Bioactive Metabolites from the Dusty Seeds of Gastrodia elata Bl., Based on Metabolomics and UPLC-Q-TOF-MS Combined with Molecular Network Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Sample Preparation and Extraction for Metabolomics Analysis
2.2.1. Seeds Extract
2.2.2. UPLC Conditions
2.2.3. ESI-Q TRAP-MS/MS
2.2.4. Metabolite Identification and Quantification
- Level 1: Secondary spectrum and RT matching score > 0.7, indicating high-confidence identification.
- Level 2: Matching score between 0.5 and 0.7, indicating medium-confidence identification.
- Level 3: Matching of Q1, Q3, RT, DP, and CE with database substances, indicating low-confidence identification.
2.2.5. UPLC-Q-TOF-MS/MS Parameters for Secondary Metabolites Analysis
2.3. Molecular Networking
2.4. Purification of Compounds
2.5. Growth Promoting Activity and Inhibition Activity Test
2.5.1. Growth Promoting Activity Test on the M. osmundicola
2.5.2. Growth Inhibition Activity Test on the F. oxysporum
2.5.3. Bioinformatic Analysis
2.6. Antioxidant Activities Assay
2.7. Anti-Inflammatory Activity Test
2.8. Cytotoxic Activity Test
3. Results and Discussion
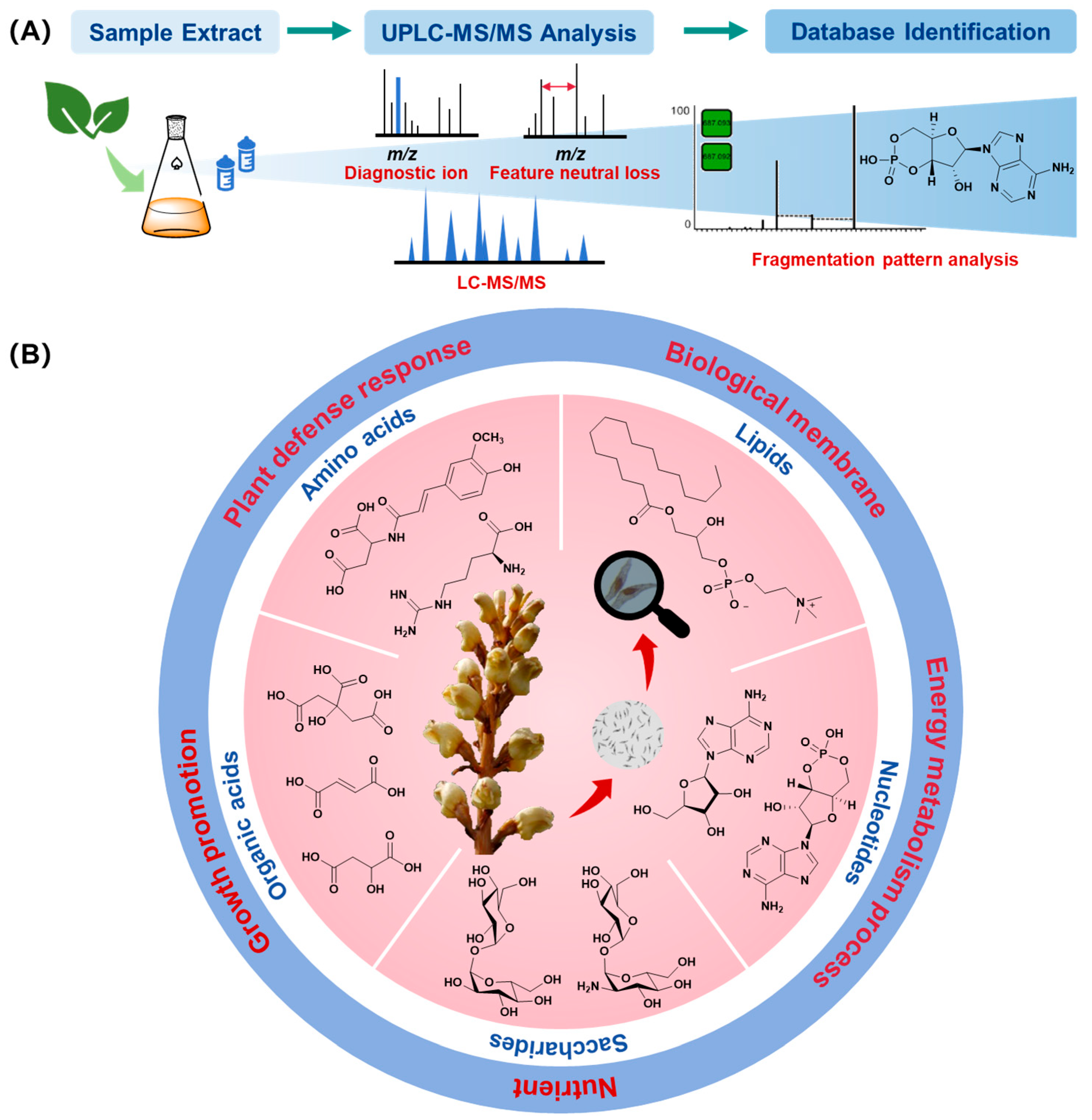
3.1. Primary Metabolite Analysis Based on Metabolomics
3.2. Molecular Networking Analysis
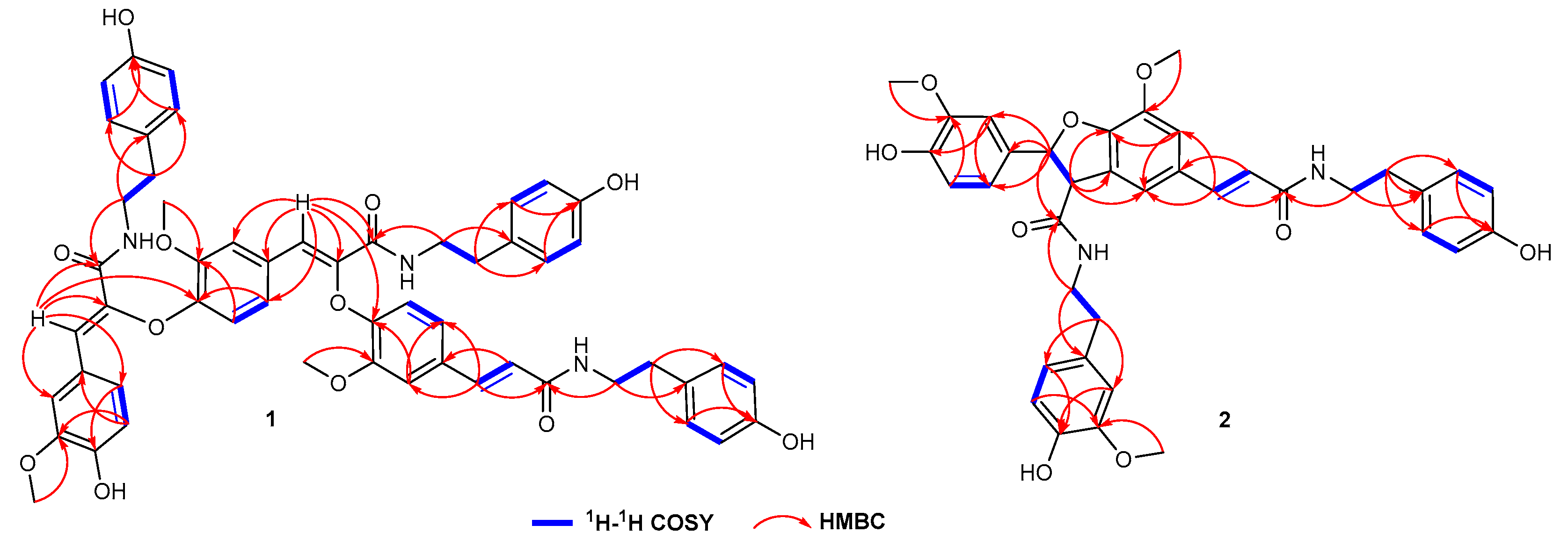
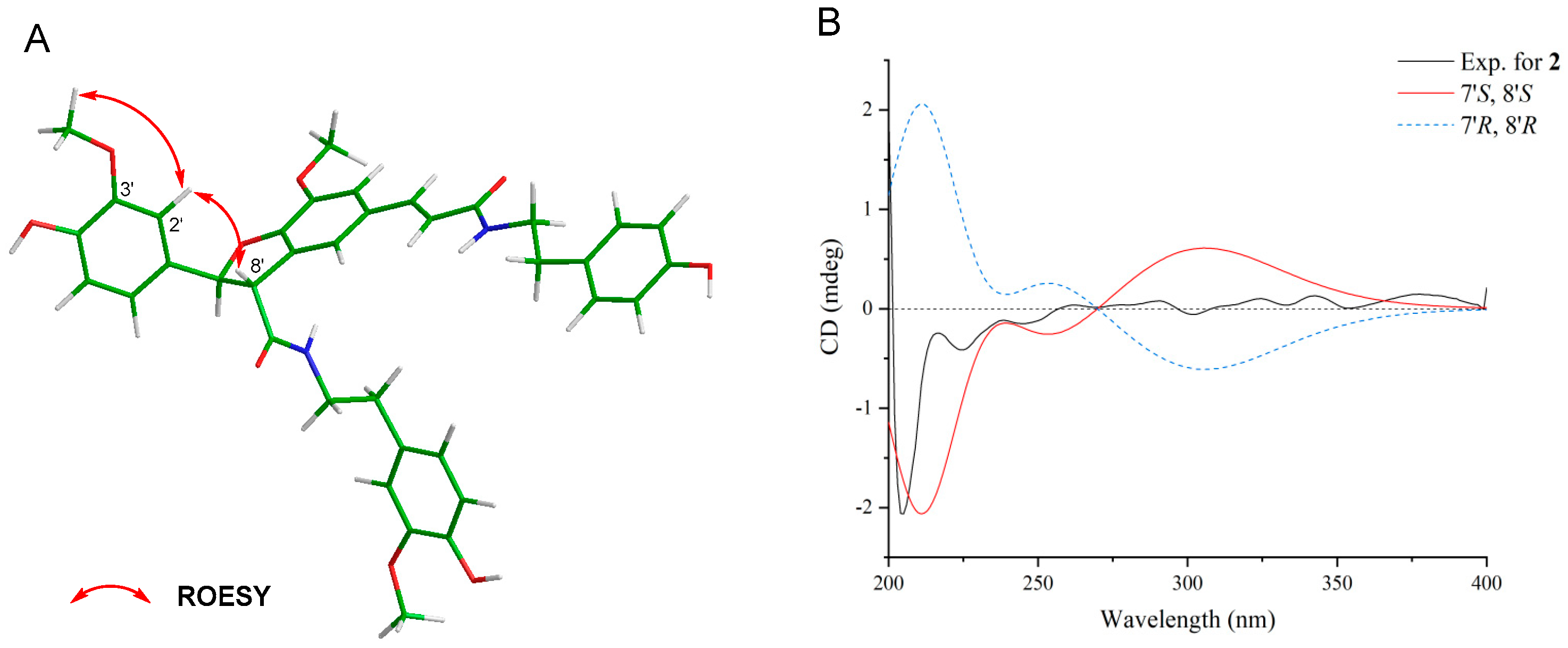
3.3. Structural Elucidation
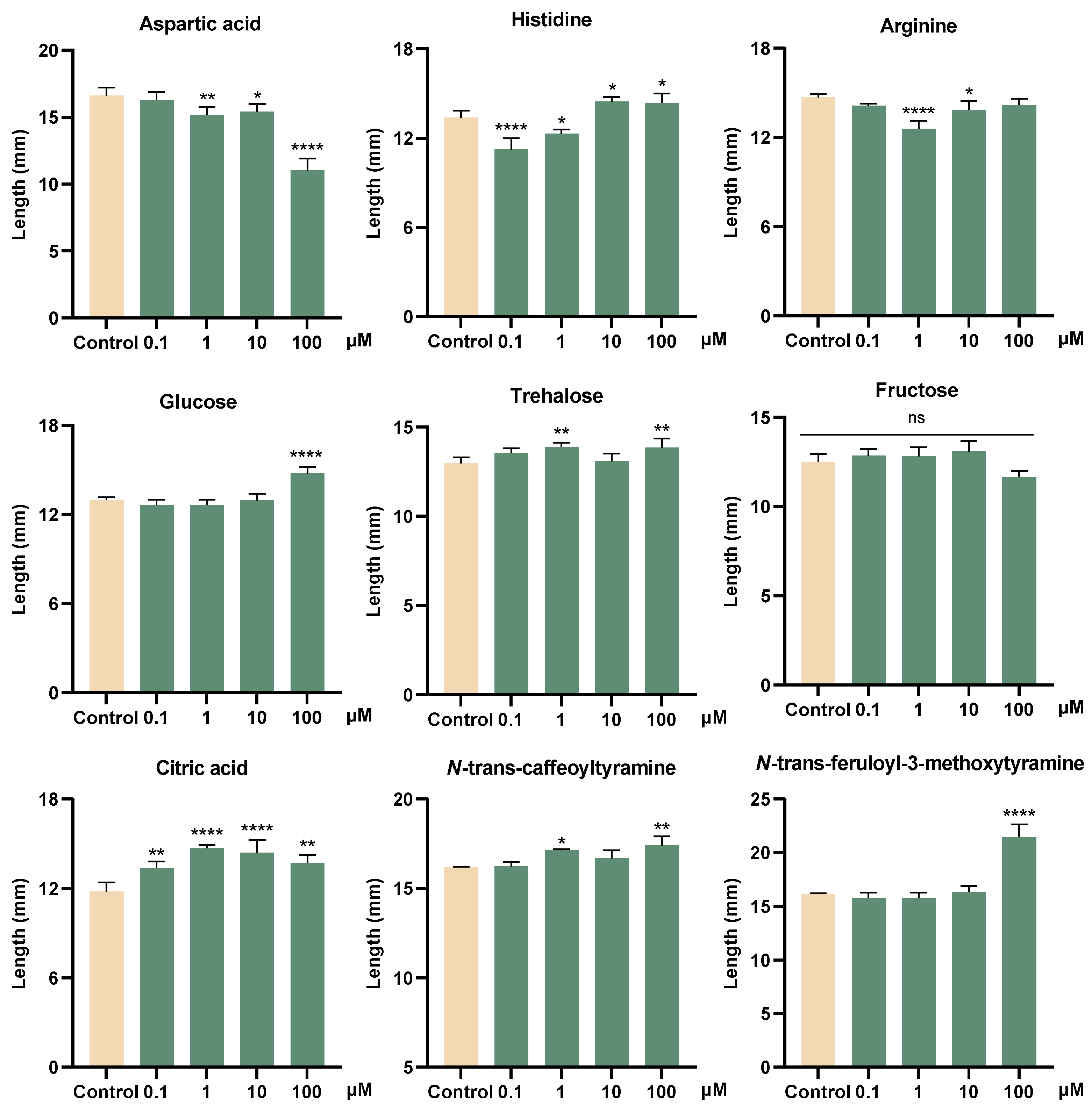
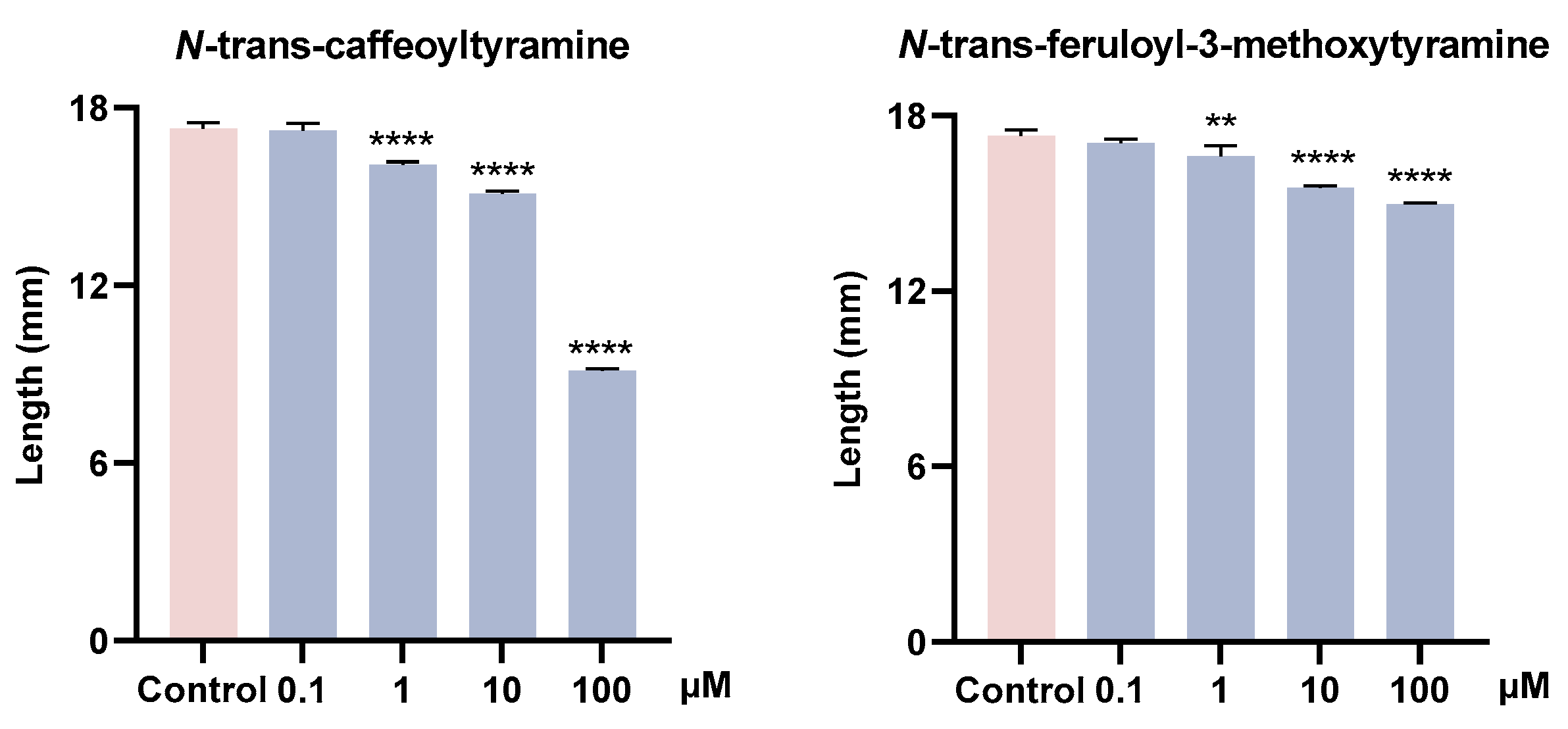
3.4. Growth-Promoting and Inhibitory Activity of Metabolites
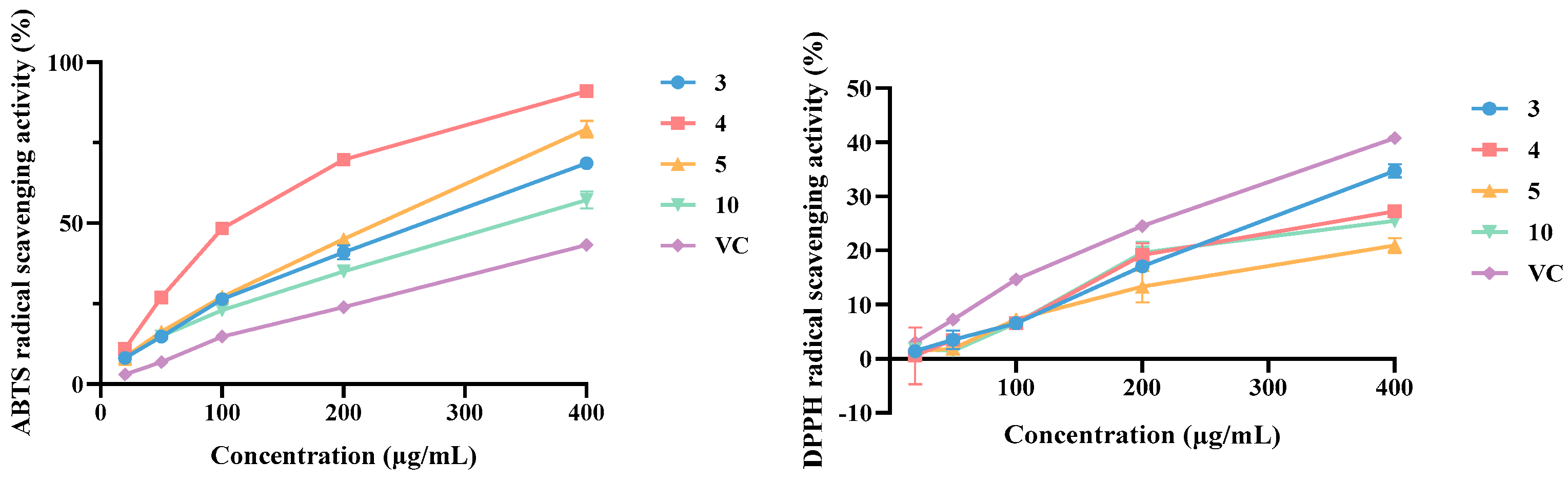
3.5. Antioxidant Activity Evaluation
3.6. Anti-Inflammatory Activity Evaluation
3.7. Cytotoxic Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Arbuscular Mycorrhizal |
