Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective
Abstract
:1. Introduction
2. Chloroplast Omics
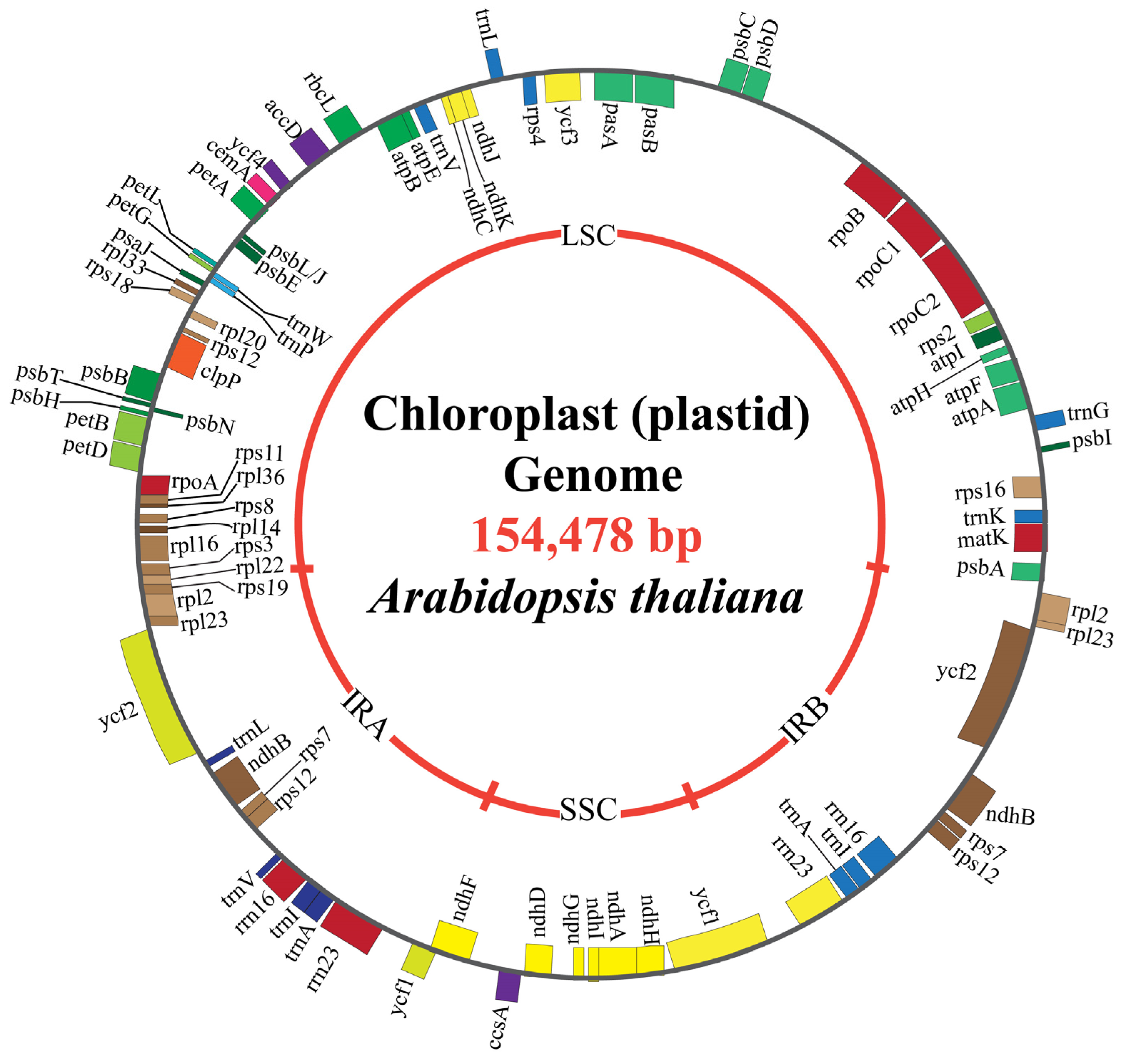
2.1. Chloroplast Genome Structural Composition
2.2. Plastid Genome Reduction in Parasitic and Non-Photosynthetic Plants
2.3. Chloroplast Proteomics and Functional Regulation
2.4. Chloroplast Transcription
2.5. Chloroplast Translation
3. Chloroplast Databases and Tools: Resources for Genomic and Functional Insight
3.1. Database Resources
3.2. Chloroplast Research Databases
4. Major Functions Performed by Chloroplast
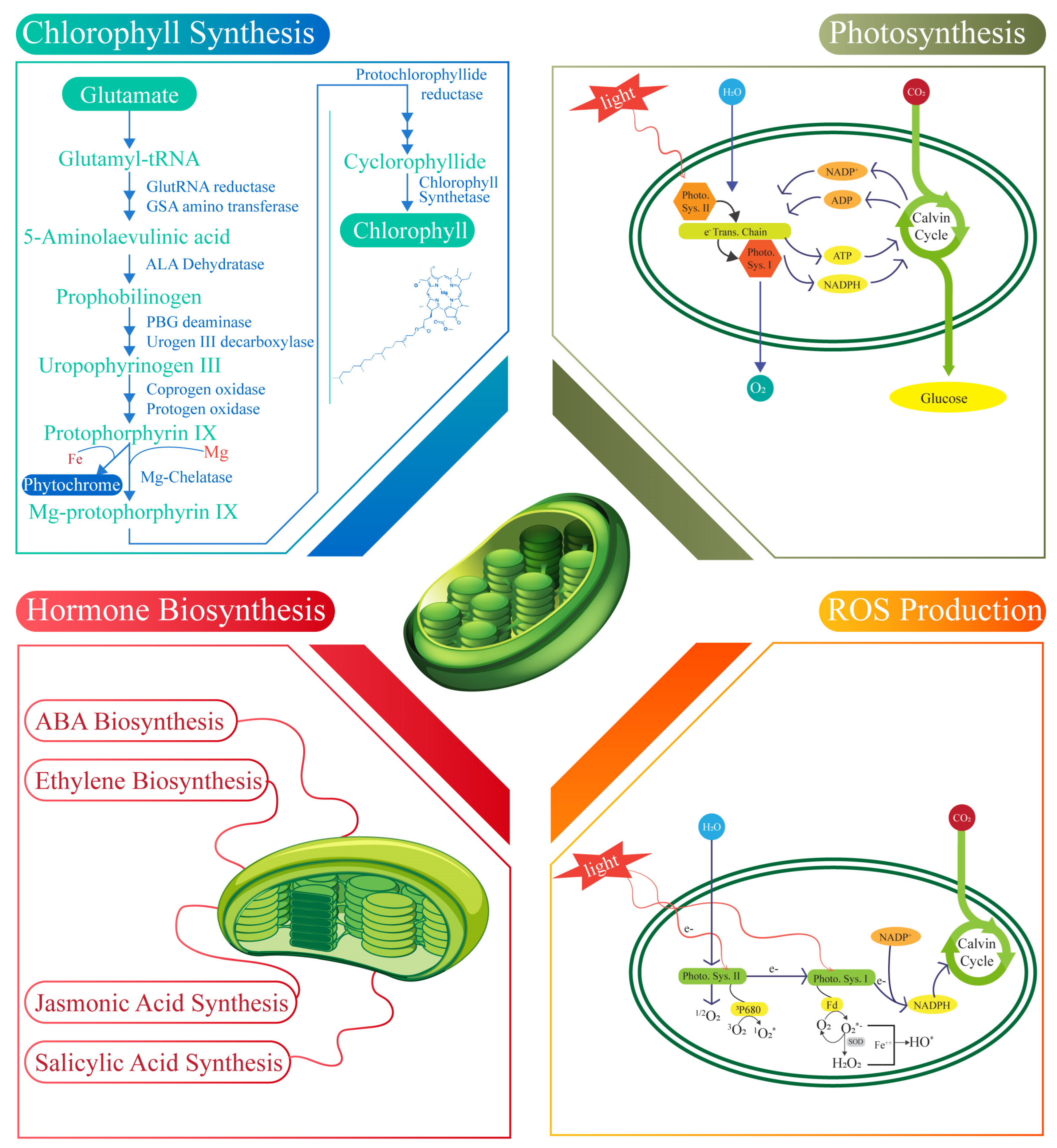
4.1. Chlorophyll Synthesis
4.2. Photosynthesis
4.3. ABA Biosynthesis
4.4. Ethylene Hormone
4.5. Jasmonic Acid Synthesis
4.6. Synthesis of Salicylic Acid
4.7. ROS Production
5. The Role of Chloroplasts in the Trade-Off Between Plant Defense and Growth
5.1. The Role of Chloroplasts in Trade-Off
5.2. Success Stories of Trade-Off Applications in Agriculture
6. Chloroplast’s Role in Plant Growth and Development
6.1. Role of Elongation Factor in Heat Stress Tolerance
6.2. Regulation of Carbon Metabolism via Phosphoglucose Isomerase (PGI)
6.3. Enhancing Drought Tolerance with Flavodiiron (FLV) Proteins
6.4. Carotenoid Biosynthesis and the Role of DCLCYB1
6.5. ZIPs and Its Homologs in Chloroplast Biogenesis
6.6. Optimizing Photosynthesis with RuBisCO and CO₂ Concentrating Mechanisms
7. Technological Innovations in Chloroplast Research
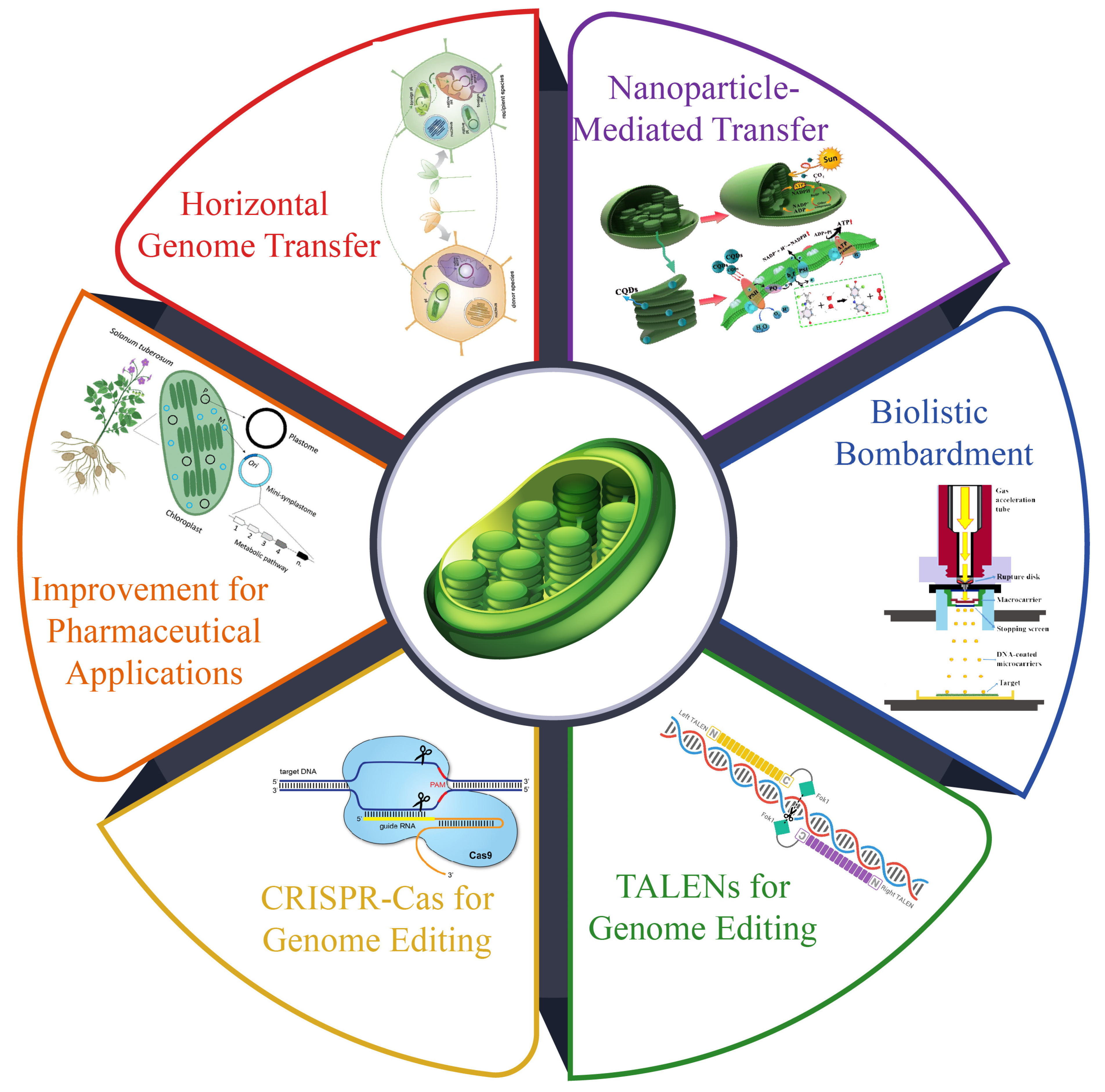
7.1. Genetic Transformations
7.2. Nanoparticle-Mediated Transfer
7.3. Horizontal Chloroplast Genome Transfer
7.4. Application of Genetic Transformation in Chloroplast
7.5. Challenges in Transplastomic Plant Development
8. Future Research Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sowden, R.G.; Watson, S.J.; Jarvis, P. The role of chloroplasts in plant pathology. Essays Biochem. 2017, 62, 21–39. [Google Scholar]
- Kachroo, P.; Burch-Smith, T.M.; Grant, M. An emerging role for chloroplasts in disease and defense. Annu. Rev. Phytopathol. 2021, 59, 423–445. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, M.; Damoo, D.; Djamei, A.; Kronstad, J. Chloroplasts and plant immunity: Where are the fungal effectors? Pathogens 2019, 9, 19. [Google Scholar] [CrossRef]
- Kusnetsov, V. Chloroplasts: Structure and expression of the plastid genome. Russ. J. Plant Physiol. 2018, 65, 465–476. [Google Scholar] [CrossRef]
- Lu, Y.; Yao, J. Chloroplasts at the crossroad of photosynthesis, pathogen infection and plant defense. Int. J. Mol. Sci. 2018, 19, 3900. [Google Scholar] [CrossRef]
- Le Provost, G.; Schenk, N.V.; Penone, C.; Thiele, J.; Westphal, C.; Allan, E.; Ayasse, M.; Blüthgen, N.; Boeddinghaus, R.S.; Boesing, A.L. The supply of multiple ecosystem services requires biodiversity across spatial scales. Nat. Ecol. Evol. 2023, 7, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 101, e02924. [Google Scholar] [CrossRef]
- Kurepa, J.; Smalle, J.A. Plant Hormone Modularity and the Survival-Reproduction Trade-Off. Biology 2023, 12, 1143. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth–defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Sahu, P.K.; Jayalakshmi, K.; Tilgam, J.; Gupta, A.; Nagaraju, Y.; Kumar, A.; Hamid, S.; Singh, H.V.; Minkina, T.; Rajput, V.D. ROS generated from biotic stress: Effects on plants and alleviation by endophytic microbes. Front. Plant Sci. 2022, 13, 1042936. [Google Scholar]
