mexico, 1982; Volume 1. [Google Scholar]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V. Phytochemical and Biological Studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Zavala, M.d.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A Natural Renewable Resource with Multiple Applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Gentry, H.S. Agaves of Continental North America; University of Arizona Press: Tucson, AZ, USA, 1982; ISBN 9780816507757. [Google Scholar]
- González-Elizondo, M.G.; Villanueva, R.G.; Socorro Gonzalez-Elizondo, M. Agaves-Magueyes, Lechuguillas y Noas-Del Estado de Durango y Sus Alrededores; Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional Unidad Durango del Instituto Politécnico Nacional-Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Durango, México, 2009. [Google Scholar]
- Ávila-Reyes, J.A.; Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; González-Valdez, L.S.; del Toro, G.V.; Durán Páramo, E. Phenol Profile and Antioxidant Capacity of Mescal Aged in Oak Wood Barrels. Food Res. Int. 2010, 43, 296–300. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; Delgado-Al, E.A.; Hernandez-, V.; Ortega-Cha, M.; Orea-Lara, G.; de Leon, A.C.-D.; Avila-Reye, J.A.; Muniz-Mart, R. Profiling of Phenolic Compounds of Somatic and Reproductive Tissues of Agave durangensis Gentry (Agavaceae). Am. J. Appl. Sci. 2009, 6, 1076–1085. [Google Scholar] [CrossRef]
- Barriada-Bernal, L.G.; Almaraz-Abarca, N.; Delgado-Alvarado, E.A.; Gallardo-Velázquez, T.; Ávila-Reyes, J.A.; Torres-Morán, M.I.; González-Elizondo, M.d.S.; Herrera-Arrieta, Y. Flavonoid Composition and Antioxidant Capacity of the Edible Flowers of Agave durangensis (Agavaceae). CyTA—J. Food 2014, 12, 105–114. [Google Scholar] [CrossRef]
- Contreras-Hernández, M.G.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, J.G.; Rocha-Guzmán, N.E.; Lara-Ceniceros, T.E.; Contreras-Esquivel, J.C.; Prado Barragán, L.A.; Rutiaga-Quiñones, O.M. Effect of Ultrasound Pre-Treatment on the Physicochemical Composition of Agave durangensis Leaves and Potential Enzyme Production. Bioresour. Technol. 2018, 249, 439–446. [Google Scholar] [CrossRef]
- Alcázar, M.; Kind, T.; Gschaedler, A.; Silveria, M.; Arrizon, J.; Fiehn, O.; Vallejo, A.; Higuera, I.; Lugo, E. Effect of Steroidal Saponins from Agave on the Polysaccharide Cell Wall Composition of Saccharomyces Cerevisiae and Kluyveromyces Marxianus. LWT 2017, 77, 430–439. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Hamdi, S.H.; Belhadj, F.; Ben Jemâa, J.M.; Messaoud, C.; Marzouki, M.N. Phytochemical Profile and Insecticidal Activity of Agave americana Leaf Extract towards Sitophilus oryzae (L.) (Coleoptera: Curculionidae). Environ. Sci. Pollut. Res. 2019, 26, 19468–19480. [Google Scholar] [CrossRef]
- Shegute, T.; Wasihun, Y. Antibacterial Activity and Phytochemical Components of Leaf Extracts of Agave americana. J. Exp. Pharmacol. 2020, 12, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Almazán-Morales, A.; Moreno-Godínez, M.E.; Hernández-Castro, E.; Vázquez-Villamar, M.; Mora-Aguilera, J.A.; Cabrera-Huerta, E.; Alvarez-Fitz, P. Phytochemical Profile and in Vitro Activity of Agave angustifolia and A. Cupreata Extracts against Phytopathogenic Fungi. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2022, 40, 169–187. [Google Scholar] [CrossRef]