| OM | Orchid Mycorrhizal |
| LCOs | Lipo-Chitooligosaccharides |
| NHCPRC | National Health Commission of the People’s Republic of China |
| CAD | Collision-Activated Dissociation |
| DP | Declustering Potential |
| CE | Collision Energy |
| LPS | Lipopolysaccharide |
| NO | Nitric Oxide |
References
- Arditti, J.; Ghani, A.K.A. Tansley review No. 110.: Numerical and physical properties of orchid seeds and their biological implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.; Van, S. The development and mobilisation of seed reserves in some African orchids. Aust. J. Bot. 1987, 35, 343–353. [Google Scholar] [CrossRef]
- Batty, A.L.; Dixon, K.W.; Brundrett, M.; Sivasithamparam, K. Constraints to symbiotic germination of terrestrial orchid seed in a mediterranean bushland. New Phytol. 2001, 152, 511–520. [Google Scholar] [CrossRef]
- Leake, J.R. Myco-heterotroph/epiparasitic plant interactions with ectomycorrhizal and arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2004, 7, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, Y.C.; Qin, L.Y.; He, H.Y.; Yu, X.L.; Yang, M.Z.; Zhang, H.B. Dynamics of fungal communities during Gastrodia elata growth. BMC Microbiol. 2019, 19, 158. [Google Scholar] [CrossRef]
- Schoser, G. Orchids and mycorrhiza—A review. In Proceedings of the 10th World Orchid Conference, Durban, South Africa, 11–17 September 1981; Stewart, J., van der Merwe, C.N., Eds.; South African Orchid Council: Edenvale, South Africa, 1981; pp. 275–277. [Google Scholar]
- Gutjahr, C. Phytohormone signaling in arbuscular mycorhiza development. Curr. Opin. Plant. Biol. 2014, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R. Orchids: A review of uses in traditional medicine, its phytochemistry and pharmacology. J. Med. Plants. 2010, 4, 592–638. [Google Scholar]