- Newkirk, G.M.; de Allende, P.; Jinkerson, R.E.; Giraldo, J.P. Nanotechnology approaches for chloroplast biotechnology advancements. Front. Plant Sci. 2021, 12, 691295. [Google Scholar]
- Tabassum, B.; Yousaf, I.; Adeyinka, O.S.; Khalid, R.; Khan, A. The Concept of Chloroplast Transformation; Its Relevance Towards Food Security. Adv. Life Sci. 2024, 11, 28–39. [Google Scholar]
- Hewage, K.A.H.; Yang, J.F.; Wang, D.; Hao, G.F.; Yang, G.F.; Zhu, J.K. Chemical manipulation of abscisic acid signaling: A new approach to abiotic and biotic stress management in agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar]
- Shikanai, T. Regulation of photosynthesis by cyclic electron transport around photosystem I. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 96, pp. 177–204. [Google Scholar]
- Sun, H.; Sun, X.; Wang, H.; Ma, X. Advances in salt tolerance molecular mechanism in tobacco plants. Hereditas 2020, 157, 5. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, J.; Yang, Z.; Li, H.; Liu, L.; Wang, H.; Sun, X.; Wu, X.; Nie, J.; Zhou, J. Chloroplast elongation factors break the growth–immunity trade-off by simultaneously promoting yield and defence. Nat. Plants 2024, 10, 1576–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Song, S.; Xu, F.; Pan, Y.; Wang, H. Transcriptomic and proteomic analyses reveal new insight into chlorophyll synthesis and chloroplast structure of maize leaves under zinc deficiency stress. J. Proteom. 2019, 199, 123–134. [Google Scholar]
- Bock, R. Engineering plastid genomes: Methods, tools, and applications in basic research and biotechnology. Annu. Rev. Plant Biol. 2015, 66, 211–241. [Google Scholar]
- Hennacy, J.H.; Jonikas, M.C. Prospects for engineering biophysical CO2 concentrating mechanisms into land plants to enhance yields. Annu. Rev. Plant Biol. 2020, 71, 461–485. [Google Scholar]
- Shahinnia, F.; Carrillo, N.; Hajirezaei, M.-R. Engineering climate-change-resilient crops: New tools and approaches. Int. J. Mol. Sci. 2021, 22, 7877. [Google Scholar] [CrossRef]
- Sharma, B.; Chaudhary, T.; Shukla, P. Combinatorial genetic engineering approaches in phytoremediation of pollutants. In Current Developments in Biotechnology And Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 55–71. [Google Scholar]
- Yarra, R. Plastome engineering in vegetable crops: Current status and future prospects. Mol. Biol. Rep. 2020, 47, 8061–8074. [Google Scholar] [CrossRef]
- Bureš, P.; Ozcan, M.; Šmerda, J.; Michálková, E.; Horová, L.; Plačková, K.; Šmarda, P.; Elliott, T.L.; Veselý, P.; Ćato, S. Evolution of genome size and GC content in the tribe Carduinae (Asteraceae): Rare descending dysploidy and polyploidy, limited environmental control and strong phylogenetic signal. Preslia 2023, 95, 185–213. [Google Scholar] [CrossRef]
- Dobrogojski, J.; Adamiec, M.; Luciński, R. The chloroplast genome: A review. Acta Physiol. Plant. 2020, 42, 98. [Google Scholar]