- Jiménez-Ferrer, E.; Vargas-Villa, G.; Martínez-Hernández, G.B.; González-Cortazar, M.; Zamilpa, A.; García-Aguilar, M.P.; Arenas-Ocampo, M.L.; Herrera-Ruiz, M. Fatty-Acid-Rich Agave angustifolia Fraction Shows Antiarthritic and Immunomodulatory Effect. Molecules 2022, 27, 7204. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guadarrama, A.B.; Díaz-Román, M.A.; Osorio-García, M.; Déciga-Campos, M.; Rios, M.Y. Chemical Constituents from Agave applanata and Its Antihyperglycemic, Anti-Inflammatory, and Antimicrobial Activities Associated with Its Tissue Repair Capability. Planta Med. 2023, 90, 397–410. [Google Scholar] [CrossRef]
- Samson, S.L.; Garber, A.J. Prevention of Type 2 Diabetes Mellitus: Potential of Pharmacological Agents. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Verma, R. Inhibitory Potential of Traditional Herbs on α-Amylase Activity. Pharm. Biol. 2012, 50, 326–331. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yang, H.; Kim, H.W.; Sung, S.H. New Polyhydroxytriterpenoid Derivatives from Fruits of Terminalia chebula Retz. and Their α-Glucosidase and α-Amylase Inhibitory Activity. Bioorg. Med. Chem. Lett. 2017, 27, 34–39. [Google Scholar] [CrossRef]
- Shengule, S.A.; Mishra, S.; Bodhale, S. Inhibitory Effect of a Standardized Hydroethanolic Extract of Terminalia arjuna Bark on Alpha-Amylase Enzyme. Asian J. Pharm. Clin. Res. 2018, 11, 366. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, C.; Ma, L.; Wei, T.; Zhao, Y.; Peng, X. Comparative Study of Inhibition Mechanisms of Structurally Different Flavonoid Compounds on α-Glucosidase and Synergistic Effect with Acarbose. Food Chem. 2021, 347, 129056. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Q.-M.; Hu, H.-J.; Yang, L.; Yang, Y.-B.; Chou, G.-X.; Wang, Z.-T. Bioactive Diterpenoids and Flavonoids from the Aerial Parts of Scoparia dulcis. J. Nat. Prod. 2014, 77, 1594–1600. [Google Scholar] [CrossRef]
- Kawabata, J.; Mizuhata, K.; Sato, E.; Nishioka, T.; Aoyama, Y.; Kasai, T. 6-Hydroxyflavonoids as α-Glucosidase Inhibitors from Marjoram (Origanum majorana) Leaves. Biosci. Biotechnol. Biochem. 2003, 67, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Amador, S.; Nieto-Camacho, A.; Ramírez-Apan, M.T.; Martínez, M.; Maldonado, E. Cytotoxic, Anti-Inflammatory, and α-Glucosidase Inhibitory Effects of Flavonoids from Lippia graveolens (Mexican oregano). Med. Chem. Res. 2020, 29, 1497–1506. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Guetat, A.; Bouajila, J.; Alzahrani, A.K.; Basha, J. Deverra tortuosa (Desf.) DC from Saudi Arabia as a New Source of Marmin and Furanocoumarins Derivatives with α-Glucosidase, Antibacterial and Cytotoxic Activities. Heliyon 2021, 7, e06656. [Google Scholar] [CrossRef]
