Plastic Responses of Iris pumila Functional and Mechanistic Leaf Traits to Experimental Warming
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Responses of SLA, LDMC, SLWC, LT, SD, and I1 to Temperature
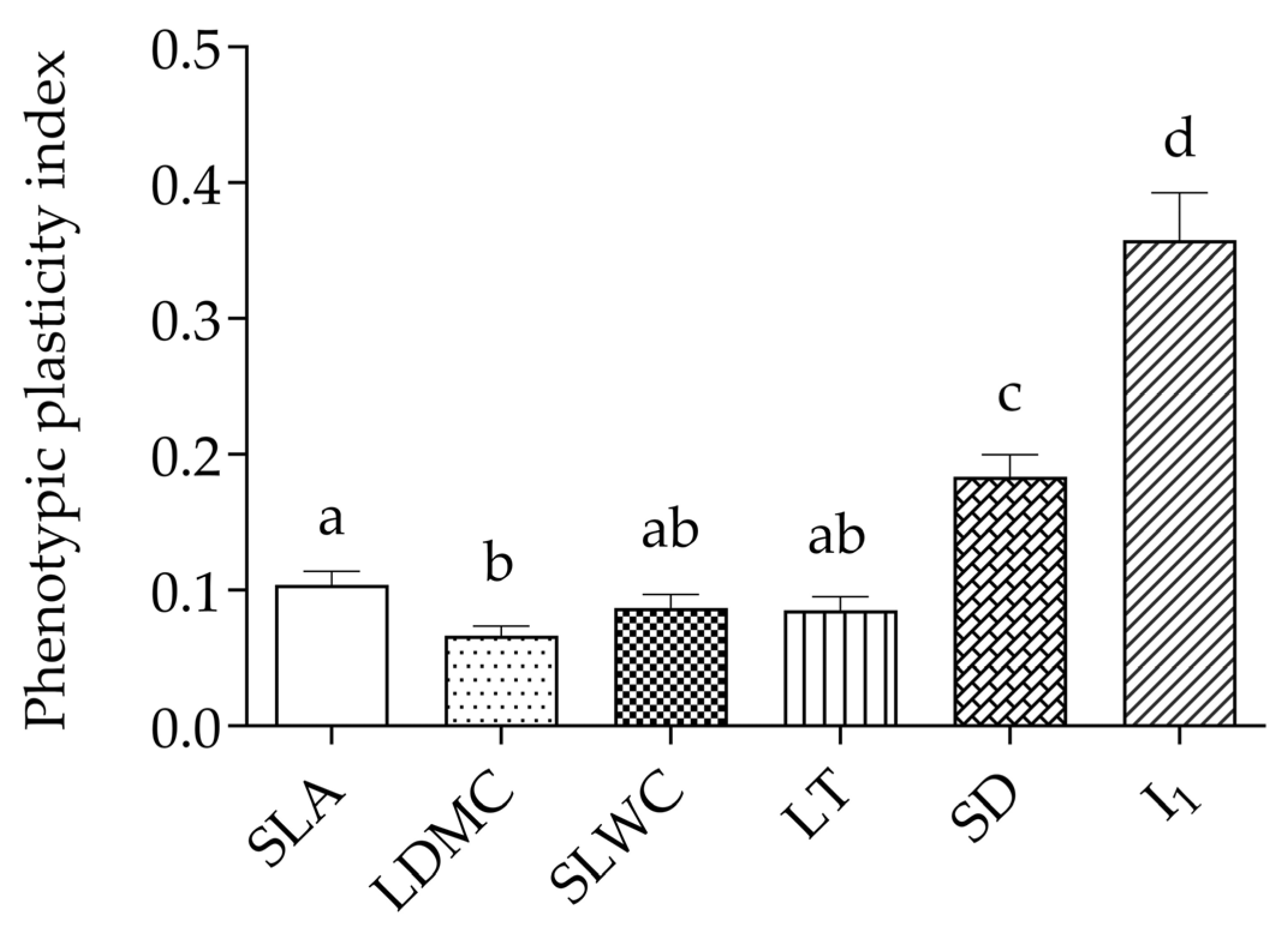
2.2. Phenotypic Plasticity of SLA, LDMC, SLWC, LT, SD, and I1 to Temperature
2.3. Phenotypic Correlations Between Functional and Mechanistic Leaf Traits in Response to Temperature
3. Discussion
3.1. Acclimation Responses to Increased Temperature
3.2. Thermal Plasticity of Functional and Mechanistic Leaf Traits
3.3. Temperature-Driven Correlation Patterns of Leaf Traits
4. Materials and Methods
4.1. The Study Species
4.2. Experimental Setup, Leaf Sampling, and Leaf Trait Measurement
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Summary for Policymakers. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 1–34. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Little, C.J.; Jägerbrand, A.K.; Molau, U. Vascular plant abundance and diversity in an alpine heath under observed and simulated global change. Sci. Rep. 2015, 5, 10197. [Google Scholar] [CrossRef] [PubMed]
- Elmendorf, S.C.; Henry, G.H.; Hollister, R.D.; Fosaae, A.M.; Gould, W.A.; Hermanutz, L.; Hofgaard, A.; Jónsdóttir, I.S.; Jorgenson, J.C.; Lévesquel, E.; et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Catullo, R.A.; Liewelyn, J.; Phillips, B.L.; Moritz, C.C. The potential for rapid evolution under anthropogenic climate change. Curr. Biol. 2019, 29, R996–R1007. [Google Scholar] [CrossRef] [PubMed]
- Greenspoon, P.B.; Spencer, H.G. Avoiding extinction under nonlinear environmental change: Models of evolutionary rescue with plasticity. Biol. Lett. 2021, 17, 20210459. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef]
- Chevin, L.M.; Collins, S.; Lefevre, F. Phenotypic plasticity and evolutionary demographic responses to climate change: Taking theory out to the field. Funct. Ecol. 2013, 27, 967–979. [Google Scholar] [CrossRef]
- Merilä, J.; Hendry, A.P. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol. Appl. 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Scheiner, S.M.; Barfield, M.; Holt, R.D. The genetics of phenotypic plasticity. XVII. Response to climate change. Evol. Appl. 2020, 13, 388–399. [Google Scholar] [CrossRef]
- Gienapp, P.; Teplitsky, C.; Alho, J.S.; Mills, J.A.; Merilä, J. Climate change and evolution: Disentangling environmental and genetic responses. Mol. Ecol. 2008, 17, 167–178. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479–485. [Google Scholar] [CrossRef]
- Nogués-Bravo, D.; Rodríguez-Sánchez, F.; Orsini, L.; de Boer, E.; Jansson, R.; Morlon, H.; Damien, A.; Jackson, S.T. Cracking the code of biodiversity responses to past climate change. Trends Ecol. Evol. 2018, 33, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Ghalambor, C.K.; McKay, J.K.; Carroll, S.P.; Reznick, D.N. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007, 21, 394–407. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef]
- Wang, S.P.; Althoff, D.M. Phenotypic plasticity facilitates initial colonization of a novel environment. Evolution 2019, 73, 303–316. [Google Scholar] [CrossRef]
- Snell-Rood, E.C.; Kobiela, M.E.; Sikkink, K.L.; Shephard, A.M. Mechanisms of plastic rescue in novel environments. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 331–354. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Vendramini, F.; Diaz, S.; Gurvich, D.E.; Wilson, P.J.; Thomson, K.; Hodgson, J.G. Leaf traits as indicator of resource-use strategy in floras with succulent species. New Phytol. 2002, 154, 147–157. [Google Scholar] [CrossRef]
