Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models
Abstract
1. Introduction
2. Results
2.1. Extraction and Phytochemical Analysis of Verbesina persicifolia
2.2. Toxicological Assessment in Artemia salina
2.3. Anticonvulsant Activity in Zebrafish Model
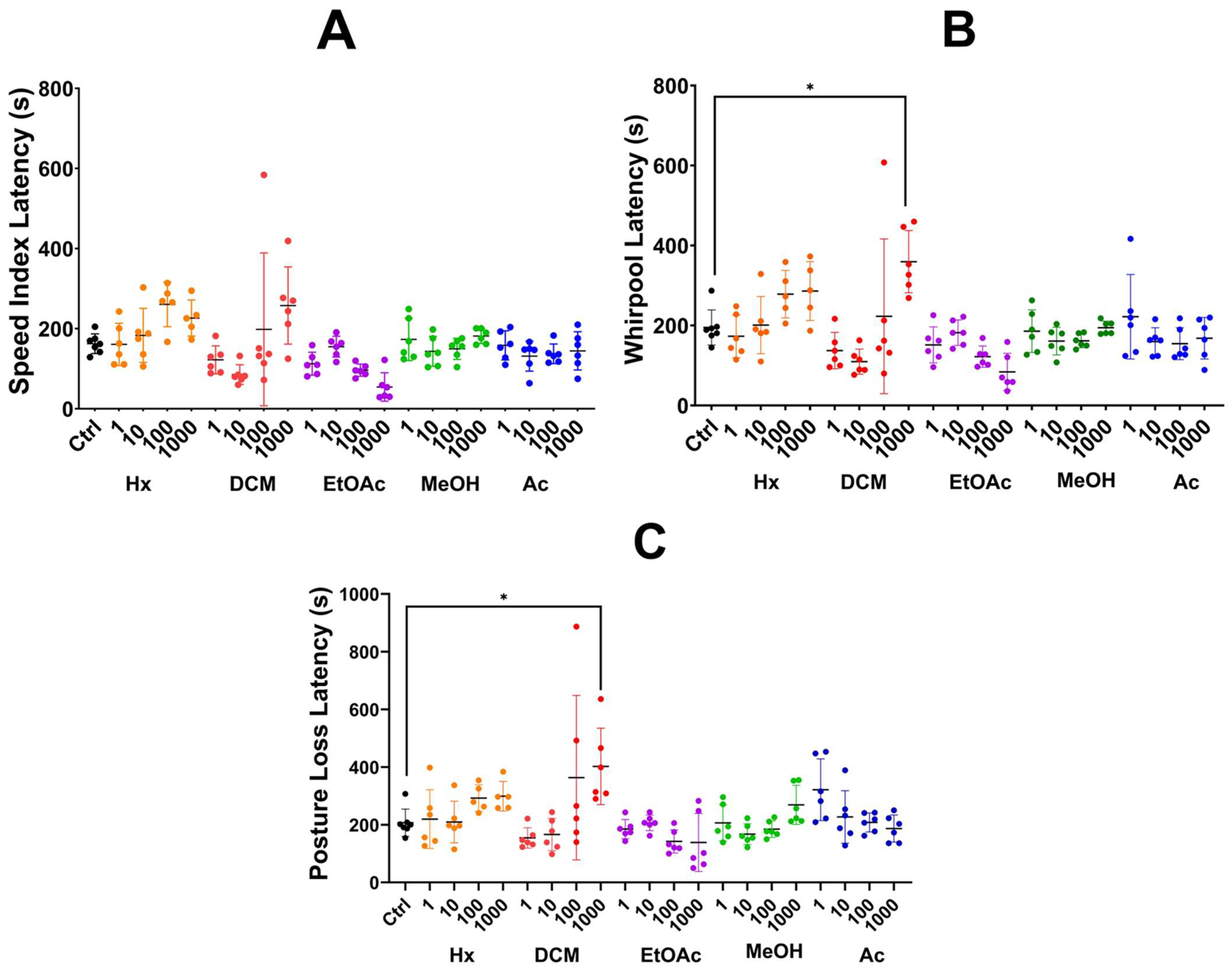
2.3.1. Partition Method
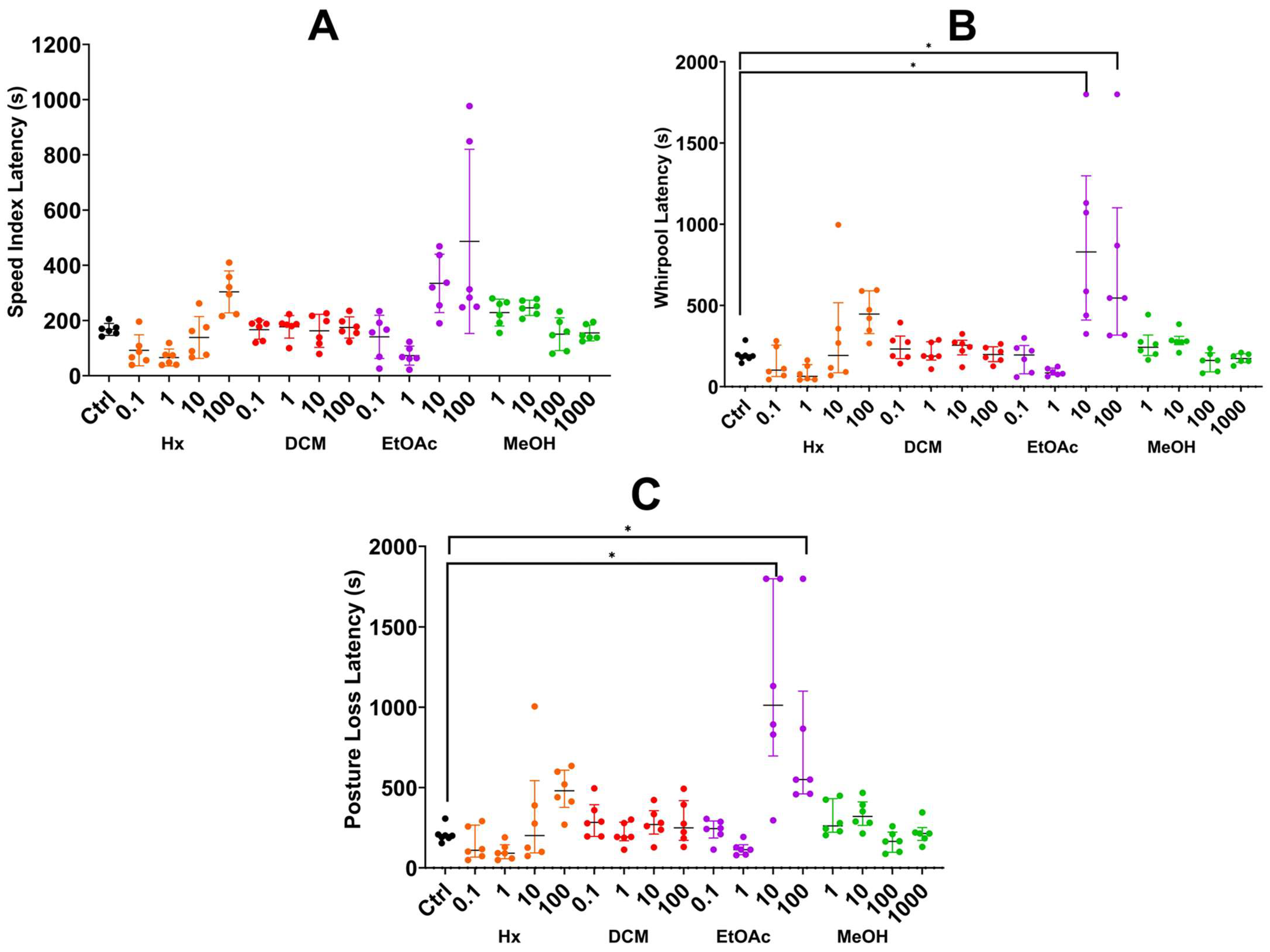
2.3.2. Sequential Method