- Ortega, R.; Valdés, M.; Alarcón-Aguilar, F.J.; Fortis-Barrera, Á.; Barbosa, E.; Velazquez, C.; Calzada, F. Antihyperglycemic Effects of Salvia polystachya Cav. and Its Terpenoids: α-Glucosidase and SGLT1 Inhibitors. Plants 2022, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Yan, Y.; Liu, D.; Wang, C.; Wang, H. Inhibition of Glycosidase by Ursolic Acid: In Vitro, in Vivo and in Silico Study. J. Sci. Food Agric. 2020, 100, 986–994. [Google Scholar] [CrossRef]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory Mechanism of Two Allosteric Inhibitors, Oleanolic Acid and Ursolic Acid on α-Glucosidase. Int. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef]
- Chen, S.; Lin, B.; Gu, J.; Yong, T.; Gao, X.; Xie, Y.; Xiao, C.; Zhan, J.Y.; Wu, Q. Binding Interaction of Betulinic Acid to α-Glucosidase and Its Alleviation on Postprandial Hyperglycemia. Molecules 2022, 27, 2517. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New Insights into the Inhibition Mechanism of Betulinic Acid on α-Glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef]
- Choi, S.B.; Ko, B.S.; Park, S.K.; Jang, J.S.; Park, S. Insulin Sensitizing and α-Glucoamylase Inhibitory Action of Sennosides, Rheins and Rhaponticin in Rhei Rhizoma. Life Sci. 2006, 78, 934–942. [Google Scholar] [CrossRef]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition of α-Glucosidase Activity by Selected Edible Seaweeds and Fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef]
- Vella, A. Mechanism of Action of DPP-4 Inhibitors—New Insights. J. Clin. Endocrinol. Metab. 2012, 97, 2626–2628. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.R.; Borde, M.; Kumar, C.S.; Maheshwari, U. Dipeptidyl Peptidase IV Inhibitory Activity of Terminalia arjuna Attributes to Its Cardioprotective Effects in Experimental Diabetes: In Silico, in Vitro and in Vivo Analyses. Phytomedicine 2019, 57, 158–165. [Google Scholar] [CrossRef]
- Lin, S.-R.; Chang, C.-H.; Tsai, M.-J.; Cheng, H.; Chen, J.-C.; Leong, M.K.; Weng, C.-F. The Perceptions of Natural Compounds against Dipeptidyl Peptidase 4 in Diabetes: From in Silico to in Vivo. Ther. Adv. Chronic Dis. 2019, 10, 2040622319875305. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Y.; Zhu, J.; Li, B.; Li, Z.; Zhu, W.; Shi, J.; Jia, Q.; Li, Y. Recent Progress in Natural Products as DPP-4 Inhibitors. Future Med. Chem. 2015, 7, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, Y.; Soeatmadji, D.W.; Sumitro, S.B.; Widodo, M.A. Anti-Diabetic Potential of Urena lobata Leaf Extract through Inhibition of Dipeptidyl Peptidase IV Activity. Asian Pac. J. Trop. Biomed. 2015, 5, 645–649. [Google Scholar] [CrossRef]
- Kalhotra, P.; Chittepu, V.C.S.R.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Phytochemicals in Garlic Extract Inhibit Therapeutic Enzyme DPP-4 and Induce Skeletal Muscle Cell Proliferation: A Possible Mechanism of Action to Benefit the Treatment of Diabetes Mellitus. Biomolecules 2020, 10, 305. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Esquivel-Gutiérrez, E.R.; Manzo-Avalos, S.; Peña-Montes, D.J.; Saavedra-Molina, A.; Morreeuw, Z.P.; Reyes, A.G. Hypolipidemic and Antioxidant Effects of Guishe Extract from Agave lechuguilla, a Mexican Plant with Biotechnological Potential, on Streptozotocin-Induced Diabetic Male Rats. Plants 2021, 10, 2492. [Google Scholar] [CrossRef]