- Madani, N.; Kimball, J.S.; Ballantyne, A.P.; Affleck, D.L.; Van Bodegom, P.M.; Reich, P.B.; Kattge, J.; Sala, A.; Nazeri, M.; Jones, M.O.; et al. Future global productivity will be affected by plant trait response to climate. Sci. Rep. 2018, 8, 2870. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, L.; Qiu, X.; Liu, B.; Chang, X.; Liu, L.; Zhang, X.; Cao, W.; Zhu, Y. Impacts of 1.5 and 2.0 °C global warming on rice production across China. Agric. For. Meteorol. 2020, 284, 107900. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, P.; Shen, W.; Rao, X.; Hu, Y. Physiological homeostasis and morphological plasticity of two tree species subjected to precipitation seasonal distribution changes. Perspect. Plant Ecol. Evol. Syst. 2017, 25, 1–19. [Google Scholar] [CrossRef]
- Kassout, J.; Terral, J.F.; Hodgson, J.G.; Ater, M. Trait-based plant ecology a flawed tool in climate studies? The leaf traits of wild olive that pattern with climate are not those routinely measured. PLoS ONE 2019, 14, e0219908. [Google Scholar] [CrossRef] [PubMed]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Atkin, O.K.; Scheurwater, I.; Pons, T.L. High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob. Change Biol. 2006, 12, 500–515. [Google Scholar] [CrossRef]
- Meziane, D.; Shipley, B. Direct and indirect relationships between specific leaf area, leaf nitrogen and leaf gas exchange. Effects of irradiance and nutrient supply. Ann. Bot. 2001, 88, 915–927. [Google Scholar] [CrossRef]
- Vuleta, A.; Manitašević-Jovanović, S.; Tucić, B. Pattern of plasticity to irradiance levels and genotypic correlations between structural and physiological leaf traits in Iris pumila. Arch. Biol. Sci. 2011, 63, 655–660. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Larigauderie, A.; Hilbert, D.W.; Oechel, W.C. Effect of CO2 enrichment and nitrogen availability on resource acquisition and resource allocation in a grass, Bromus mollis. Oecologia 1988, 77, 544–549. [Google Scholar] [CrossRef]
- Bussotti, F. Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: A critical overview. Glob. Change Biol. 2008, 14, 2727–2739. [Google Scholar] [CrossRef]
- Poorter, H.; De Jong, R.O.B. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytol. 1999, 143, 163–176. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billè, G.; Navas, M.L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.B.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Manitašević-Jovanović, S.; Vuleta, A.; Tucić, B. Does physiological integration among intraclonal ramets of Iris pumila enhance stress tolerance in heterogeneous environments? Arch. Biol. Sci. 2014, 66, 713–720. [Google Scholar] [CrossRef]
- Rosbakh, S.; Römermann, C.; Poschlod, P. Specific leaf area correlates with temperature: New evidence of trait variation at the population, species and community levels. Alp. Bot. 2015, 125, 79–86. [Google Scholar] [CrossRef]
- Shipley, B.; Vu, T.T. Dry matter content as a measure of dry matter concentration in plants and their parts. New Phytol. 2002, 153, 359–364. [Google Scholar] [CrossRef]
- Duru, M.; Al Haj Khaled, R.; Ducourtieux, C.; Theau, J.P.; De Quadros, F.L.F.; Cruz, P. Do plant functional types based on leaf dry matter content allow characterizing native grass species and grasslands for herbage growth pattern? In Herbaceous Plant Ecology; Van Der Valk, A.G., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 57–69. ISBN 978-90-481-2797-9. [Google Scholar]