- Molina, J.; Hazzouri, K.M.; Nickrent, D.; Geisler, M.; Meyer, R.S.; Pentony, M.M.; Flowers, J.M.; Pelser, P.; Barcelona, J.; Inovejas, S.A.; et al. Possible loss of the chloroplast genome in the parasitic flowering plant Rafflesia lagascae (Rafflesiaceae). Mol. Biol. Evol. 2014, 31, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Ems, S.C.; Morden, C.W.; Dixon, C.K.; Wolfe, K.H.; de Pamphilis, C.W.; Palmer, J.D. Transcription, splicing and editing of plastid RNAs in the nonphotosynthetic plant Epifagus virginiana. Plant Mol. Biol. 1995, 29, 721–733. [Google Scholar] [CrossRef]
- Azim, M.K. Date Palm (Phoenix dactylifera L.) Chloroplast Genome. In The Date Palm Genome, Vol. 1: Phylogeny, Biodiversity and Mapping; Springer: Berlin/Heidelberg, Germany, 2021; pp. 201–209. [Google Scholar]
- Fang, J.; Chen, Y.; Liu, G.; Verbruggen, H.; Zhu, H. Chloroplast genome traits correlate with organismal complexity and ecological traits in Chlorophyta. Front. Ecol. Evol. 2021, 9, 791166. [Google Scholar] [CrossRef]
- Nobis, M.; Klichowska, E.; Vintsek, L.; Wróbel, A.; Nobis, A.; Zalewska-Gałosz, J.; Nowak, A. Evolutionary response of cold-adapted chasmophytic plants to Quaternary climatic oscillations in the Mountains of Central Asia (a world hotspot of biodiversity). Divers. Distrib. 2023, 29, 1458–1477. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Li, X.; Wang, G.; Wan, Y. Detecting of chloroplast circular RNAs in Arabidopsis thaliana. Plant Signal. Behav. 2019, 14, 1621088. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, Q.; Dong, Y.; Wang, X.; Zhang, S.; Mu, Z. Chloroplast genome structure and phylogenetic analysis of 13 Lamiaceae plants in Tibet. Front. Biosci.-Landmark 2023, 28, 110. [Google Scholar] [CrossRef]
- López, M.G.; Fass, M.; Rivas, J.G.; Carbonell-Caballero, J.; Vera, P.; Puebla, A.; Defacio, R.; Dopazo, J.; Paniego, N.; Hopp, H.E. Plastome genomics in South American maize landraces: Chloroplast lineages parallel the geographical structuring of nuclear gene pools. Ann. Bot. 2021, 128, 115–125. [Google Scholar]
- Tian, S.; Lu, P.; Zhang, Z.; Wu, J.Q.; Zhang, H.; Shen, H. Chloroplast genome sequence of Chongming lima bean (Phaseolus lunatus L.) and comparative analyses with other legume chloroplast genomes. BMC Genom. 2021, 22, 194. [Google Scholar]
- Peredo, E.L.; King, U.M.; Les, D.H. The plastid genome of Najas flexilis: Adaptation to submersed environments is accompanied by the complete loss of the NDH complex in an aquatic angiosperm. PLoS ONE 2013, 8, e68591. [Google Scholar] [CrossRef]
- Miglani, G.S. Plastid structure and genomics. In Plant Cells Their Organelles; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 239–299. [Google Scholar]
- Wang, T.-R.; Wang, Z.-W.; Song, Y.-G.; Kozlowski, G. The complete chloroplast genome sequence of Quercus ningangensis and its phylogenetic implication. Plant Fungal Syst. 2021, 66, 155–165. [Google Scholar] [CrossRef]
- Kousar, M.; Park, J. Comparative Analysis of the Chloroplast Genome of Sicyos angulatus with Other Seven Species of Cucurbitaceae Family. Genes 2023, 14, 1776. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Ebrahimi, A.; Mathur, S.; Lawson, S. The first complete chloroplast genome sequence and phylogenetic analysis of pistachio (Pistacia vera). Diversity 2022, 14, 577. [Google Scholar] [CrossRef]
- Hu, Q.; Qian, R.; Zhang, Y.; Ma, X.; Ye, Y.; Zhang, X.; Lin, L.; Liu, H.; Zheng, J. Complete chloroplast genome molecular structure, comparative and phylogenetic analyses of Sphaeropteris lepifera of Cyatheaceae family: A tree fern from China. Sci. Rep. 2023, 13, 1356. [Google Scholar]