- Aleem, A.; Shahnaz, S.; Javaid, S.; Ashraf, W.; Rasool, M.F.; Ahmad, T.; Alotaibi, A.F.; Albeshri, K.S.; Alqahtani, F.; Imran, I. Chronically Administered Agave americana Var. Marginata Extract Ameliorates Diabetes Mellitus, Associated Behavioral Comorbidities and Biochemical Parameters in Alloxan-Induced Diabetic Rats. Saudi Pharm. J. 2022, 30, 1373–1386. [Google Scholar] [CrossRef]
- Rameshrad, M.; Razavi, B.M.; Ferns, G.A.A.; Hosseinzadeh, H. Pharmacology of Dipeptidyl Peptidase-4 Inhibitors and Its Use in the Management of Metabolic Syndrome: A Comprehensive Review on Drug Repositioning. DARU J. Pharm. Sci. 2019, 27, 341–360. [Google Scholar] [CrossRef]
- Monami, M.; Lamanna, C.; Desideri, C.M.; Mannucci, E. DPP-4 Inhibitors and Lipids: Systematic Review and Meta-Analysis. Adv. Ther. 2012, 29, 14–25. [Google Scholar] [CrossRef]
- Mandić, M.R.; Oalđe, M.M.; Lunić, T.M.; Sabovljević, A.D.; Sabovljević, M.S.; Gašić, U.M.; Duletić-Laušević, S.N.; Božić, B.D.; Božić Nedeljković, B.D. Chemical Characterization and in Vitro Immunomodulatory Effects of Different Extracts of Moss Hedwigia ciliata (Hedw.) P. Beauv. from the Vršačke Planine Mts., Serbia. PLoS ONE 2021, 16, e0246810. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ordoñez, A.; Gomez, J.; Vattuone, M.; LSLA, M. Antioxidant Activities of Sechium edule (Jacq.) Swartz Extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic Constituents in the Leaves of Northern Willows: Methods for the Analysis of Certain Phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Zhou, T.; Li, Z.; Kang, O.-H.; Mun, S.-H.; Seo, Y.-S.; Kong, R.; Shin, D.-W.; Liu, X.-Q.; Kwon, D.-Y. Antimicrobial Activity and Synergism of Ursolic Acid 3-O-α-L-Arabinopyranoside with Oxacillin against Methicillin-Resistant Staphylococcus Aureus. Int. J. Mol. Med. 2017, 40, 1285–1293. [Google Scholar] [CrossRef]
- Miyakoshi, M.; Isoda, S.; Sato, H.; Hirai, Y.; Shoji, J.; Ida, Y. 3α-Hydroxy-Oleanene Type Triterpene Glycosyl Esters from Leaves of Acanthopanax spinosus. Phytochemistry 1997, 46, 1255–1259. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- American Veterinary Medical Association. American Veterinary Medical Association Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020; pp. 1–121. [Google Scholar]
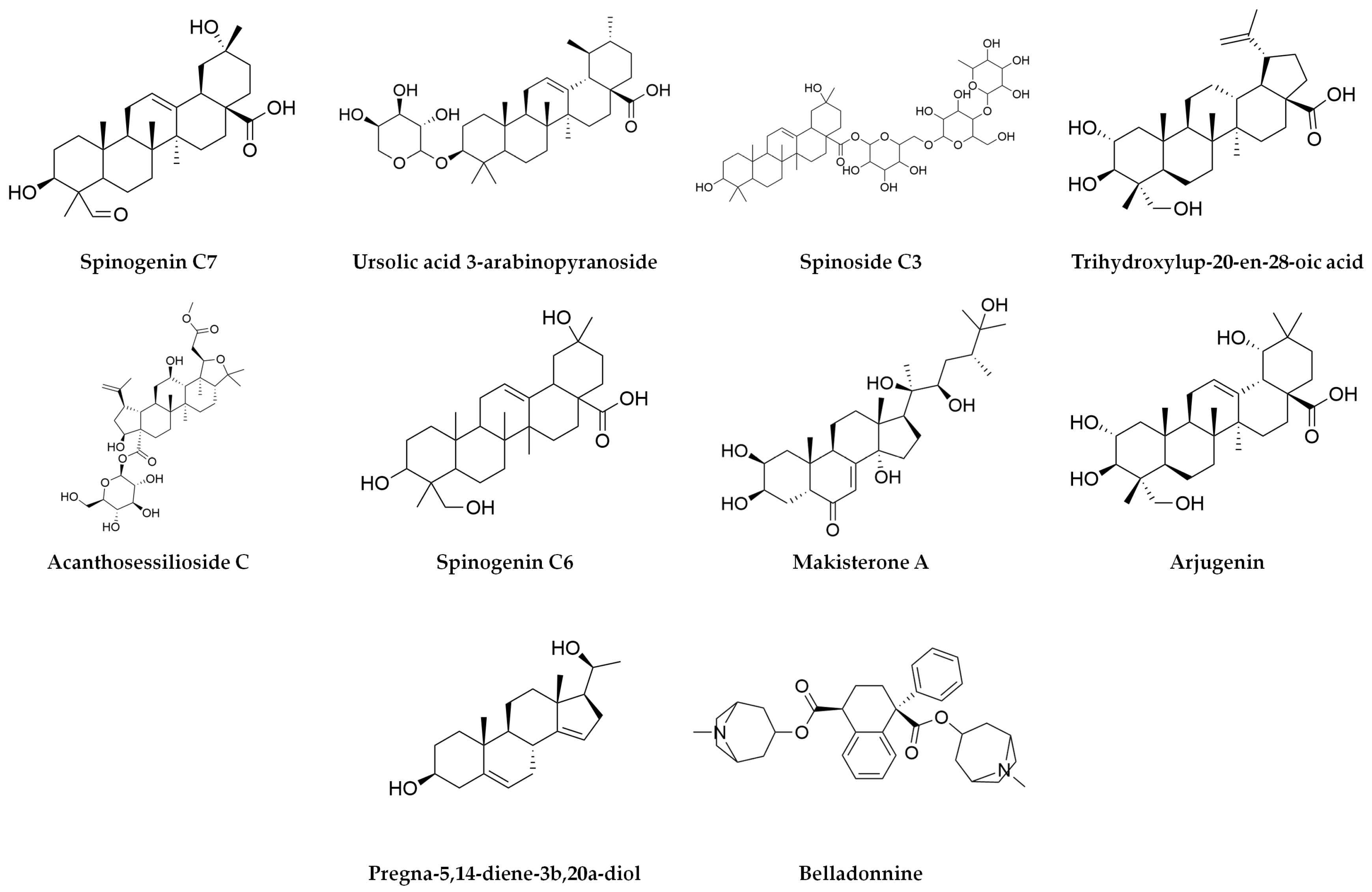
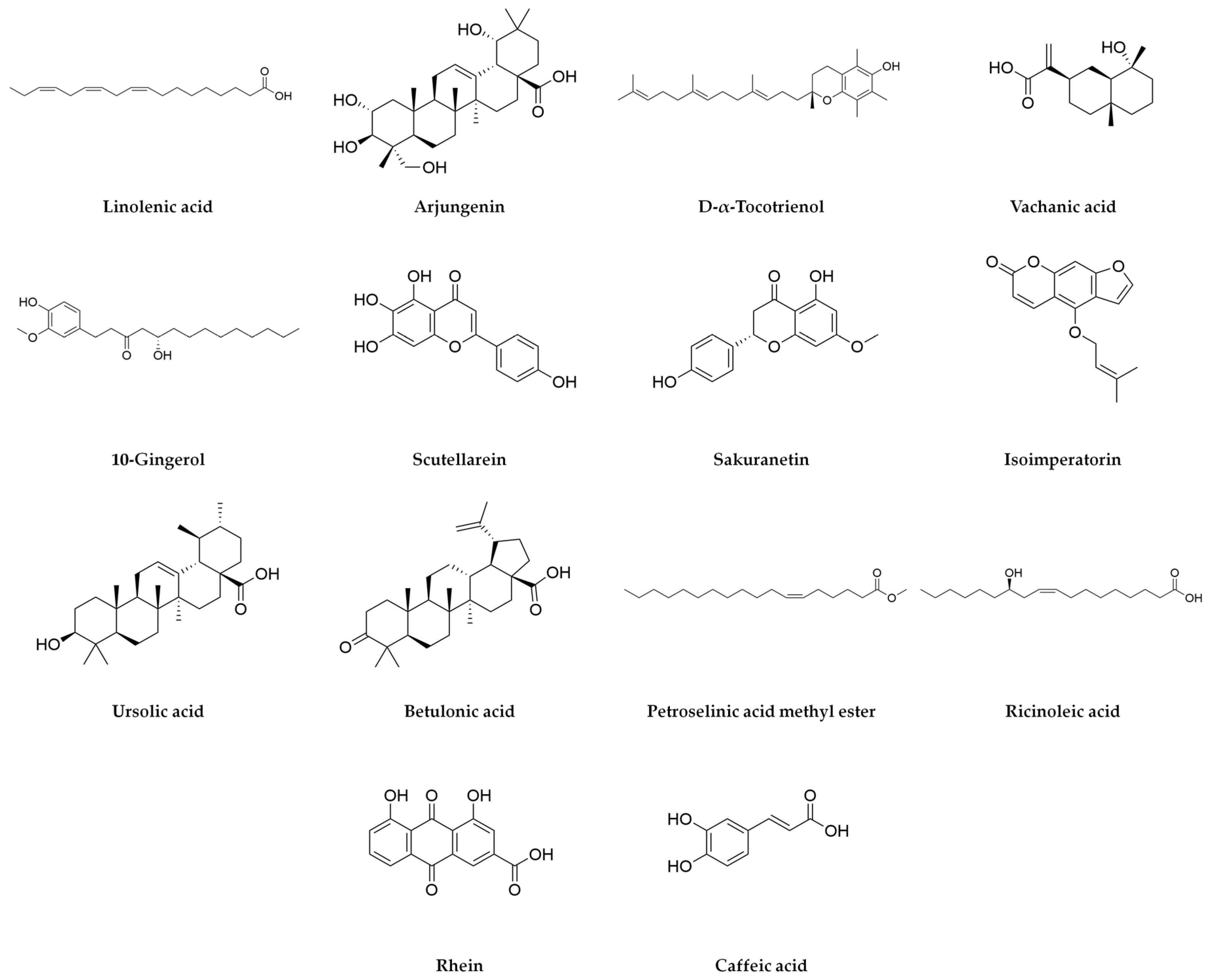
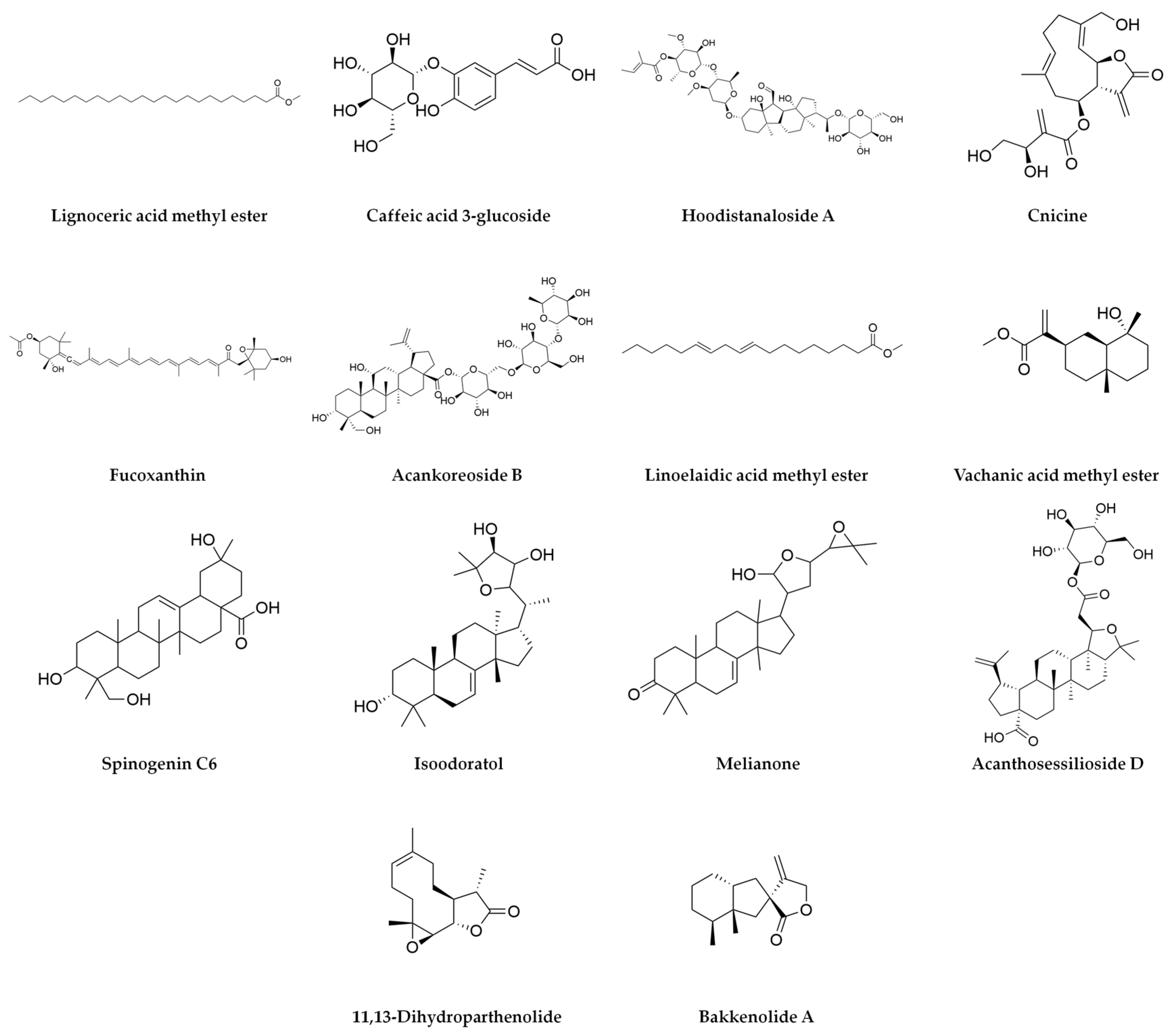

| Adduct | Compound | Observed Mass (g/mol) | Expected Mass (g/mol) | Mass Error (ppm) | Tr (min) | Response | Fragment Ions (Positive Mode) (m/z) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (+H) | Spinogenin C7 | 472.3192 | 472.32 | 0.8 | 19.84 | 73,110 | 455.3159 | 437.30634 | 341.24792 | ||||||
| (+H) | Ursolic acid 3-arabinopyranoside | 588.4022 | 588.4 | −0.6 | 21.67 | 10,557 | 119.08552 | 105.07013 | 221.1335 | 303.30564 | |||||
| (+H) | Spinoside C3 | 928.5034 | 928.5 | 0.3 | 14.7 | 6801 | 207.03239 | ||||||||
| (+H) | Trihydroxylup-20-en-28-oic acid | 488.3521 | 488.35 | 3.9 | 23.89 | 5853 | 335.25784 | 261.22124 | 333.24281 | 259.20575 | 173.13234 | 145.10116 | |||
| (+H) | Acanthosessilioside C | 694.3909 | 694.8 | −2.7 | 11.45 | 5834 | 661.39018 | ||||||||