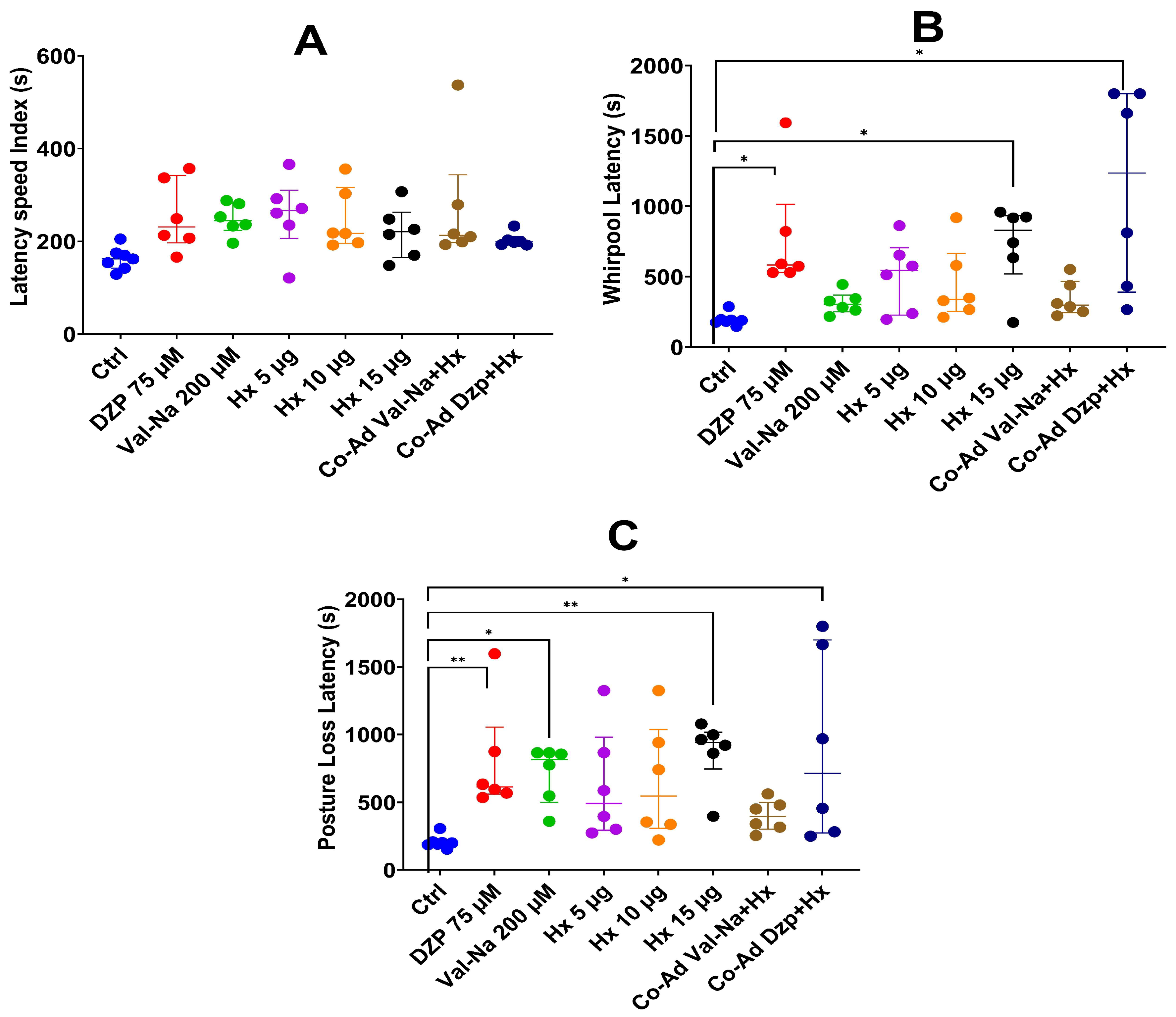
2.4. Co-Administration with Pharmacological Controls
2.5. Survival Rate
3. Discussion
3.1. Phytochemical Contributions to Anticonvulsant Activity
3.2. Efficacy of Extracts in Reducing Seizure Parameters
3.3. Synergistic Effects in Co-Administration with Standard Anticonvulsants
3.4. Toxicity and Safety Profile
3.5. Implications and Future Directions
4. Materials and Methods
4.1. Plant Material Collection
4.2. Preparation of Extracts
4.3. Phytochemical Screening
4.4. Toxicological Evaluation in Artemia salina
4.5. Anticonvulsant Activity in Zebrafish Model
4.6. Co-Administration with Pharmacological Controls
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Epilepsia; 2024. Available online: https://www.who.int/es/news-room/fact-sheets/detail/epilepsy (accessed on 20 December 2024).