- Wang, T.; Song, Z.; Wang, X.; Xu, L.; Sun, Q.; Li, L. Functional insights into the roles of hormones in the Dendrobium officinale-Tulasnella sp. germinated seed symbiotic association. Int. J. Mol. Sci. 2018, 19, 3484. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.T.; Guo, S.X. Fungus associated with nutrition of seed germination of Gastrodia elata-Mycena osmundicola Lange. Mycosystema 1989, 8, 221–226. [Google Scholar]
- Xu, J.T.; Mu, C. The relation between growth of Gastrodia elata a protocorms and fungi. Acta Bot. Sin. 1990, 32, 26–31. [Google Scholar]
- Xu, J.T.; Ran, X.Z.; Guo, S.X. Studies on nutrition source of seeds germination of Gastrodia elata Bl. Acta Acad. Med. Sin. 1990, 6, 431–434. [Google Scholar]
- Xu, J.T.; Ran, X.Z.; Guo, S.X. Studies on the life cycle of Gastrodia elata. Acta Acad. Med. Sin. 1989, 4, 237–241. [Google Scholar]
- Su, Z.H.; Yang, Y.G.; Chen, S.Z. The processing methods, phytochemistry and pharmacology of Gastrodia elata Bl.: A comprehensive review. J. Ethnopharmacol. 2023, 314, 116467. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, J.; dos Santos Passos, C.; Nurisso, A.; Simões-Pires, C.A.; Ji, M.; Lou, H.; Fan, P. Characterization of lignanamides from Hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015, 63, 10611–10619. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gu, Y.F.; Su, X.Q.; Li, M.M.; Huo, H.X.; Zhang, J.; Zeng, K.W.; Zhang, Q.; Zhao, Y.F.; Li, J.; et al. Anti-inflammatory lignanamides from the roots of Solanum melongena L. Fitoterapia 2014, 98, 110–116. [Google Scholar] [CrossRef]