| (+H) | Spinogenin C6 | 474.3353 | 474.33 | 1.7 | 19.94 | 5740 | 89.06008 | 335.25978 | 379.28394 | 439.32245 | |||||
| (+H) | Makisterone A | 494.3223 | 494.7 | −4.1 | 22.11 | 5700 | 255.23218 | 221.08592 | 165.09117 | ||||||
| (+H) | Arjungenin | 504.3456 | 504.7 | 1 | 21.28 | 5557 | 333.24176 | 437.33973 | |||||||
| (+H) | Pregna-5,14-diene-3b,20a-diol | 316.2409 | 316.24 | 2.2 | 16.21 | 4951 | 299.23659 | 281.22635 | 187.14858 | 173.13401 | 145.10141 | 105.07021 | 215.14367 | 225.16449 | 269.19088 |
| (+H) | Belladonnine | 542.3141 | 542.7 | −0.6 | 12.19 | 3833 | 189.09477 | 101.04227 | 233.12259 | ||||||
| Adduct | Compound | Observed Mass (g/mol) | Expected Mass (g/mol) | Mass Error (ppm) | Tr (min) | Response | Fragment Ions (Negative Mode) (m/z) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (−H) | Linolenic acid | 278.2248 | 278.4 | 0.8 | 18.35 | 622,876 | 278.22081 | 277.21751 | 259.20662 | ||||
| (−H) | Arjungenin | 504.3443 | 504.7 | −1.6 | 21.25 | 55,882 | 277.21753 | 293.21236 | 419.3143 | 471.30954 | 235.16928 | 471.30954 | 443.3136 |
| (−H) | D-alpha-Tocotrienol | 424.3344 | 424.7 | 0.5 | 21.7 | 38,951 | 205.15989 | 145.02954 | |||||
| (−H) | Vachanic acid | 252.1726 | 252.35 | 0.3 | 18.36 | 23,819 | 221.15407 | 220.14654 | 149.09725 | 171.10206 | |||
| (−H) | 10-Gingerol | 350.244 | 350.5 | −5 | 20.86 | 21,577 | 149.09705 | 236.1041 | 275.20213 | ||||
| (−H) | Scutellarein | 286.0477 | 286.24 | 0 | 8.95 | 19,940 | 285.0406 | 255.02907 | 283.02385 | ||||
| (−H) | Sakuranetin | 286.0843 | 286.28 | 0.8 | 9.44 | 19,333 | 179.03465 | 285.07816 | 177.01968 | ||||
| (−H) | Isoimperatorin | 270.0893 | 270.28 | 0.2 | 12.31 | 15,674 | 269.08187 | 254.05786 | |||||
| (−H) | Ursolic acid | 456.3604 | 456.7 | 0.2 | 23.92 | 15,275 | 455.35314 | 456.35654 | 457.36112 | 453.33631 | |||
| (−H) | Betulonic acid | 454.3449 | 454.7 | 0.4 | 23.19 | 11,434 | 277.2178 | 339.20073 | 438.31414 | ||||
| (−H) | Petroselinic acid methyl ester | 296.2718 | 296.5 | 1 | 21.43 | 8831 | 149.09754 | ||||||
| (−H) | Ricinoleic acid | 298.2509 | 298.5 | 0.3 | 16.15 | 7396 | 279.23256 | 297.24312 | 221.15402 | ||||
| (−H) | Rhein | 284.0322 | 284.22 | 0.2 | 8.96 | 7153 | 183.01199 | 283.02385 | 211.03993 | ||||
| (−H) | Caffeic acid | 180.04226 | 180.16 | 1.5 | 9.44 | 5464 | 179.03465 | 135.0449 | |||||
| (−H) | Lignoceric acid methyl ester | 382.3808 | 382.7 | −0.9 | 24.77 | 4934 | 367.35847 | 339.32682 | 253.21758 | 225.22208 | |||
| (−H) | Caffeic acid 3-glucoside | 342.0955 | 342.3 | 1.3 | 3.78 | 4443 | 179.03453 | 135.04567 | |||||
| (−H) | Hoodistanaloside A | 914.4909 | 915.1 | 3.7 | 7.24 | 4339 | 623.41572 | 867.47919 | 837.43031 | ||||
| (−H) | Cnicine | 378.1693 | 378.4 | 3.7 | 15.72 | 4247 | 309.17442 | 119.05023 | |||||
| (−H) | Fucoxanthin | 658.4262 | 658.9 | 4.4 | 21.9 | 4163 | 605.40462 | 537.3761 | 486.28544 | 275.20176 | |||
| (−H) | Acankoreoside B | 958.5157 | 959.1 | 2 | 7.37 | 3903 | 881.45779 | 911.50138 | |||||
| (−H) | Linoelaidic acid methyl ester | 294.2559 | 294.5 | 0 | 20.18 | 3894 | 277.21713 | 279.23301 | 149.09723 | ||||