- Liancourt, P.; Boldgiv, B.; Song, D.S.; Spence, L.A.; Helliker, B.R.; Petraitis, P.S.; Casper, B.B. Leaf-trait plasticity and species vulnerability to climate change in a Mongolian Steppe. Glob. Change Biol. 2015, 21, 3489–3498. [Google Scholar] [CrossRef]
- Niklas, K.J.; Shi, P.; Gielis, J.; Schrader, J.; Niinemets, Ü. Leaf functional traits: Ecological and evolutionary implications. Front. Plant Sci. 2023, 14, 1169558. [Google Scholar] [CrossRef]
- Garnier, E.; Laurent, G. Leaf anatomy, specific mass and water content in congeneric annual and perennial grass species. New Phytol. 1994, 128, 725–736. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, H.; Wang, H.; Peñuelas, J.; Sardans, J.; Niinemets, Ü.; Niklas, K.J.; Li, Y.; Xie, J.; Wright, I.J. Leaf water content contributes to global leaf trait relationships. Nat. Commun. 2022, 13, 5525. [Google Scholar] [CrossRef]
- Agusti, S.; Enriquez, S.; Frost-Christensen, H.; Sand-Jensen, K.; Duarte, C.M. Light harvesting among photosynthetic organisms. Funct. Ecol. 1994, 8, 273–279. [Google Scholar] [CrossRef]
- Syvertsen, J.P.; Lloyd, J.; McConchie, C.; Kriedemann, P.E.; Farquhar, G.D. On the relationship between leaf anatomy and CO2 diffusion through the mesophyll of hypostomatous leaves. Plant Cell Environ. 1995, 18, 149–157. [Google Scholar] [CrossRef]
- Garnier, E.; Salager, J.L.; Laurent, G.; Sonié, L. Relationship between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 1999, 143, 119–149. [Google Scholar] [CrossRef]
- Enríquez, S.; Sand-Jensen, K. Variation in light absorption properties of Mentha aquatica L. as a function of leaf form: Implications for plant growth. Int. J. Plant Sci. 2003, 164, 125–136. [Google Scholar] [CrossRef]
- Poorter, H.; Remkes, C.; Lambers, H. Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol. 1990, 94, 621–627. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Enríquez, S.; Duarte, C.M.; Sand-Jensen, K. Scaling maximum growth rates across photosynthetic organisms. Funct. Ecol. 1996, 10, 167–175. [Google Scholar] [CrossRef]
- White, J.W.; Montes-R, C. Variation in parameters related to leaf thickness in common bean (Phaseolus vulgaris L.). Field Crops Res. 2005, 91, 7–21. [Google Scholar] [CrossRef]
- Díaz, S.; Hodgson, J.G.; Thompson, K.; Cabido, M.; Cornelissen, J.H.; Jalili, A.; Montserrat-Martí, G.; Grime, J.P.; Zarrinkamar, F.; Asri, Y.; et al. The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 2004, 15, 295–304. [Google Scholar] [CrossRef]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef]
- Beerling, D.J.; Woodward, F.I. Changes in land plant function over the Phanerozoic: Reconstructions based on the fossil record. Bot. J. Linn. 1997, 124, 137–153. [Google Scholar] [CrossRef]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef]
- Vuleta, A.; Manitašević-Jovanović, S.; Tucić, B. Light intensity influences variations in the structural and physiological traits in the leaves of Iris pumila L. Arch. Biol. Sci. 2011, 63, 1099–1110. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.; Kang, H.; Hui, N.; Yin, S.; Chen, Z.; Du, B.; Liu, C. Spatial variations in leaf trichomes and their coordination with stomata in Quercus variabilis across Eastern Asia. J. Plant Ecol. 2024, 17, rtae023. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Drake, P.L.; Froend, R.H.; Franks, P.J. Smaller, faster stomata: Scaling of stomatal size, rate of response, and stomatal conductance. J. Exp. Bot. 2013, 64, 495–505. [Google Scholar] [CrossRef]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Change Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Llusia, J.; Siscart, D.; Piñol, J. Comparative field study of spring and summer leaf gas exchange and photobiology of the Mediterranean trees Quercus ilex and Phillyrea latifolia. J. Exp. Bot. 1998, 49, 229–238. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Zhang, J.; Yang, H.; Xu, L.; Wang, Q.; Sack, L.; Wu, X.; Hou, J.; He, N. Variation in leaf chlorophyll concentration from tropical to cold-temperate forests: Association with gross primary productivity. Ecol. Indic. 2018, 85, 383–389. [Google Scholar] [CrossRef]