- Fisher, R.S.; Cross, J.H.; D’Souza, C.; French, J.A.; Haut, S.R.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia 2017, 58, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef]
- SSA. Egresos Hospitalarios Datos Abiertos. 2025. Available online: http://www.dgis.salud.gob.mx/contenidos/basesdedatos/da_egresoshosp_gobmx.html (accessed on 7 March 2025).
- SSA. Defunciones (Mortalidad) Cubos Dinámicos. 2025. Available online: http://www.dgis.salud.gob.mx/contenidos/basesdedatos/bdc_defunciones_gobmx.html (accessed on 7 March 2025).
- Kanner, A.M.; Bicchi, M.M. Antiseizure Medications for Adults With Epilepsy: A Review. JAMA 2022, 327, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Zhang, J.; Wang, J.; Sha, L.; Xia, Y.; Ortyl, T.C.; Tian, X.; Chen, L. Advances in Epilepsy: Mechanisms, Clinical Trials, and Drug Therapies. J. Med. Chem. 2023, 66, 4434–4467. [Google Scholar] [CrossRef]
- Brodie, M.J.; Besag, F.; Ettinger, A.B.; Mula, M.; Gobbi, G.; Comai, S.; Aldenkamp, A.P.; Steinhoff, B.J. Epilepsy, Antiepileptic Drugs, and Aggression: An Evidence-Based Review. Pharmacol. Rev. 2016, 68, 563. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Ding, D.; Kwan, P. Editorial: Epidemiology of epilepsy and seizures. Front. Epidemiol. 2023, 3, 1273163. [Google Scholar] [CrossRef]
- Springer, C.; Nappe, T. Anticonvulsants Toxicity; 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537206/ (accessed on 5 March 2025).