- Liu, J.Z.; Wang, Y.D.; Fang, H.Q.; Sun, G.B.; Ding, G. UPLC-Q-TOF-MS/MS-based targeted discovery of chetomin analogues from Chaetomium cochliodes. J. Nat. Prod. 2024, 87, 1660–1665. [Google Scholar] [CrossRef]
- Liu, X.; Xie, Z.; Wang, Y.; Sun, Y.; Dang, X.; Sun, H. A dual role of amino acids from Sesbania rostrata seed exudates in the chemotaxis response of Azorhizobium caulinodans ORS571. Mol. Plant Microbe Interact. 2019, 32, 1134–1147. [Google Scholar] [CrossRef]
- Yang, C.; Bai, Y.; Halitschke, R.; Gase, K.; Baldwin, G.; Baldwin, I.T. Exploring the metabolic basis of growth/defense trade-offs in complex environments with Nicotiana attenuata plants cosilenced in NaMYC2a/b expression. New Phytol. 2023, 238, 349–366. [Google Scholar] [CrossRef]
- Pacheco-Moreno, A.; Bollmann-Giolai, A.; Chandra, G.; Brett, P.; Davies, J.; Thornton, O.; Poole, P.; Ramachandran, V.; Brown, J.K.M.; Nicholson, P.; et al. The genotype of barley cultivars influences multiple aspects of their associated microbiota via differential root exudate secretion. PLoS Biol. 2024, 22, e3002232. [Google Scholar] [CrossRef]
- Wen, T.; Yuan, J.; He, X.; Lin, Y.; Huang, Q.; Shen, Q. Enrichment of beneficial cucumber rhizosphere microbes mediated by organic acid secretion. Hortic. Res. 2020, 7, 154. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Bao, H.Y. Nutrient composition of seeds of Gastrodia elata Bl. and its antioxidant effect on convulsive mice. Chin. J. Gerontol. 2019, 39, 2458–2462. [Google Scholar]
- Sun, J.; Tong, L.T.; Tu, P.F.; Chen, L.L.; Xu, X.; Song, Y.; Yang, X.X.; Guo, Z.B.; Zou, X.; Sun, C.X.; et al. Lignanamides: A comprehensive review of chemical constituents, biological activities, extraction methods and synthetic pathway. Food Chem. 2024, 460, 140459. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Liu, Y.; Xiang, L.; Zhou, F.; Li, H.; Su, Y.; Xu, X.; Wang, Q. Metabolites identification of bioactive compounds daturataturin A, daturametelin I, N-trans-feruloyltyramine, and cannabisin F from the seeds of Datura metel in rats. Front. Pharmacol. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- King, R.R.; Calhoun, L.A. Characterization of cross-linked hydroxycinnamic acid amides isolated from potato common scab lesions. Phytochemistry 2005, 66, 2468–2473. [Google Scholar] [CrossRef]
- Seca, A.M.; Silva, A.M.; Silvestre, A.J.; Cavaleiro, J.A.; Domingues, F.M.; Pascoal-Neto, C. Lignanamides and other phenolic constituents from the bark of kenaf (Hibiscus cannabinus). Phytochemistry 2001, 58, 1219–1223. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Huang, H.; Li, Q.; Wang, X.; Sun, Q.; Wang, Q.; Li, N. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. 2023, 407, 135198. [Google Scholar] [CrossRef]
- Hao, R.; Li, M.; Li, F.; Sun-Waterhouse, D.; Li, D. Protective effects of the phenolic compounds from mung bean hull against H2O2-induced skin aging through alleviating oxidative injury and autophagy in HaCaT cells and HSF cells. Sci. Total Environ. 2022, 841, 156669. [Google Scholar] [CrossRef]
- Tuohongerbieke, A.L.J.; Sabir, G.; Xin, X.; Hu, M.; Duan, X.; Liu, L.; Tang, D.; Zhu, J.; Aisa, H.A. Lignanamides from the roots of Limonium gmelinii (Willd.) Kuntze and their anti-diabetic, cytotoxic and anti-inflammatory activities. Phytochemistry 2021, 184, 112648. [Google Scholar] [CrossRef]


| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH a (Mult, J in Hz) | δC b, Type | δH a (Mult, J in Hz) | δC b, Type | |