- Cutolo, E.A.; Guardini, Z.; Dall’Osto, L.; Bassi, R. A paler shade of green: Engineering cellular chlorophyll content to enhance photosynthesis in crowded environments. New Phytol. 2023, 239, 1567–1583. [Google Scholar] [CrossRef]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Matesanz, S.; Gianoli, E.; Valladares, F. Global change and the evolution of phenotypic plasticity in plants. Ann. N. Y. Acad. Sci. 2010, 1206, 35–55. [Google Scholar] [CrossRef]
- Manitašević Jovanović, S.; Hočevar, K.; Vuleta, A.; Tucić, B. Predicting the responses of functional leaf traits to global warming: An in situ temperature manipulation design using Iris pumila L. Plants 2023, 12, 3114. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zuo, X.; Griffin-Nolan, R.J.; Xu, C.; Ma, W.; Song, L.; Helsen, K.; Lin, Y.; Cai, J.; Yu, Q.; et al. Long term experimental drought alters community plant trait variation, not trait means, across three semiarid grasslands. Plant Soil 2019, 442, 343–353. [Google Scholar] [CrossRef]
- Manitašević, S.; Dunđerski, J.; Matić, G.; Tucić, B. Seasonal variation in heat shock proteins Hsp70 and Hsp90 expression in an exposed and a shaded habitat of Iris pumila. Plant Cell Environ. 2007, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, N.; Zhang, H.; Zhao, M.; Ren, T.; Liu, C.; Westerband, A.; He, N. Divergent long- and short-term responses to environmental gradients in specific leaf area of grassland species. Ecol. Indic. 2021, 130, 108058. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Summerhayes, B.; Westoby, M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999, 69, 569–588. [Google Scholar] [CrossRef]
- McDonald, P.G.; Fonseca, C.R.; Overton, J.M.C.; Westoby, M. Leaf-size divergence along rainfall and soil-nutrient gradients: Is the method of size reduction common among clades? Funct. Ecol. 2003, 17, 50–57. [Google Scholar] [CrossRef]
- Ackerly, D.; Knight, C.; Weiss, S.; Barton, K.; Starmer, K. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: Contrasting patterns in species level and community level analyses. Oecologia 2002, 130, 449–457. [Google Scholar] [CrossRef]
- Correia, P.M.; da Silva, A.B.; Vaz, M.; Carmo-Silva, E.; Marques da Silva, J. Efficient regulation of CO2 assimilation enables greater resilience to high temperature and drought in maize. Front. Plant Sci. 2021, 12, 675546. [Google Scholar] [CrossRef]
- Allahverdiyev, T.; Huseynova, I. Influence of water deficit on photosynthetic activity, dry matter partitioning and grain yield of different durum and bread wheat genotypes. Cereal Res. Commun. 2017, 45, 432–441. [Google Scholar] [CrossRef]
- Atkin, O.K.; Atkinson, L.J.; Fisher, R.A.; Campbell, C.D.; Zaragoza-Castells, J.; Pitchford, J.W.; Woodward, F.I.; Hurry, V. Using temperature-dependent changes in leaf scaling relationships to quantitatively account for thermal acclimation of respiration in a coupled global climate–vegetation model. Glob. Change Biol. 2008, 14, 2709–2726. [Google Scholar] [CrossRef]
- Kühn, N.; Tovar, C.; Carretero, J.; Vandvik, V.; Enquist, B.J.; Willis, K.J. Globally important plant functional craits for coping with climate change. Front. Biogeogr. 2021, 13, e53774. [Google Scholar] [CrossRef]