- Su, H.; Ding, X.; Liao, B.; Zhang, D.; Huang, J.; Bai, J.; Xu, S.; Zhang, J.; Xu, W.; Qiu, X. Comparative chloroplast genomes provided insights into the evolution and species identification on the Datureae plants. Front. Plant Sci. 2023, 14, 1270052. [Google Scholar]
- Manzo, E.; Nillies, S.; Gutiérrez, D.G.; Panero, J.L.; Bräuchler, C.; Tomasello, S. Species delimitation in Xanthium sect. Acanthoxanthium (Heliantheae, Asteraceae) and the neglected species Xanthium argenteum. TAXON 2024, 73, 1251–1271. [Google Scholar]
- Yan, M.; Dong, S.; Gong, Q.; Xu, Q.; Ge, Y. Comparative chloroplast genome analysis of four Polygonatum species insights into DNA barcoding, evolution, and phylogeny. Sci. Rep. 2023, 13, 16495. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, N. Chloroplast Proteomics: Potentials and Challenges. J. Exp. Bot. 2024, 13, 84. [Google Scholar]
- Jarvis, P.; López-Juez, E. Biogenesis and homeostasis of chloroplasts and other plastids. Nat. Rev. Mol. Cell Biol. 2013, 14, 787–802. [Google Scholar]
- Wang, S.; Gao, J.; Chao, H.; Li, Z.; Pu, W.; Wang, Y.; Chen, M. Comparative chloroplast genomes of Nicotiana Species (Solanaceae): Insights into the genetic variation, phylogenetic relationship, and polyploid speciation. Front. Plant Sci. 2022, 13, 899252. [Google Scholar]
- Yoo, M.-J.; Jin, D.-P.; Lee, H.-O.; Lim, C.E. Complete plastome of three Korean Asarum (Aristolochiaceae): Confirmation tripartite structure within Korean Asarum and comparative analyses. Plants 2021, 10, 2056. [Google Scholar] [CrossRef]
- Robison, T.; Nelson, J.M.; Hauser, D.A.; Lewis, L.A.; Li, F.W. Dynamic plastid and mitochondrial genomes in Chaetopeltidales (Chlorophyceae) and characterization of a new chlorophyte taxon. Am. J. Bot. 2022, 109, 939–951. [Google Scholar]
- Lu, L.; Hou, Q.; Wang, L.; Zhang, T.; Zhao, W.; Yan, T.; Zhao, L.; Li, J.; Wan, X. Genome-wide identification and characterization of polygalacturonase gene family in maize (Zea mays L.). Int. J. Mol. Sci. 2021, 22, 10722. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zagorchev, L.; Chen, L.; Tao, Y.; Cai, C.; Jiang, M.; Sun, Z.; Li, J. Complete chloroplast genomes of five Cuscuta species and their evolutionary significance in the Cuscuta genus. BMC Genom. 2023, 24, 310. [Google Scholar] [CrossRef]
- Kurata, S.; Sakaguchi, S.; Kurashima, O.; Ogawa, R.; Suyama, Y.; Nishida, S.; Ito, M. Refugia within refugium of Geranium yesoense varieties: A follow-up study using chloroplast genome sequencing data of specimens from Mt. Asama, Japan. Biol. J. Linn. Soc. 2024, 142, 1–7. [Google Scholar]
- Li, B.; Huang, K.; Chen, X.; Qin, C.; Zhang, X. Comparative and phylogenetic analysis of chloroplast genomes from four species in Quercus section Cyclobalanopsis. BMC Genom. Data 2024, 25, 57. [Google Scholar]
- Yagi, Y.; Shiina, T. The chloroplast gene-expression system. In Applied Molecular Biotechnology: The Next Generation of Genetic Engineering; CRC Press: Boca Raton, FL, USA, 2016; p. 91. [Google Scholar]
- Miandoab, L.Z. Transcription Flexibility of Dunaliella Chloroplast Genome. In Progress in Microalgae Research—A Path for Shaping Sustainable Futures; IntechOpen: London, UK, 2022. [Google Scholar]