| 1 | 129.0, C | 130.5, C | ||
| 2 | 7.42, d, (2.0) | 114.2, CH | 6.70, d, (2.0) | 117.8, CH |
| 3 | 150.1, C | 146.0, C | ||
| 4 | 147.2, C | 151.2, C | ||
| 5 | 6.64, d, (8.5) | 114.6, CH | 129.4, C | |
| 6 | 7.03, dd, (8.5, 2.0) | 125.5, CH | 7.11, d, (2.0) | 113.4, CH |
| 7 | 7.27, s | 124.3, CH | 7.40, d, (15.5) | 141.7, CH |
| 8 | 143.1, C | 6.36, d, (15.5) | 119.5, CH | |
| 9 | 165.2, C | 169.0, C | ||
| 1′ | 132.0, C | 132.6, C | ||
| 2′ | 7.22, d, (2.0) | 112.5, CH | 6.91, d, (2.0) | 110.5, CH |
| 3′ | 15.0.5, C | 149.0, C | ||
| 4′ | 147.3, C | 148.1, C | ||
| 5′ | 6.70, d, (8.0) | 115.1, CH | 6.79, d, (8.5) | 116.3, C |
| 6′ | 6.96, dd, (8.0, 2.0) | 122.3, CH | 6.75, dd, (8.5, 2.0) | 120.0, C |
| 7′ | 7.44, d, (15.5) | 141.1, CH | 5.88, d, (8.5) | 90.0, CH |
| 8′ | 6.47, d, (15.5) | 121.1, CH | 4.14, d, (8.5) | 58.8, CH |
| 9′ | 168.7, C | 172.9, C | ||
| 1″ | 125.4, C | 131.3, C | ||
| 2″ | 7.18, d, (2.0) | 113.4, CH | 7.07, m | 130.8, CH |
| 3″ | 148.9, C | 6.71, m | 116.4, CH | |
| 4″ | 149.5, C | 157.0, C | ||
| 5″ | 6.70, d, (8.0) | 116.3, CH | 6.71, m | 116.4, CH |
| 6″ | 6.97, dd, (8.0, 2.0) | 126.4, CH | 7.07, m | 130.8, CH |
| 7″ | 7.20, s | 125.3, CH | 2.76, t, (7.0) | 35.8, CH2 |
| 8″ | 141.2, C | 3.49, t, (7.0) | 42.6, CH2 | |
| 1‴ | 130.8, C | 131.8, C | ||
| 2‴ | 6.86, m | 130.7, CH | 6.81, d, (2.0) | 113.4, CH |
| 3‴ | 6.59, m | 116.3, CH | 149.3, C | |
| 4‴ | 157.0, C | 146.0, C | ||
| 5‴ | 6.59, m | 116.3, CH | 6.74, d, (8.0) | 116.4, CH |
| 6‴ | 6.86, m | 130.7, CH | 6.65, dd, (8.0, 2.0) | 122.4, CH |
| 7‴ | 2.65, t, (7.0) | 35.5, CH2 | 2.82, dt, (14.0, 7.0) 2.74, dt, (14.0, 7.0) | 35.7, CH2 |
| 8‴ | 3.46, t, (7.0) | 42.2, CH2 | 3.59, dt, (14.0, 7.0) 3.47, dt, (14.0, 7.0) | 42.0, CH2 |