- Henn, J.J.; Buzzard, V.; Enquist, B.J.; Halbritter, A.H.; Klanderud, K.; Maitner, B.S.; Michaletz, S.T.; Pötsch, C.; Seltzer, L.; Telford, R.J.; et al. Intraspecific trait variation and phenotypic plasticity mediate alpine plant species response to climate change. Front. Plant Sci. 2018, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Michaletz, S.T.; Weiser, M.D.; Zhou, J.; Kaspari, M.; Helliker, B.R.; Enquist, B.J. Plant thermoregulation: Energetics, trait–environment interactions, and carbon economics. Trends Ecol. Evol. 2015, 30, 714–724. [Google Scholar] [CrossRef]
- Gong, H.; Gao, J. Soil and climatic drivers of plant SLA (Specific Leaf Area). Glob. Ecol. Conserv. 2019, 20, e00696. [Google Scholar] [CrossRef]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Pontes, L.D.S.; Soussana, J.F.; Louault, F.; Andueza, D.; Carrère, P. Leaf traits affect the above-ground productivity and quality of pasture grasses. Funct. Ecol. 2007, 21, 844–853. [Google Scholar] [CrossRef]
- Gorné, L.D.; Díaz, S.; Minden, V.; Onoda, Y.; Kramer, K.; Muir, C.; Michaletz, S.T.; Lavorel, S.; Sharpe, J.; Jansen, S.; et al. The acquisitive–conservative axis of leaf trait variation emerges even in homogeneous environments. Ann. Bot. 2022, 129, 709–722. [Google Scholar] [CrossRef]
- Hulshof, C.M.; Swenson, N.G. Variation in leaf functional trait values within and across individuals and species: An example from a Costa Rican dry forest. Funct. Ecol. 2010, 24, 217–223. [Google Scholar] [CrossRef]
- Wang, R.; He, N.; Li, S.; Xu, L.; Li, M. Variation and adaptation of leaf water content among species, communities, and biomes. Environ. Res. Lett. 2021, 16, 124038. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Pence, H.E.; Hasegawa, P.M.; Mickelbart, M.V. Regulation of transpiration to improve crop water use. Crit. Rev. Plant Sci. 2009, 28, 410–431. [Google Scholar] [CrossRef]
- Sikorska, D.; Papierowska, E.; Szatyłowicz, J.; Sikorski, P.; Suprun, K.; Hopkins, R.J. Variation in leaf surface hydrophobicity of wetland plants: The role of plant traits in water retention. Wetlands 2017, 37, 997–1002. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.; Ingwers, M.; McGuire, M.A.; Teskey, R.O. Stomatal Conductance Increases with Rising Temperature. Plant Signal. Behav. 2017, 12, e1356534. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Feild, T.S. Evolution of stomatal responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytol. 2009, 183, 839–847. [Google Scholar] [CrossRef]
- Leigh, A.; Sevanto, S.; Close, J.D.; Nicotra, A.B. The influence of leaf size and shape on leaf thermal dynamics: Does theory hold up under natural conditions? Plant Cell Environ. 2017, 40, 237–248. [Google Scholar] [CrossRef]
- Tardy, F.; Créach, A.; Havaux, M. Photosynthetic pigment concentration, organization and interconversions in a pale green syrian landrace of barley (Hordeum vulgare L., Tadmor) adapted to harsh climatic conditions. Plant Cell Environ. 1998, 21, 479–489. [Google Scholar] [CrossRef]
- Silla, F.; González-Gil, A.; González-Molina, M.E.; Mediavilla, S.; Escudero, A. Estimation of chlorophyll in Quercus leaves using a portable chlorophyll meter: Effects of species and leaf age. Ann. For. Sci. 2010, 67, 108. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Hu, X.; Gu, T.; Khan, I.; Zada, A.; Jia, T. Research progress in the interconversion, turnover and degradation of chlorophyll. Cells 2021, 10, 3134. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. In Advances in Genetics; Elsevier: Amsterdam, The Netherlands, 1965; Volume 13, pp. 115–155. ISBN 978-0-12-017613-7. [Google Scholar]