- Weihe, A.; Liere, K.; Börner, T. Transcription and transcription regulation in chloroplasts and mitochondria of higher plants. In Organelle Genetics: Evolution of Organelle Genomes and Gene Expression; Springer: Berlin/Heidelberg, Germany, 2011; pp. 297–325. [Google Scholar]
- Yin, C.; Richter, U.; Börner, T.; Weihe, A. Evolution of plant phage-type RNA polymerases: The genome of the basal angiosperm Nuphar advena encodes two mitochondrial and one plastid phage-type RNA polymerases. BMC Evol. Biol. 2010, 10, 379. [Google Scholar]
- Börner, T.; Zhelyazkova, P.; Legen, J.; Schmitz-Linneweber, C. Chloroplast gene expression—RNA synthesis and processing. In Plastid Biology; Springer: New York, NY, USA, 2014; pp. 3–47. [Google Scholar]
- Depp, S.L.J. Processing, Stability and Translation of the Chloroplast PsbB/T/H Transcripts of Chlamydomonas Reinhardtii. Ph.D. Thesis, Verlag Nicht Ermittelbar, Berlin, Germany, 2007. [Google Scholar]
- Germain, A.; Hotto, A.M.; Barkan, A.; Stern, D.B. RNA processing and decay in plastids. Wiley Interdiscip. Rev. RNA 2013, 4, 295–316. [Google Scholar]
- Richly, E. Structural and Functional Genomics in Semi-Autonomous Organelles: Composition and Origin of Proteomes of Chloroplasts and Mitochondria and Related Transcriptomics. Ph.D. Thesis, Universität zu Köln, Köln, Germany, 2003. [Google Scholar]
- Xiong, H.-B.; Pan, H.-M.; Long, Q.-Y.; Wang, Z.-Y.; Qu, W.-T.; Mei, T.; Zhang, N.; Xu, X.-F.; Yang, Z.-N.; Yu, Q.-B. AtNusG, a chloroplast nucleoid protein of bacterial origin linking chloroplast transcriptional and translational machineries, is required for proper chloroplast gene expression in Arabidopsis thaliana. Nucleic Acids Res. 2022, 50, 6715–6734. [Google Scholar] [CrossRef]
- Williams-Carrier, R.; Zoschke, R.; Belcher, S.; Pfalz, J.; Barkan, A. A major role for the plastid-encoded RNA polymerase complex in the expression of plastid transfer RNAs. Plant Physiol. 2014, 164, 239–248. [Google Scholar] [PubMed]
- Stoppel, R.; Lezhneva, L.; Schwenkert, S.; Torabi, S.; Felder, S.; Meierhoff, K.; Westhoff, P.; Meurer, J. Recruitment of a ribosomal release factor for light-and stress-dependent regulation of petB transcript stability in Arabidopsis chloroplasts. Plant Cell 2011, 23, 2680–2695. [Google Scholar] [CrossRef] [PubMed]
- Chotewutmontri, P.; Barkan, A. Light-induced psbA translation in plants is triggered by photosystem II damage via an assembly-linked autoregulatory circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 21775–21784. [Google Scholar]
- Pulido, P.; Zagari, N.; Manavski, N.; Gawronski, P.; Matthes, A.; Scharff, L.B.; Meurer, J.; Leister, D. CHLOROPLAST RIBOSOME ASSOCIATED supports translation under stress and interacts with the ribosomal 30S subunit. Plant Physiol. 2018, 177, 1539–1554. [Google Scholar]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar]
- Shi, L.-X.; Theg, S.M. The motors of protein import into chloroplasts. Plant Signal. Behav. 2011, 6, 1397–1401. [Google Scholar]
- Rochaix, J.D. Chloroplast protein import machinery and quality control. FEBS J. 2022, 289, 6908–6918. [Google Scholar]
- Andrès, C.; Agne, B.; Kessler, F. The TOC complex: Preprotein gateway to the chloroplast. Biochim. Et Biophys. Acta-Mol. Cell Res. 2010, 1803, 715–723. [Google Scholar]