| (−H) | Vachanic acid methyl ester | 266.1881 | 266.38 | −0.3 | 18.35 | 1872 | 221.15407 | 233.11473 | 149.09725 | 205.15939 | 171.10206 | ||
| (−H) | Spinogenin C6 | 474.3342 | 474.33 | −0.6 | 23.17 | 1830 | 277.2178 | 339.20073 | 391.28602 | 438.31414 | |||
| (−H) | Isoodoratol | 474.372 | 474.7 | 2.3 | 22.41 | 1674 | 279.23321 | 367.29661 | 293.21262 | 227.20168 | |||
| (−H) | Melianone | 470.3391 | 470.7 | −1.2 | 20.93 | 1503 | 293.21165 | 353.20613 | 275.20213 | 185.11715 | |||
| (−H) | Acanthosessilioside D | 648.3885 | 648.8 | 1.8 | 20.47 | 1498 | 577.3741 | 503.33659 | 485.32631 | 309.20769 | 291.1963 | ||
| (−H) | 11,13-Dihydroparthenolide | 250.1566 | 250.33 | −1 | 16.79 | 1490 | 149.09753 | 205.15907 | |||||
| (−H) | Bakkenolide A | 234.1619 | 234.33 | −0.4 | 20.79 | 1292 | 221.15462 | 205.16 | |||||
| Compound | AMPK | SUR1 | α-Glucosidases | PPARγ | DPP4 | SGLT2 | GLP1-R | |||
|---|---|---|---|---|---|---|---|---|---|---|
| C-Terminal Subunit of Maltase-Glucoamylase | N-Terminal Subunit of Maltase-Glucoamylase | Sucrase-Isomaltase | Pancreatic α-Amylase | |||||||
| Linolenic acid | −5.4 | −6.1 | −4.9 | −4.7 | −5.1 | −4.9 | −4.8 | −5.4 | −5.2 | −3.9 |
| Spinogenin C7 | −7.3 | −8.5 | −8.8 | −7.8 | −6.5 | −9.1 | −8.1 | −7.9 | −8 | −8.4 |
| Arjungenin | −7.5 | −9.2 | −8.5 | −6.9 | −7 | −9 | −8 | −8.6 | −7.9 | −8.2 |
| D-α-Tocotrienol | −5.4 | −8.8 | −8.2 | −8 | −7.1 | −8.5 | −8.4 | −8 | −10.8 | −7.1 |
| Vachanic acid | −6.1 | −7.2 | −6.8 | −6.6 | −6.9 | −7.7 | −7 | −7.4 | −7.2 | −9 |
| 10-Gingerol | −7 | −6.7 | −6.9 | −5.8 | −6.4 | −6.3 | −5.8 | −5.9 | −8.3 | −8.3 |
| Scutellarein | −9.4 | −8.2 | −9 | −7.3 | −7.7 | −8.4 | −7.4 | −7.8 | −8.8 | −9.1 |
| Sakuranetin | −10.6 | −8.9 | −9.3 | −8.3 | −8.5 | −9.7 | −8.7 | −8.6 | −10.5 | −9.6 |
| Isoimperatorin | −8 | −7.5 | −7.8 | −7.1 | −7.5 | −7.8 | −7.1 | −7.7 | −9.6 | −6.8 |
| Ursolic acid | −7.7 | −9.4 | −8.1 | −7.5 | −7 | −9.3 | −7.9 | −8.7 | −9.5 | −8.9 |
| Betulonic acid | −7.8 | −9.1 | −8.8 | −7 | −7 | −9.1 | −8 | −8.3 | −8.2 | −8.2 |
| Ursolic acid 3-arabinopyranoside | −7.5 | −9.8 | −9.2 | −7.6 | −8.1 | −9 | −8.8 | −8.6 | −9.2 | −8.9 |
| Petroselinic acid methyl ester | −5.3 | −5.3 | −5.7 | −4.4 | −4.1 | −3.8 | −3.8 | −4.6 | −6.7 | −5.5 |
| Ricinoleic acid | −5.6 | −5.8 | −5.7 | −5.4 | −5.5 | −5.1 | −6.2 | −5.2 | −6.7 | −6.8 |
| Rhein | −9.3 | −8.6 | −9 | −6.9 | −7.4 | −8.7 | −7.7 | −8.5 | −9 | −9.6 |
| Spinoside C3 | −7.3 | −10.7 | −9.2 | −7.6 | −8.6 | −9 | −9.4 | −9.4 | −9.6 | −9 |
| Trihydroxylup-20-en-28-oic acid | −7.4 | −8.6 | −8.6 | −7.1 | −7.1 | −8.7 | −8.2 | −8.4 | −8.1 | −7.6 |
| Acanthosessilioside C | −7.1 | −8.6 | −7.9 | −7.4 | −7.2 | −8.5 | −8 | −8.4 | −8.3 | −8.1 |
| Makisterone A | −7.7 | −8.5 | −8.4 | −6.7 | −6.4 | −8.5 | −8.9 | −8.2 | −9.1 | −9 |
| Caffeic acid | −6.7 | −6.5 | −6.9 | −6.2 | −6.3 | −6.5 | −6.6 | −6.8 | −7.6 | −6.7 |
| Pregna-5,14-diene-3b,20a-diol | −7.2 | −8.2 | −7.6 | −7 | −6.5 | −8.7 | −7.1 | −8.1 | −8 | −10.3 |
| Lignoceric acid methyl ester | −3.3 | −5.5 | −5 | −4.7 | −4.6 | −4.4 | −5.1 | −5.2 | −6.7 | −7.1 |
| Caffeic acid 3-glucoside | −7.9 | −7.5 | −8.3 | −7.3 | −6.6 | −7.6 | −6.7 | −8.4 | −9 | −7.6 |