- Knight, C.A.; Ackerly, D.D. Evolution and plasticity of photosynthetic thermal tolerance, specific leaf area and leaf size: Congeneric species from desert and coastal environments. New Phytol. 2003, 160, 337–347. [Google Scholar] [CrossRef]
- Huang, W.; Gielis, J.; Shi, P. The adaptation, plasticity and extinction of forest plants to climate change: Mechanisms behind the morphological, physiological, phenological and ecological traits. Front. Ecol. Evol. 2024, 12, 1488465. [Google Scholar] [CrossRef]
- Kumarathunge, D.P.; Medlyn, B.E.; Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Battaglia, M.; Cano, F.J.; Carter, K.R.; Cavaleri, M.A.; Cernusak, L.A.; et al. Acclimation and adaptation components of the temperature dependence of plant photosynthesis at the global scale. New Phytol. 2019, 222, 768–784. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L.F.M. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [PubMed]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Volaire, F.; Gleason, S.M.; Delzon, S. What do you mean “functional” in ecology? Patterns versus processes. Ecol. Evol. 2020, 10, 11875–11885. [Google Scholar] [CrossRef]
- Reich, P.B.; Wright, I.J.; Cavender-Bares, J.; Craine, J.M.; Oleksyn, J.; Westoby, M.; Walters, M.B. The Evolution of plant functional variation: Traits, spectra, and strategies. Int. J. Plant Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Davidson, A. Adaptive phenotypic plasticity and plant water use. Funct. Plant Biol. 2010, 37, 117. [Google Scholar] [CrossRef]
- Burns, J.H.; Strauss, S.Y. Effects of competition on phylogenetic signal and phenotypic plasticity in plant functional traits. Ecology 2012, 93, S126–S137. [Google Scholar] [CrossRef]
- Schlichting, C.D. Phenotypic integration and environmental change. BioScience 1989, 39, 460–464. [Google Scholar] [CrossRef]
- Shaar-Moshe, L.; Hayouka, R.; Roessner, U.; Peleg, Z. Phenotypic and metabolic plasticity shapes life-history strategies under combinations of abiotic stresses. Plant Direct 2019, 3, e00113. [Google Scholar] [CrossRef]
- Sheehy, K.A.; Laskowski, K.L. Correlated behavioural plasticities: Insights from plasticity evolution, the integrated phenotype and behavioural syndromes. Anim. Behav. 2023, 200, 263–271. [Google Scholar] [CrossRef]
- Gong, H.; Yang, M.; Wang, C.; Tian, C. Leaf phenotypic variation and its response to environmental factors in natural populations of Eucommia ulmoides. BMC Plant Biol. 2023, 23, 562. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Pal, N.; Arora, A.; Prashant, B.D.; Venadan, S. Plant functional traits in crop breeding: Advancement and challenges. In Plant Functional Traits for Improving Productivity; Kumar, N., Singh, H., Eds.; Springer Nature: Singapore, 2024; pp. 169–202. ISBN 978-981-97-1509-1. [Google Scholar]
- Franks, S.J.; Sim, S.; Weis, A.E. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl. Acad. Sci. USA 2007, 104, 1278–1282. [Google Scholar] [CrossRef]
- Mitchell, P.J.; Veneklaas, E.J.; Lambers, H.; Burgess, S.S.O. Leaf water relations during summer water deficit: Differential responses in turgor maintenance and variation in leaf structure among different plant communities in south-western Australia. Plant Cell Environ. 2008, 31, 1791–1802. [Google Scholar] [CrossRef]
- Napier, J.D.; Heckman, R.W.; Juenger, T.E. Gene-by-environment interactions in plants: Molecular mechanisms, environmental drivers, and adaptive plasticity. Plant Cell 2023, 35, 109–124. [Google Scholar] [CrossRef]