- Simm, S.; Papasotiriou, D.G.; Ibrahim, M.; Leisegang, M.S.; Müller, B.; Schorge, T.; Karas, M.; Mirus, O.; Sommer, M.S.; Schleiff, E. Defining the core proteome of the chloroplast envelope membranes. Front. Plant Sci. 2013, 4, 11. [Google Scholar]
- Ali, S.M.; Li, N.; Soufi, Z.; Yao, J.; Johnson, E.; Ling, Q.; Jarvis, R.P. Multiple ubiquitin E3 ligase genes antagonistically regulate chloroplast-associated protein degradation. Curr. Biol. 2023, 33, 1138–1146.e5. [Google Scholar]
- Li, J.; Yuan, J.; Li, Y.; Sun, H.; Ma, T.; Huai, J.; Yang, W.; Zhang, W.; Lin, R. The CDC48 complex mediates ubiquitin-dependent degradation of intra-chloroplast proteins in plants. Cell Rep. 2022, 39, 110664. [Google Scholar] [PubMed]
- Sebastin, R.; Kim, J.; Jo, I.-H.; Yu, J.-K.; Jang, W.; Han, S.; Park, H.-S.; AlGarawi, A.M.; Hatamleh, A.A.; So, Y.-S. Comparative chloroplast genome analyses of cultivated and wild Capsicum species shed light on evolution and phylogeny. BMC Plant Biol. 2024, 24, 797. [Google Scholar]
- Otálora-Otálora, B.A.; Osuna-Garzón, D.A.; Carvajal-Parra, M.S.; Cañas, A.; Montecino, M.; López-Kleine, L.; Rojas, A. Identifying general tumor and specific lung cancer biomarkers by transcriptomic analysis. Biology 2022, 11, 1082. [Google Scholar] [CrossRef]
- Jerkovic Gulin, S.; Kravvas, G.; Seifert, O. Regional Variations in the Incidence of Lichen Sclerosus in Sweden: Insights from a Nationwide Register Study (2001–2021). J. Clin. Med. 2024, 13, 7836. [Google Scholar] [CrossRef]
- Tian, D.; Xu, T.; Kang, H.; Luo, H.; Wang, Y.; Chen, M.; Li, R.; Ma, L.; Wang, Z.; Hao, L. Plant genomic resources at National Genomics Data Center: Assisting in data-driven breeding applications. Abiotech 2024, 5, 94–106. [Google Scholar]
- Qiao, D.-H. Tea Plant Chloroplast and Mitochondrial Genome. In The Tea Plant Genome; Springer: Berlin/Heidelberg, Germany, 2024; pp. 243–261. [Google Scholar]
- Kaduchová, M.K. Analysis of dynamics and 3D organization of the nuclear genome in barley (Hordeum vulgare). Ph.D. Thesis, Palacký University Olomouc, Olomouc, Czechia, 2023. [Google Scholar]
- Liu, S.; Ni, Y.; Li, J.; Zhang, X.; Yang, H.; Chen, H.; Liu, C. CPGView: A package for visualizing detailed chloroplast genome structures. Mol. Ecol. Resour. 2023, 23, 694–704. [Google Scholar]
- Liu, F.; Yuan, L.; Zhang, Y. The complete chloroplast genome of the Syzygium buxifolium Hook. Et Arn. 1833 and its phylogenetic analysis. Mitochondrial DNA Part B 2024, 9, 851–855. [Google Scholar]
- Arshad, A.; Aditama, R.; Rahayu, M.S.; Natawijaya, A.; Matra, D.D.; Sudarsono, S. Comparative study of chloroplast genomes across seven Salacca species. Biodiversitas J. Biol. Divers. 2024, 25, 11. [Google Scholar]
- Leavitt, S.D.; DeBolt, A.; McQuhae, E.; Allen, J.L. Genomic Resources for the First Federally Endangered Lichen: The Florida Perforate Cladonia (Cladonia perforata). J. Fungi 2023, 9, 698. [Google Scholar] [CrossRef]
- Zhong, X. Assembly, Annotation and Analysis of Chloroplast Genomes. Ph.D. Thesis, The University of Western Australia, Claremont, Australia, 2020. [Google Scholar]
- Li, J.; Lin, M. Ensemble learning with diversified base models for fault diagnosis in nuclear power plants. Ann. Nucl. Energy 2021, 158, 108265. [Google Scholar]
- UniProt: The Universal protein knowledgebase in 2025. Nucleic Acids Res. 2024, 53, gkae1010.