- Tavakol, S.; Royer, J.; Lowe, A.J.; Bonilha, L.; Tracy, J.I.; Jackson, G.D.; Duncan, J.S.; Bernasconi, A.; Bernasconi, N.; Bernhardt, B.C. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: From focal lesions to macroscale networks. Epilepsia 2019, 60, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Cui, X.; Su, S.; Li, L.; Chen, Y.; Wang, N.; Sun, L.; Zhao, J.; Zhang, J.; et al. Oridonin exerts anticonvulsant profile and neuroprotective activity in epileptic mice by inhibiting NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2024, 134, 112247. [Google Scholar] [CrossRef]
- Bezerra, L.D.A.; Mangabeira, P.A.O.; de Oliveira, R.A.; Costa, L.; Da Cunha, M. Leaf blade structure of Verbesina macrophylla (Cass.) F. S. Blake (Asteraceae): Ontogeny, duct secretion mechanism and essential oil composition. Plant Biol. 2018, 20, 433–443. [Google Scholar] [CrossRef]
- He, X.; Chen, X.; Yang, Y.; Xie, Y.; Liu, Y. Medicinal plants for epileptic seizures: Phytoconstituents, pharmacology and mechanisms revisited. J. Ethnopharmacol. 2024, 320, 117386. [Google Scholar] [CrossRef]
- Zamora-Bello, I.; Rivadeneyra-Dominguez, E.; Rodriguez-Landa, J.F. Anticonvulsant Effect of Turmeric and Resveratrol in Lithium/Pilocarpine-Induced Status Epilepticus in Wistar Rats. Molecules 2022, 27, 3835. [Google Scholar] [CrossRef]
- Viegas, F.P.D.; Gontijo, V.S.; de Freitas Silva, M.; Ortiz, C.J.C.; Dos Reis Rosa Franco, G.; Ernesto, J.T.; Damasio, C.M.; Silva, I.M.F.; Campos, T.G.; Viegas, C. Curcumin, Resveratrol and Cannabidiol as Natural Key Prototypes in Drug Design for Neuroprotective Agents. Curr. Neuropharmacol. 2022, 20, 1297–1328. [Google Scholar] [CrossRef]
- Olguín Guerrero, M.C.; Saavedra Vélez, M.V.; López Rosas, C.A.; Camacho Pernas, M.Á.; Ramos Morales, F.R.; Alcántara López, M.G. Huichín (Verbesina persicifolia DC), planta medicinal con potencial farmacológico. Cienc. Lat. Rev. Científica Multidiscip. 2024, 8, 13461–13477. [Google Scholar] [CrossRef]
- Wang, S.; Dong, G.; Sheng, C. Structural Simplification of Natural Products. Chem. Rev. 2019, 119, 4180–4220. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.Á.M.; Oliva, V.E.; Cruz, M.M.; García, G.M.; Olazcoaga, G.T.; León, A.W. Catálogo de Plantas Útiles de la Sierra Norte de Puebla, México. 1995. Available online: https://www.academia.edu/75837762/Cat%C3%A1logo_de_plantas_%C3%BAtiles_de_la_Sierra_Norte_de_Puebla_M%C3%A9xico?f_ri=50212 (accessed on 20 February 2025).
- Domínguez-Barradas, C.; Cruz-Morales, G.E.; González-Gándara, C. Plantas de uso medicinal de la Reserva Ecológica “Sierra de Otontepec”, municipio de Chontla, Veracruz, México. CienciaUAT 2015, 9, 41–52. [Google Scholar] [CrossRef]
- Samokhina, E.; Samokhin, A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int. J. Neurosci. 2018, 128, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Kajita, Y.; Fukuda, Y.; Kawamatsu, R.; Oyanagi, T.; Mushiake, H. Pentylenetetrazole kindling induces dynamic changes in GAD65 expression in hippocampal somatostatin interneurons. Pharmacol. Biochem. Behav. 2024, 239, 173755. [Google Scholar] [CrossRef]
- Murugan, R.; Ramya Ranjan Nayak, S.P.; Haridevamuthu, B.; Priya, D.; Chitra, V.; Almutairi, B.O.; Arokiyaraj, S.; Saravanan, M.; Kathiravan, M.K.; Arockiaraj, J. Neuroprotective potential of pyrazole benzenesulfonamide derivative T1 in targeted intervention against PTZ-induced epilepsy-like condition in in vivo zebrafish model. Int. Immunopharmacol. 2024, 131, 111859. [Google Scholar] [CrossRef]