| 1‴ | 131.2, C | |||
| 2‴ | 7.06, m | 130.7, CH | ||
| 3‴ | 6.72, m | 116.3, CH | ||
| 4‴ | 156.9, C | |||
| 5‴ | 6.72, m | 116.3, CH | ||
| 6‴ | 7.06, m | 130.7, CH | ||
| 7‴ | 2.76, t, (7.0) | 35.8, CH2 | ||
| 8‴ | 3.47, t, (7.0) | 42.6, CH2 | ||
| 1′′′′′ | 130.8, C | |||
| 2′′′′′ | 6.77, m | 130.7, CH | ||
| 3′′′′′ | 6.56, m | 116.3, CH | ||
| 4′′′′′ | 156.9, C | |||
| 5′′′′′ | 6.56, m | 116.3, CH | ||
| 6′′′′′ | 6.77, m | 130.7, CH | ||
| 7′′′′′ | 2.56, (7.0) | 35.6, CH2 | ||
| 8′′′′′ | 3.40, (7.0) | 42.2, CH2 | ||
| 3-OCH3 | 3.72, s | 56.1, CH3 | 3.89, s | 56.8, CH3 |
| 3′-OCH3 | 3.91, s | 56.4, CH3 | 3.76, s | 56.3, CH3 |
| 3″-OCH3 | 3.49, s | 55.9, CH3 | ||
| 3‴-OCH3 | 3.82, s | 56.4, CH3 | ||
| Compound | ABTS (100 μg/mL) | DPPH (100 μg/mL) |
|---|---|---|
| 1 | 9.22 ± 0.40 | 6.28 ± 0.83 |
| 2 | 19.29 ± 1.13 | 4.45 ± 0.95 |
| 3 | 27.43 ± 1.30 | 4.90 ± 0.59 |
| 4 | 48.49 ± 1.75 | 8.79 ± 0.89 |
| 5 | 27.38 ± 0.80 | 5.18 ± 0.46 |
| 6 | 11.55 ± 0.52 | 5.34 ± 0.31 |
| 7 | 15.05 ± 0.89 | 5.33 ± 0.63 |
| 8 | 14.30 ± 1.69 | 3.59 ± 0.52 |
| 9 | 11.15 ± 0.19 | 8.17 ± 0.22 |
| 10 | 22.04 ± 1.31 | 6.15 ± 0.76 |
| 11 | 16.34 ± 0.63 | 8.49 ± 0.33 |
| 12 | 10.84 ± 0.99 | 5.60 ± 0.90 |
| VC | 14.93 ± 0.36 | 11.42 ± 0.08 |
| Compound | NO Inhibition (%) |
|---|---|
| 1 | 35.42 ± 1.24 |
| 2 | 28.16 ± 2.56 |
| 3 | 21.87 ± 1.64 |
| 4 | 25.77 ± 1.09 |
| 5 | 17.80 ± 3.25 |
| 6 | 9.73 ± 1.54 |
| 7 | 23.64 ± 2.41 |
| 8 | 14.25 ± 1.41 |
| 9 | 38.21 ± 1.48 |
| 10 | 14.66 ± 3.22 |
| 11 | 11.79 ± 0.07 |
| 12 | 12.67 ± 3.44 |
| Dexamethasone sodium acetate | 49.01 ± 1.96 |
| Compound | IC50 (μM) | ||
|---|---|---|---|
| A549 | Hela | HepG2 | |
| 1 | 33 ± 1.63 | 35 ± 1.34 | 25 ± 1.34 |
| 2 | 49 ± 2.74 | 43 ± 2.12 | 36 ± 2.42 |