- Huckvale, E.D.; Moseley, H.N. Predicting the pathway involvement of all pathway and associated compound entries defined in the kyoto encyclopedia of genes and genomes. Metabolites 2024, 14, 582. [Google Scholar] [CrossRef]
- Grigoriev, I.V.; Hayes, R.D.; Calhoun, S.; Kamel, B.; Wang, A.; Ahrendt, S.; Dusheyko, S.; Nikitin, R.; Mondo, S.J.; Salamov, A. PhycoCosm, a comparative algal genomics resource. Nucleic Acids Res. 2021, 49, D1004–D1011. [Google Scholar] [PubMed]
- Hawkins, C.; Ginzburg, D.; Zhao, K.; Dwyer, W.; Xue, B.; Xu, A.; Rice, S.; Cole, B.; Paley, S.; Karp, P. Plant Metabolic Network 15: A resource of genome-wide metabolism databases for 126 plants and algae. J. Integr. Plant Biol. 2021, 63, 1888–1905. [Google Scholar] [PubMed]
- Tripathy, B.C.; Pattanayak, G.K. Chlorophyll biosynthesis in higher plants. In Photosynthesis: Plastid Biology, Energy Conversion and Carbon Assimilation; Springer: Dordrecht, The Netherlands, 2012; pp. 63–94. [Google Scholar]
- Wu, Y.; Liao, W.; Dawuda, M.M.; Hu, L.; Yu, J. 5-Aminolevulinic acid (ALA) biosynthetic and metabolic pathways and its role in higher plants: A review. Plant Growth Regul. 2019, 87, 357–374. [Google Scholar]
- Schultz, M.J.; Hall, P.L.; Tortorelli, S. Porphyrins, Porphobilinogen, and δ-Aminolevulinic Acid. In Laboratory Guide to the Methods in Biochemical Genetics; Springer: Berlin/Heidelberg, Germany, 2024; pp. 283–305. [Google Scholar]
- Mingers, T.; Barthels, S.; Mass, V.; Acuña, J.M.B.-d.; Biedendieck, R.; Cooke, A.; Dailey, T.A.; Gerdes, S.; Blankenfeldt, W.; Dailey, H.A. The alternative coproporphyrinogen III oxidase (CgoN) catalyzes the oxygen-independent conversion of coproporphyrinogen III into coproporphyrin III. Front. Microbiol. 2024, 15, 1378989. [Google Scholar] [CrossRef]
- Fölsche, V.; Großmann, C.; Richter, A.S. Impact of Porphyrin Binding to GENOMES UNCOUPLED 4 on Tetrapyrrole Biosynthesis in planta. Front. Plant Sci. 2022, 13, 850504. [Google Scholar]
- Vedalankar, P.; Tripathy, B.C. Evolution of light-independent protochlorophyllide oxidoreductase. Protoplasma 2019, 256, 293–312. [Google Scholar]
- Wang, Y.-T.; Yang, C.-H.; Huang, K.-S.; Shaw, J.-F. Chlorophyllides: Preparation, purification, and application. Biomolecules 2021, 11, 1115. [Google Scholar] [CrossRef]
- Nelson, N.; Junge, W. Structure and energy transfer in photosystems of oxygenic photosynthesis. Annu. Rev. Biochem. 2015, 84, 659–683. [Google Scholar] [CrossRef]
- Wege, S. Plants Increase Photosynthesis Efficiency by Lowering the Proton Gradient across the Thylakoid Membrane. Plant Physiology 2020, 182, 1812–1813. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.-J.; Zhang, S.-B. Specific roles of cyclic electron flow around photosystem I in photosynthetic regulation in immature and mature leaves. J. Plant Physiol. 2017, 209, 76–83. [Google Scholar] [CrossRef]
- Shikanai, T. Central role of cyclic electron transport around photosystem I in the regulation of photosynthesis. Curr. Opin. Biotechnol. 2014, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, J.; Wang, Y.; He, M.; He, M.; Liu, W.; Shu, S.; Sun, J.; Guo, S. The key cyclic electron flow protein PGR5 associates with cytochrome b6f, and its function is partially influenced by the LHCII state transition. Hortic. Res. 2021, 8, 55. [Google Scholar] [CrossRef]
- Cook, G.; Teufel, A.; Kalra, I.; Li, W.; Wang, X.; Priscu, J.; Morgan-Kiss, R. The Antarctic psychrophiles Chlamydomonas spp. UWO241 and ICE-MDV exhibit differential restructuring of photosystem I in response to iron. Photosynth. Res. 2019, 141, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Losey, N.A.; Poudel, S.; Boyd, E.S.; McInerney, M.J. The beta subunit of non-bifurcating NADH-dependent [FeFe]-hydrogenases differs from those of multimeric electron-bifurcating [FeFe]-hydrogenases. Front. Microbiol. 2020, 11, 1109. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.S.; Semchonok, D.A.; Nosek, L.; Kouřil, R.; Fucile, G.; Boekema, E.J.; Eichacker, L.A. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys. Acta-Bioenerg. 2017, 1858, 12–20. [Google Scholar] [CrossRef]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic electron flow around photosystem I via chloroplast NAD (P) H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011, 68, 966–976. [Google Scholar] [CrossRef]
- Shikanai, T. Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim. Biophys. Acta-Bioenerg. 2016, 1857, 1015–1022. [Google Scholar] [CrossRef]
- Gorski, C.; Riddle, R.; Toporik, H.; Da, Z.; Dobson, Z.; Williams, D.; Mazor, Y. The structure of the Physcomitrium patens photosystem I reveals a unique Lhca2 paralogue replacing Lhca4. Nat. Plants 2022, 8, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Seng, S.; Ponce, G.E.; Andreas, P.; Kisiala, A.; De Clerck-Floate, R.; Miller III, D.G.; Chen, M.-S.; Price, P.W.; Tooker, J.F.; Emery, R.N. Abscisic acid: A potential secreted effector synthesized by phytophagous insects for host-plant manipulation. Insects 2023, 14, 489. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Pan, X.; Kakar, K.U.; Nawaz, Z. Regulation of carotenoid metabolism and ABA biosynthesis during blueberry fruit ripening. Plant Physiol. Biochem. 2024, 206, 108232. [Google Scholar] [CrossRef]
- Dar, N.A.; Amin, I.; Wani, W.; Wani, S.A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant Gene 2017, 11, 106–111. [Google Scholar]
- Che, Y.; Yao, T.; Wang, H.; Wang, Z.; Zhang, H.; Sun, G.; Zhang, H. Potassium ion regulates hormone, Ca2+ and H2O2 signal transduction and antioxidant activities to improve salt stress resistance in tobacco. Plant Physiol. Biochem. 2022, 186, 40–51. [Google Scholar] [CrossRef]
- Ali, S.; Baloch, A.M. Overview of sustainable plant growth and differentiation and the role of hormones in controlling growth and development of plants under various stresses. Recent Pat. Food Nutr. Agric. 2020, 11, 105–114. [Google Scholar] [PubMed]