- Ciubotaru, A.D.; Leferman, C.-E.; Ignat, B.-E.; Knieling, A.; Esanu, I.M.; Salaru, D.L.; Foia, L.G.; Minea, B.; Hritcu, L.D.; Dimitriu, C.D.; et al. Behavioral and Biochemical Insights into the Therapeutic Potential of Mitocurcumin in a Zebrafish–Pentylenetetrazole (PTZ) Epilepsy Model. Pharmaceuticals 2025, 18, 382. [Google Scholar] [CrossRef]
- Dalla Via, L.; Mejia, M.; Garcia-Argaez, A.N.; Braga, A.; Toninello, A.; Martinez-Vazquez, M. Anti-inflammatory and antiproliferative evaluation of 4beta-cinnamoyloxy,1beta,3alpha-dihydroxyeudesm-7,8-ene from Verbesina persicifolia and derivatives. Bioorg. Med. Chem. 2015, 23, 5816–5828. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Ellmauerer, E.; Jia, Y.; Bohlmann, F.; Dominguez, X.A.; Hirschmann, G.S. Further eudesmane derivatives from verbesina species. Planta Med. 1987, 53, 39–42. [Google Scholar] [CrossRef]
- Ramseyer, J.; Thuerig, B.; De Mieri, M.; Scharer, H.J.; Oberhansli, T.; Gupta, M.P.; Tamm, L.; Hamburger, M.; Potterat, O. Eudesmane Sesquiterpenes from Verbesina lanata with Inhibitory Activity against Grapevine Downy Mildew. J. Nat. Prod. 2017, 80, 3296–3304. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.A.; Da Silva, H.C.; De Almeida, W.B. Structural Determination of Antioxidant and Anticancer Flavonoid Rutin in Solution through DFT Calculations of (1)H NMR Chemical Shifts. ChemistryOpen 2018, 7, 902–913. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; de Vivar, A.R.; Ortega, A.; de Lourdes Quintero, M.; Garcia, C.; Fronczek, F.R. Eudesmane triols from Verbesina virgata. Phytochemistry 1983, 22, 979–982. [Google Scholar] [CrossRef]
- Bohlmann, F.; Lonitz, M. Natürlich vorkommende Terpen-Derivate, 109: Neue Eudesman-Derivate und andere Sesquiterpene aus Verbesina-Arten. Chem. Berichte 2006, 111, 254–263. [Google Scholar] [CrossRef]
- Wagner, H.; Iyengar, M.A.; Seligmann, O.; Herz, W. Rhamnocitrin-3-glucuronid in Verbesina myricephala. Phytochemistry 1974, 13, 493–494. [Google Scholar] [CrossRef]
- Copmans, D.; Orellana-Paucar, A.M.; Steurs, G.; Zhang, Y.; Ny, A.; Foubert, K.; Exarchou, V.; Siekierska, A.; Kim, Y.; De Borggraeve, W.; et al. Methylated flavonoids as anti-seizure agents: Naringenin 4′,7-dimethyl ether attenuates epileptic seizures in zebrafish and mouse models. Neurochem. Int. 2018, 112, 124–133. [Google Scholar] [CrossRef]
- Jager, A.K.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef]
- Johnston, G.A. Flavonoid nutraceuticals and ionotropic receptors for the inhibitory neurotransmitter GABA. Neurochem. Int. 2015, 89, 120–125. [Google Scholar] [CrossRef]
- Sucher, N.J.; Carles, M.C. A pharmacological basis of herbal medicines for epilepsy. Epilepsy Behav. 2015, 52 Pt B, 308–318. [Google Scholar] [CrossRef]
- Nieoczym, D.; Socała, K.; Gawel, K.; Esguerra, C.V.; Wyska, E.; Wlaź, P. Anticonvulsant Activity of Pterostilbene in Zebrafish and Mouse Acute Seizure Tests. Neurochem. Res. 2019, 44, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.F.; de Andrade, J.P.; Bevilaqua, L.R.; de Souza, M.M.; Izquierdo, I.; Henriques, A.T.; Zuanazzi, J.A. Anxiolytic-, antidepressant- and anticonvulsant-like effects of the alkaloid montanine isolated from Hippeastrum vittatum. Pharmacol. Biochem. Behav. 2006, 85, 148–154. [Google Scholar] [CrossRef]