- Yang, J.; Gao, Y.; Zhao, C.; Chen, H. Leaf phenotypic plasticity and integration balance plant adaptation to water table decline: A mesocosm experiment. Plant Soil 2024, 497, 611–627. [Google Scholar] [CrossRef]
- Wang, X.; Ji, M.; Zhang, Y.; Zhang, L.; Akram, M.A.; Dong, L.; Hu, W.; Xiong, J.; Sun, Y.; Li, H.; et al. Plant trait networks reveal adaptation strategies in the drylands of China. BMC Plant Biol. 2023, 23, 266. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Kazakou, E.; Violle, C.; Roumet, C.; Navas, M.; Vile, D.; Kattge, J.; Garnier, E. Are trait-based species rankings consistent across data sets and spatial scales? J. Veg. Sci. 2014, 25, 235–247. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Arnold, P.A.; Wang, S.; Catling, A.A.; Kruuk, L.E.B.; Nicotra, A.B. Patterns of phenotypic plasticity along a thermal gradient differ by trait type in an alpine plant. Funct. Ecol. 2022, 36, 2412–2428. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dawson, W.; Prati, D.; Haeuser, E.; Feng, Y.; Van Kleunen, M. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? Ann. Bot. 2016, 118, 1329–1336. [Google Scholar] [CrossRef]
- Pierce, S.; Maffi, D.; Faoro, F.; Cerabolini, B.E.L.; Spada, A. The leaf anatomical trade-offs associated with plant ecological strategy variation. Plant Ecol. 2022, 223, 1233–1246. [Google Scholar] [CrossRef]
- Randolph, L.F.; Mitra, J. Karyotypes of Iris pumila and related species. Am. J. Bot. 1959, 46, 93–102. [Google Scholar] [CrossRef]
- Randolph, L.F. The geographic distribution of European and eastern Mediterranean species of bearded Iris. In Iris Year Book; British Iris Society: Tunbridge Wells, UK, 1955; pp. 35–46. [Google Scholar]
- Dalmady, J. Iris species on Slovenski Kras area. Nat. Prot 1972, 3, 64–65. [Google Scholar]
- Randuška, D.; Križo, M. Chránené Rastliny [“Protected Plants”]; Príroda: Bratislava, Slovakia, 1986; pp. 1–430. (In Slovak) [Google Scholar]
- Teixeira da Silva, J.A. Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; Global Science Books: London, UK, 2006; Volume I–IV, p. 2506. [Google Scholar]
- Eliáš, P.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th Edition (October 2014). Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Parnikoza, I.Y.; Andreev, I.O.; Bublyk, O.M.; Spiridonova, K.V.; Gołębiewska, J.; Kubiak, M.; Kuczyńska, A.; Mystkowska, K.; Olędrzyńska, N.; Urasińska, B.; et al. The current state of steppe perennial plants populations: A case study on Iris pumila. Biologia 2017, 72, 24–35. [Google Scholar] [CrossRef]
- Tucić, B.; Milojković, S.; Vujčić, S.; Tarasjev, A. Clonal diversity and dispersion in Iris pumila. Acta Oecol. 1988, 9, 211–219. [Google Scholar]
- Kadović, R.; Spasov, P.; Bohajar, Y.; Belanovic-Simić, S.; Košanin, O. Analysis of aridity indicators in the Deliblato Sands. Glas. Sumar. Fak. 2014, 109, 97–112. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. Corrigendum to: New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2016, 64, 715–716. [Google Scholar] [CrossRef]
- Yulin, L.I.; Johnson, D.A.; Yongzhong, S.U.; Jianyuan, C.U.I.; Zhang, T. Specific leaf area and leaf dry matter content of plants growing in sand dunes. Bot. Bull. Acad. Sin. 2005, 46, 127–134. [Google Scholar] [CrossRef]
- Vile, D.; Garnier, É.; Shipley, B.; Laurent, G.; Navas, M.-L.; Roumet, C.; Lavorel, S.; Díaz, S.; Hodgson, J.G.; Lloret, F.; et al. Specific leaf area and dry matter content estimate thickness in laminar leaves. Ann. Bot. 2005, 96, 1129–1136. [Google Scholar] [CrossRef]