| Hoodistanaloside A | −7.6 | −9.6 | −10.1 | −7.8 | −7.5 | −9.9 | −8.9 | −9.8 | −8.7 | −8.2 |
| Cnicine | −6.4 | −8.4 | −7.4 | −7 | −6.6 | −7.7 | −6.8 | −8.4 | −7.3 | −7.2 |
| Fucoxanthin | −7.9 | −2.3 | −8.3 | −7.2 | −7.6 | −8.8 | −7.5 | −9.2 | −11 | −8.6 |
| Acankoreoside B | −9.4 | −10.3 | −10.2 | −8.5 | −7.7 | −11.1 | −10.1 | −11.5 | −10.2 | −10.5 |
| Linoelaidic acid methyl ester | −3.8 | −5.8 | −5.2 | −5.1 | −5 | −5.4 | −5.5 | −5.1 | −6.2 | −7.1 |
| Belladonnine | −7.7 | −10.1 | −10.5 | −8.4 | −8.8 | −8.5 | −9 | −9.6 | −8.9 | −8.3 |
| Vachanic acid methyl ester | −5.7 | −6.9 | −6.9 | −6.2 | −6.4 | −7.7 | −6.9 | −6.8 | −7.9 | −9 |
| Spinogenin C6 | −6.9 | −8 | −8.2 | −7.5 | −7.7 | −10.4 | −7.6 | −8.4 | −8.5 | −8.4 |
| Isoodoratol | −7.4 | −9.1 | −9.3 | −7.7 | −7 | −9.6 | −8.8 | −8.9 | −9.6 | −8.6 |
| Melianone | −8.3 | −9.6 | −8.9 | −8.2 | −8.1 | −10.4 | −8.5 | −9.2 | −9.6 | −8.7 |
| Acanthosessilioside D | −8 | −8.7 | −8.5 | −8.2 | −7.1 | −9.3 | −8.7 | −9.2 | −8.9 | −8.9 |
| 11β,13-Dihydroparthenolide | −8.5 | −7.3 | −7.2 | −6.1 | −5.8 | −7.4 | −6.9 | −7.8 | −7.6 | −9 |
| Bakkenolide A | −7 | −7.6 | −6.5 | −5.5 | −5.9 | −7.6 | −6.8 | −8.3 | −7.6 | −7.1 |
| Metformin | −4.9 | |||||||||
| CG7 | −10.9 | |||||||||
| Glibenclamide | −9.6 | |||||||||
| Acarbose | −7.6 | −6.7 | −6.7 | −7.8 | ||||||
| Pioglitazone | −8.7 | |||||||||
| Sitagliptin | −8.5 | |||||||||
| Empagliflozin | −10.7 | |||||||||
| Danuglipron | −13.1 | |||||||||
| Healthy | Diabetic | Sitagliptin | Metformin | EAD | EAD + Sitagliptin | EAD + Metformin | |
|---|---|---|---|---|---|---|---|
| Glucose (mg/dL) | 104.30 ± 5.54 *** | 407.00 ± 60.21 | 171.70 ± 25.83 ** | 195.00 ± 50.32 ** | 176.00 ± 52.58 ** | 157.30 ± 8.34 ** | 154.70 ± 11.70 ** |
| HbA1c (%) | 4.80 ± 0.28 ** | 9.68 ± 1.14 | 6.27 ± 0.82 * | 7.45 ± 1.01 | 4.62 ± 0.54 *** | 6.55 ± 0.23 * | 6.43 ± 0.32 |
| Insulin (ng/mL) | 0.18 ± 0.02 | 0.10 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.10 ± 0.02 | 0.14 ± 0.02 | 0.08 ± 0.02 |
| Urea (mg/dL) | 90.67 ± 6.64 ** | 265.00 ± 36.67 | 182.00 ± 19.05 | 225.00 ± 35.04 | 236.30 ± 38.34 | 181.00 ± 9.31 | 200.30 ± 41.06 |
| Creatinine (mg/dL) | 1.00 ± 0.05 | 1.97 ± 0.51 | 1.26 ± 0.06 | 1.26 ± 0.08 | 2.02 ± 0.53 | 1.15 ± 0.08 | 1.13 ± 0.18 |
| Total cholesterol (mg/dL) | 55.00 ± 6.50 | 77.25 ± 7.60 | 39.33 ± 4.41 ** | 48.33 ± 7.31 * | 45.67 ± 4.25 * | 42.50 ± 9.02 ** | 51.00 ± 5.68 |
| HDL (mg/dL) | 14.00 ± 1.52 | 19.25 ± 1.93 | 10.00 ± 1.15 ** | 12.33 ± 1.76 | 11.67 ± 1.20 * | 10.50 ± 2.25 ** | 13.00 ± 1.52 |
| LDL (mg/dL) | 26.33 ± 2.60 | 36.00 ± 1.68 | 21.67 ± 2.02 | 26.00 ± 5.85 | 25.00 ± 3.78 | 24.25 ± 5.89 | 29.00 ± 4.72 |
| VLDL (mg/dL) | 14.67 ± 3.18 | 22.00 ± 4.02 | 7.66 ± 2.18 ** | 10.00 ± 2.88 * | 8.75 ± 2.05 ** | 7.75 ± 1.31 ** | 9.00 ± 2.08 * |
| Triglycerides (mg/dL) | 72.33 ± 15.59 | 109.30 ± 20.34 | 38.67 ± 10.37 ** | 49.67 ± 13.59 * | 44.50 ± 10.52 ** | 39.00 ± 7.29 ** | 44.67 ± 9.61 * |
| Total bilirubin (mg/dL) | 0.16 ± 0.03 ** | 0.35 ± 0.01 | 0.23 ± 0.03 | 0.29 ± 0.03 | 0.29 ± 0.04 | 0.41 ± 0.04 | 0.32 ± 0.03 |