- Fahmy, N.M.; Al-Sayed, E.; El-Shazly, M.; Nasser Singab, A. Alkaloids of genus Erythrina: An updated review. Nat. Prod. Res. 2020, 34, 1891–1912. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Punia, J.K.; Bladen, C.; Zamponi, G.W.; Goel, R.K. Anticonvulsant mechanisms of piperine, a piperidine alkaloid. Channels 2015, 9, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Glennie, C.W.; Jain, S.C. Flavonol 3, 7-diglycosides of Verbesina encelioides. Phytochemistry 1980, 19, 157–158. [Google Scholar] [CrossRef]
- Garcia-Bores, A.M.; Alvarez-Santos, N.; Lopez-Villafranco, M.E.; Jacquez-Rios, M.P.; Aguilar-Rodriguez, S.; Grego-Valencia, D.; Espinosa-Gonzalez, A.M.; Estrella-Parra, E.A.; Hernandez-Delgado, C.T.; Serrano-Parrales, R.; et al. Verbesina crocata: A pharmacognostic study for the treatment of wound healing. Saudi J. Biol. Sci. 2020, 27, 3113–3124. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for Structural Modification of Small Molecules to Improve Blood-Brain Barrier Penetration: A Recent Perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Calcaterra, N.E.; Barrow, J.C. Classics in Chemical Neuroscience: Diazepam (Valium). ACS Chem. Neurosci. 2014, 5, 253–260. [Google Scholar] [CrossRef]
- Akinpelu, L.A.; Akanmu, M.A.; Obuotor, E.M. Mechanism of Anticonvulsant Effects of Ethanol Leaf Extract and Fractions of Milicia excelsa (Moraceae) in Mice. J. Pharm. Res. Int. 2018, 23, 1–11. [Google Scholar] [CrossRef]
- Sulaimon, L.A.; Anise, E.O.; Obuotor, E.M.; Samuel, T.A.; Moshood, A.I.; Olajide, M.; Fatoke, T. In vitro antidiabetic potentials, antioxidant activities and phytochemical profile of african black pepper (Piper guineense). Clin. Phytosci. 2020, 6. [Google Scholar] [CrossRef]
- Cseke, L.J.; Kirakosyan, A.; Kaufman, P.B.; Warber, S.; Duke, J.A.; Brielmann, H.L. Natural Products from Plants; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Ntungwe, N.E.; Dominguez-Martin, E.M.; Roberto, A.; Tavares, J.; Isca, V.M.S.; Pereira, P.; Cebola, M.J.; Rijo, P. Artemia species: An Important Tool to Screen General Toxicity Samples. Curr. Pharm. Des. 2020, 26, 2892–2908. [Google Scholar] [CrossRef]
- Hamidi, M.R.; Jovanova, B.; Panovska, T.K. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced. Pharm. Bull. 2014, 60, 9–18. [Google Scholar] [CrossRef]
- Almeida, E.R.; Lima-Rezende, C.A.; Schneider, S.E.; Garbinato, C.; Pedroso, J.; Decui, L.; Aguiar, G.P.S.; Muller, L.G.; Oliveira, J.V.; Siebel, A.M. Micronized Resveratrol Shows Anticonvulsant Properties in Pentylenetetrazole-Induced Seizure Model in Adult Zebrafish. Neurochem. Res. 2021, 46, 241–251. [Google Scholar] [CrossRef]
- Mussulini, B.H.; Leite, C.E.; Zenki, K.C.; Moro, L.; Baggio, S.; Rico, E.P.; Rosemberg, D.B.; Dias, R.D.; Souza, T.M.; Calcagnotto, M.E.; et al. Seizures induced by pentylenetetrazole in the adult zebrafish: A detailed behavioral characterization. PLoS ONE 2013, 8, e54515. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, D.; Kim, Y.H.; Lee, H.; Lee, C.J. Improvement of pentylenetetrazol-induced learning deficits by valproic acid in the adult zebrafish. Eur. J. Pharmacol. 2010, 643, 225–231. [Google Scholar] [CrossRef]
- UNAM. Atlas de las Plantas de la Medicina Tradicional Mexicana. Huichín. 2009. Available online: http://www.medicinatradicionalmexicana.unam.mx/apmtm/termino.php?l=3&t=huichin (accessed on 20 February 2025).