- Hilu, K.W.; Randall, J.L. Convenient method for studying grass leaf epidermis. TAXON 1984, 33, 413–415. [Google Scholar] [CrossRef]
- Wu, Q.G.; Cutler, D.F. Taxonomic, evolutionary and ecological implications of the leaf anatomy of rhizomatous Iris species. Bot. J. Linn. 1985, 90, 253–303. [Google Scholar] [CrossRef]
- Sánchez-Sastre, L.F.; Alte Da Veiga, N.M.S.; Ruiz-Potosme, N.M.; Carrión-Prieto, P.; Marcos-Robles, J.L.; Navas-Gracia, L.M.; Martín-Ramos, P. Assessment of RGB vegetation indices to estimate chlorophyll content in sugar beet leaves in the final cultivation stage. AgriEngineering 2020, 2, 128–149. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 25 December 2024).
- Wickham, H.; Vaughan, D.; Girlich, M. Tidyr: Tidy Messy Data. R package Version 1.3.0. 2023. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 25 December 2024).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. R package Version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 25 December 2024).
- Wickham, H. Reshaping Data with the reshape Package. J. Stat. Softw. 2007, 21, 1–20. Available online: http://www.jstatsoft.org/v21/i12/ (accessed on 25 December 2024). [CrossRef]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.2. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 25 December 2024).
- Wei, T.; Simko, V. Corrplot: Visualization of a Correlation Matrix. R Package Version 0.92. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 25 December 2024).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 25 December 2024).
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
- Ali, P.J.M.; Faraj, R.H.; Koya, E.; Ali, P.J.M.; Faraj, R.H. Data normalization and standardization: A technical report. Mach. Learn. Tech. Rep. 2014, 1, 1–6. [Google Scholar]
- Singh, D.; Singh, B. Investigating the impact of data normalization on classification performance. Appl. Soft Comput. 2020, 97, 105524. [Google Scholar] [CrossRef]
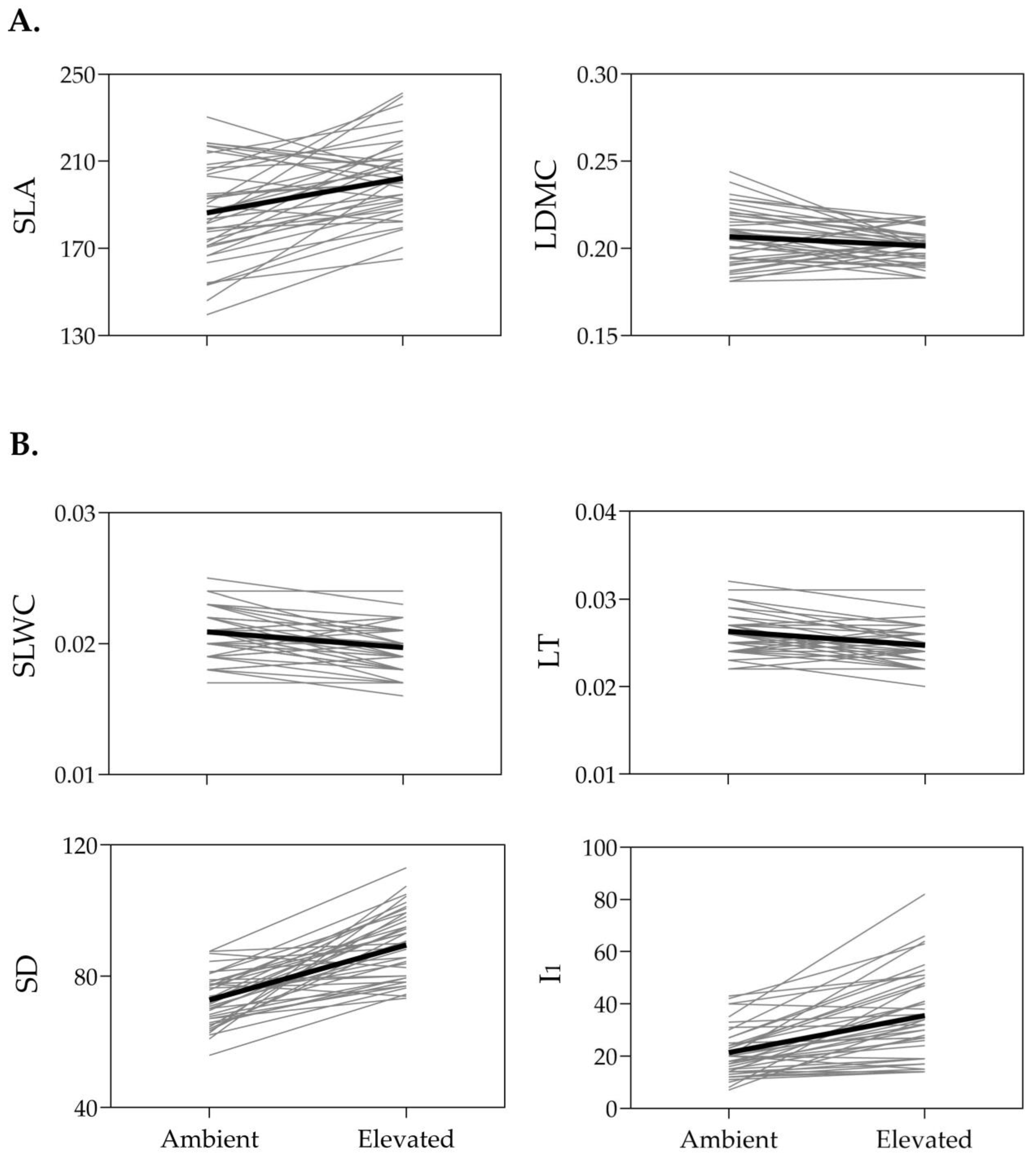

| Leaf Trait | Ambient Temperature | Elevated Temperature | F for Comparison of Trait Means | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SE | CV% | SE | CV% | |||||||
| Functional | ||||||||||
| SLA | 186.4 | 3.5 | 11.9 | 202.3 | 2.8 | 8.7 | 24.63 **** | |||
| LDMC | 0.2066 | 0.0026 | 7.8 | 0.2014 | 0.0016 | 4.9 | 4.86 * | |||
| Mechanistic | ||||||||||
| SLWC | 0.0209 | 0.0003 | 9.2 | 0.0197 | 0.0003 | 9.4 | 18.95 **** | |||
| LT | 0.0263 | 0.0004 | 9.2 | 0.0247 | 0.0004 | 8.9 | 25.37 **** | |||
| SD | 72.8 | 1.2 | 10.5 | 89.5 | 1.6 | 11.6 | 83.89 **** | |||
| I1 | 21.3 | 1.5 | 44.3 | 35.5 | 2.6 | 47.1 | 45.14 **** | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hočevar, K.; Vuleta, A.; Manitašević Jovanović, S. Plastic Responses of Iris pumila Functional and Mechanistic Leaf Traits to Experimental Warming. Plants 2025, 14, 960. https://doi.org/10.3390/plants14060960
Hočevar K, Vuleta A, Manitašević Jovanović S. Plastic Responses of Iris pumila Functional and Mechanistic Leaf Traits to Experimental Warming. Plants. 2025; 14(6):960. https://doi.org/10.3390/plants14060960
Chicago/Turabian StyleHočevar, Katarina, Ana Vuleta, and Sanja Manitašević Jovanović. 2025. "Plastic Responses of Iris pumila Functional and Mechanistic Leaf Traits to Experimental Warming" Plants 14, no. 6: 960. https://doi.org/10.3390/plants14060960
APA StyleHočevar, K., Vuleta, A., & Manitašević Jovanović, S. (2025). Plastic Responses of Iris pumila Functional and Mechanistic Leaf Traits to Experimental Warming. Plants, 14(6), 960. https://doi.org/10.3390/plants14060960