| Direct bilirubin (mg/dL) | 0.08 ± 0.02 | 0.11 ± 0.03 | 0.15 ± 0.05 | 0.20 ± 0.04 | 0.11 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| Indirect bilirubin (mg/dL) | 0.07 ± 0.01 * | 0.23 ± 0.03 | 0.08 ± 0.02 * | 0.09 ± 0.00 * | 0.18 ± 0.04 | 0.23 ± 0.03 | 0.15 ± 0.03 |
| AST (U/L) | 244.00 ± 8.08 | 812.50 ± 217.50 | 686.70 ± 159.20 | 649.30 ± 155.50 | 582.50 ± 122.80 | 400.00 ± 169.40 | 475.00 ± 95.00 |
| ALT (U/L) | 280.70 ± 11.10 | 463.00 ± 91.14 | 317.30 ± 23.25 | 378.70 ± 73.54 | 380.50 ± 63.54 | 185.00 ± 35.71 * | 275.00 ± 15.00 |
| Healthy | Diabetic | Sitagliptin | Metformin | EAD | EAD + Sitagliptin | EAD + Metformin |
|---|---|---|---|---|---|---|
| 100.00% | 110.90 ± 1.44% | 7.13 ± 1.70% *** | 128.40 ± 8.68% | 74.72 ± 3.58% *** | 4.54 ± 1.97% *** | 94.86 ± 7.89% |
| Time (Minutes) | Phase A | Phase B |
|---|---|---|
| 0 | 95 | 5 |
| 2 | 95 | 5 |
| 22 | 5 | 95 |
| 25 | 5 | 95 |
| 27 | 95 | 5 |
| 30 | 95 | 5 |
| Drug Family | Enzyme Target | PDB | Control | Coordinates of the Grid Box | Size of the Grid Box | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Biguanides | AMPK | 6B1U | CG7 | −48.97 | 27.95 | −67.13 | 40 Å × 40 Å × 40 Å |
| Sulfonylureas | SUR1 | 7S5V | Glibenclamide | 198 | 296 | 232 | 40 Å × 40 Å × 40 Å |
| α-Glucosidase inhibitor | Maltase-glucoamylase | 3TOP | Acarbose | −30.61 | 35.65 | 26.44 | 40 Å × 40 Å × 40 Å |
| 2QMJ | −20.8 | −6.58 | −5.07 | 40 Å × 40 Å × 40 Å | |||
| Sucrase-isomaltase | 3LPP | 39.89 | 58.74 | 78.87 | 40 Å × 40 Å × 40 Å | ||
| Pancreatic α-amylase | 4W93 | −9.63 | 4.34 | −23.1 | 40 Å × 40 Å × 40 Å | ||
| Thiazolidinedione | PPARγ | 5Y2O | Pioglitazone | −48.34 | −2.16 | 78.2 | 40 Å × 40 Å × 40 Å |
| DPP-4 inhibitor | DPP4 | 1X70 | Sitagliptin | 41.18 | 51.04 | 35.62 | 40 Å × 40 Å × 40 Å |
| SGLT2 inhibitor | SGLT2 | 7VSI | Empagliflozin | 38.3 | 50.24 | 46.38 | 40 Å × 40 Å × 40 Å |
| GLP-1R agonists | GLP-1 receptor | 6X1A | Danuglipron | 131.34 | 116.77 | 155.03 | 40 Å × 40 Å × 40 Å |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bermudes-Contreras, J.D.; Gutiérrez-Velázquez, M.V.; Delgado-Alvarado, E.A.; Torres-Ricario, R.; Cornejo-Garrido, J. Hypoglycemic and Hypolipidemic Effects of Triterpenoid Standardized Extract of Agave durangensis Gentry. Plants 2025, 14, 894. https://doi.org/10.3390/plants14060894
Bermudes-Contreras JD, Gutiérrez-Velázquez MV, Delgado-Alvarado EA, Torres-Ricario R, Cornejo-Garrido J. Hypoglycemic and Hypolipidemic Effects of Triterpenoid Standardized Extract of Agave durangensis Gentry. Plants. 2025; 14(6):894. https://doi.org/10.3390/plants14060894
Chicago/Turabian StyleBermudes-Contreras, Juan David, Marcela Verónica Gutiérrez-Velázquez, Eli Amanda Delgado-Alvarado, René Torres-Ricario, and Jorge Cornejo-Garrido. 2025. "Hypoglycemic and Hypolipidemic Effects of Triterpenoid Standardized Extract of Agave durangensis Gentry" Plants 14, no. 6: 894. https://doi.org/10.3390/plants14060894
APA StyleBermudes-Contreras, J. D., Gutiérrez-Velázquez, M. V., Delgado-Alvarado, E. A., Torres-Ricario, R., & Cornejo-Garrido, J. (2025). Hypoglycemic and Hypolipidemic Effects of Triterpenoid Standardized Extract of Agave durangensis Gentry. Plants, 14(6), 894. https://doi.org/10.3390/plants14060894