| 3 | 60 ± 2.34 | 73 ± 1.84 | 64 ± 3.57 |
| 4 | 24 ± 4.75 | 31 ± 2.92 | 28 ± 1.58 |
| 5 | 25 ± 1.76 | 25 ± 2.31 | 31 ± 2.37 |
| 6 | 37 ± 1.30 | 29 ± 2.50 | 29 ± 3.42 |
| 7 | 52 ± 2.36 | 67 ± 4.58 | 26 ± 1.65 |
| 8 | 44 ± 3.56 | 37 ± 2.56 | 33 ± 2.56 |
| 9 | 78 ± 2.46 | 64 ± 1.34 | 57 ± 2.58 |
| 10 | 25 ± 1.75 | 31 ± 1.34 | 41 ± 3.76 |
| 11 | 54 ± 3.57 | 47 ± 2.33 | 65 ± 1.46 |
| 12 | >100 | >100 | >100 |
| cisplatin | 14 ± 1.24 | 12 ± 2.75 | 16 ± 1.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhong, L.; Fang, H.; Liu, Z.; Wang, P.; Li, L.; Chen, L.; Ding, G. Bioactive Metabolites from the Dusty Seeds of Gastrodia elata Bl., Based on Metabolomics and UPLC-Q-TOF-MS Combined with Molecular Network Strategy. Plants 2025, 14, 916. https://doi.org/10.3390/plants14060916
Wang Y, Zhong L, Fang H, Liu Z, Wang P, Li L, Chen L, Ding G. Bioactive Metabolites from the Dusty Seeds of Gastrodia elata Bl., Based on Metabolomics and UPLC-Q-TOF-MS Combined with Molecular Network Strategy. Plants. 2025; 14(6):916. https://doi.org/10.3390/plants14060916
Chicago/Turabian StyleWang, Yanduo, Liwen Zhong, Huiqi Fang, Zhao Liu, Peng Wang, Longfei Li, Lin Chen, and Gang Ding. 2025. "Bioactive Metabolites from the Dusty Seeds of Gastrodia elata Bl., Based on Metabolomics and UPLC-Q-TOF-MS Combined with Molecular Network Strategy" Plants 14, no. 6: 916. https://doi.org/10.3390/plants14060916
APA StyleWang, Y., Zhong, L., Fang, H., Liu, Z., Wang, P., Li, L., Chen, L., & Ding, G. (2025). Bioactive Metabolites from the Dusty Seeds of Gastrodia elata Bl., Based on Metabolomics and UPLC-Q-TOF-MS Combined with Molecular Network Strategy. Plants, 14(6), 916. https://doi.org/10.3390/plants14060916