- Fonseca-Chávez, R.E.; Rivera-Levario, L.A.; Vázquez-García, L. Guía Ilustrada de Plantas Medicinales en el Valle de México; Instituto Nacional de los Pueblos Indígenas, 2020. Available online: https://www.gob.mx/cms/uploads/attachment/file/568378/guia-ilustrada-de-plantas-medicinales-valle-de-mexico-inpi.pdf (accessed on 20 February 2025).

| Metabolite Family | 2019 Extract | 2020 Extract | 2021 Extract |
|---|---|---|---|
| Alkaloids | + | + | + |
| Saponins | + | − | + |
| Tannins | − | + | + |
| Quinones | + | − | − |
| Triterpenoids | − | + | + |
| Flavonoids | + | + | + |
| Steroids | + | + | + |
| Metabolite Family | Extraction Method | Literature Source |
|---|---|---|
| Eudesmane sesquiterpenes | Hexane, methanol | [26] |
| Flavonoids | Ethyl acetate, methanol | [18] |
| Alkaloids | Methanol | [18] |
| Steroids | Hexane | [18] |
| Saponins | Methanol | [18] |
| Tannins | Methanol, aqueous extracts | [18] |
| Essential oils | Hexane | [27] |
| Extract Type | Fraction | LC50 (mg/mL) | Toxicity Classification 1 |
|---|---|---|---|
| Methanolic extract partition | Hexane | 0.499 | Low toxicity |
| Dichloromethane | >1 | Non-toxic | |
| Ethyl acetate | 0.633 | Low toxicity | |
| Methanol | >1 | Non-toxic | |
| Aqueous | >1 | Non-toxic | |
| Sequential extract | Hexane | 0.073 | High toxicity |
| Dichloromethane | 0.105 | Moderate toxicity | |
| Ethyl acetate | 0.421 | Moderate toxicity | |
| Methanol | 0.421 | Moderate toxicity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rosas, C.A.; González-Periañez, S.; Pawar, T.J.; Zurutuza-Lorméndez, J.I.; Ramos-Morales, F.R.; Olivares-Romero, J.L.; Saavedra Vélez, M.V.; Hernández-Rosas, F. Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models. Plants 2025, 14, 1078. https://doi.org/10.3390/plants14071078
López-Rosas CA, González-Periañez S, Pawar TJ, Zurutuza-Lorméndez JI, Ramos-Morales FR, Olivares-Romero JL, Saavedra Vélez MV, Hernández-Rosas F. Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models. Plants. 2025; 14(7):1078. https://doi.org/10.3390/plants14071078
Chicago/Turabian StyleLópez-Rosas, Carlos Alberto, Santiago González-Periañez, Tushar Janardan Pawar, Jorge Iván Zurutuza-Lorméndez, Fernando Rafael Ramos-Morales, José Luís Olivares-Romero, Margarita Virginia Saavedra Vélez, and Fabiola Hernández-Rosas. 2025. "Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models" Plants 14, no. 7: 1078. https://doi.org/10.3390/plants14071078
APA StyleLópez-Rosas, C. A., González-Periañez, S., Pawar, T. J., Zurutuza-Lorméndez, J. I., Ramos-Morales, F. R., Olivares-Romero, J. L., Saavedra Vélez, M. V., & Hernández-Rosas, F. (2025). Anticonvulsant Potential and Toxicological Profile of Verbesina persicifolia Leaf Extracts: Evaluation in Zebrafish Seizure and Artemia salina Toxicity Models. Plants, 14(7), 1078. https://doi.org/10.3390/plants14071078