- Ahmadizadeh, M.; Chen, J.-T.; Hasanzadeh, S.; Ahmar, S.; Heidari, P. Insights into the genes involved in the ethylene biosynthesis pathway in Arabidopsis thaliana and Oryza sativa. J. Genet. Eng. Biotechnol. 2020, 18, 62. [Google Scholar]
- Xie, X.-l.; Xia, X.-j.; Kuang, S.; Zhang, X.-l.; Yin, X.-r.; Yu, J.-q.; Chen, K.-s. A novel ethylene responsive factor CitERF13 plays a role in photosynthesis regulation. Plant Sci. 2017, 256, 112–119. [Google Scholar]
- Iqbal, N.; Nazar, R.; Syeed, S.; Masood, A.; Khan, N.A. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J. Exp. Bot. 2011, 62, 4955–4963. [Google Scholar]
- Khan, S.; Alvi, A.F.; Khan, N.A. Role of Ethylene in the Regulation of Plant Developmental Processes. Stresses 2024, 4, 28–53. [Google Scholar] [CrossRef]
- Ravanbakhsh, M.; Sasidharan, R.; Voesenek, L.A.; Kowalchuk, G.A.; Jousset, A. Microbial modulation of plant ethylene signaling: Ecological and evolutionary consequences. Microbiome 2018, 6, 52. [Google Scholar]
- Mohorović, P.; Vaughan-Hirsch, J.; Ceusters, J.; Van de Poel, B. The role of ethylene in photosynthate partitioning and source-sink modulation in crops. In The Plant Hormone Ethylene; Elsevier: Amsterdam, The Netherlands, 2023; pp. 23–39. [Google Scholar]
- Bhatla, S.C.; Lal, M.A. Jasmonic acid. In Plant Physiology, Development and Metabolism; Springer: Berlin/Heidelberg, Germany, 2023; pp. 473–478. [Google Scholar]
- Wasternack, C.; Hause, B. The missing link in jasmonic acid biosynthesis. Nat. Plants 2019, 5, 776–777. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.; Zarandi, M.A.; Zhou, X.; Jayawickramarajah, J. Synthesis and applications of porphyrin-biomacromolecule conjugates. Front. Chem. 2021, 9, 764137. [Google Scholar] [CrossRef] [PubMed]
- Efimova, M.; Mukhamatdinova, E.; Kovtun, I.; Kabil, F.; Medvedeva, Y.V.; Kuznetsov, V.V. Jasmonic acid enhances the potato plant resistance to the salt stress in vitro. In Doklady Biological Sciences; Pleiades Publishing: New York, NY, USA, 2019; pp. 149–152. [Google Scholar]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Gupta, A.; Hisano, H.; Hojo, Y.; Matsuura, T.; Ikeda, Y.; Mori, I.C.; Senthil-Kumar, M. Global profiling of phytohormone dynamics during combined drought and pathogen stress in Arabidopsis thaliana reveals ABA and JA as major regulators. Sci. Rep. 2017, 7, 4017. [Google Scholar] [CrossRef]
- Shin, S.; Lv, J.; Fazio, G.; Mazzola, M.; Zhu, Y. Transcriptional regulation of ethylene and jasmonate mediated defense response in apple (Malus domestica) root during Pythium ultimum infection. Hortic. Res. 2014, 1, 14053. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef]
- Sambyal, K.; Singh, R.V. Production of salicylic acid; a potent pharmaceutically active agent and its future prospects. Crit. Rev. Biotechnol. 2021, 41, 394–405. [Google Scholar] [CrossRef]
- Macaulay, K.M.; Heath, G.A.; Ciulli, A.; Murphy, A.M.; Abell, C.; Carr, J.P.; Smith, A.G. The biochemical properties of the two Arabidopsis thaliana isochorismate synthases. Biochem. J. 2017, 474, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Scholten, N.; Hartmann, M.; Abts, S.; Abts, L.; Reinartz, E.; Altavilla, A.; Müller, T.J.; Zeier, J. In-depth analysis of isochorismate synthase-derived metabolism in plant immunity: Identification of meta-substituted benzoates and salicyloyl-malate. J. Biol. Chem. 2024, 300, 107667. [Google Scholar]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Biosynthetic pathways of hormones in plants. Metabolites 2023, 13, 884. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and defense responses in plants. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 191–219. [Google Scholar]
- Nazar, R.; Umar, S.; Khan, N.; Sareer, O. Salicylic acid supplementation improves photosynthesis and growth in mustard through changes in proline accumulation and ethylene formation under drought stress. S. Afr. J. Bot. 2015, 98, 84–94. [Google Scholar]
- Amoanimaa-Dede, H.; Su, C.; Yeboah, A.; Zhou, H.; Zheng, D.; Zhu, H. Growth regulators promote soybean productivity: A review. PeerJ 2022, 10, e12556. [Google Scholar]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar]
- Pospíšil, P.; Kumar, A.; Prasad, A. Reactive oxygen species in photosystem II: Relevance for oxidative signaling. Photosynth. Res. 2022, 152, 245–260. [Google Scholar]
- Galvez-Valdivieso, G.; Mullineaux, P.M. The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plant. 2010, 138, 430–439. [Google Scholar]
- Pierella Karlusich, J.J.; Zurbriggen, M.D.; Shahinnia, F.; Sonnewald, S.; Sonnewald, U.; Hosseini, S.A.; Hajirezaei, M.-R.; Carrillo, N. Chloroplast redox status modulates genome-wide plant responses during the non-host interaction of tobacco with the hemibiotrophic bacterium Xanthomonas campestris pv. vesicatoria. Front. Plant Sci. 2017, 8, 1158. [Google Scholar]
- Littlejohn, G.R.; Breen, S.; Smirnoff, N.; Grant, M. Chloroplast immunity illuminated. New Phytol. 2021, 229, 3088–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, A.; Li, X.; Lu, C. The role of chloroplast gene expression in plant responses to environmental stress. Int. J. Mol. Sci. 2020, 21, 6082. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Hu, S.; Wu, Q.; Feng, X.; Zhang, Y.; Jiang, Q.; Ma, J.; Qi, P.; Chen, G.; Jiang, Y. An effector protein of Fusarium graminearum targets chloroplasts and suppresses cyclic photosynthetic electron flow. Plant Physiol. 2024, 196, kiae538. [Google Scholar] [CrossRef] [PubMed]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Wu, M.; Rosas-Diaz, T.; Wang, L.; Ding, X.; Zhang, D.; Fu, X.; Kim, C. A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 2020, 182, 1109–1124.e1125. [Google Scholar] [CrossRef]
- Liu, M.; Sui, Y.; Yu, C.; Wang, X.; Zhang, W.; Wang, B.; Yan, J.; Duan, L. Coronatine-Induced Maize Defense against Gibberella Stalk Rot by Activating Antioxidants and Phytohormone Signaling. J. Fungi 2023, 9, 1155. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Allahverdiyeva, Y.; Alvarez, C.E.; Büchel, C.; Burlacot, A.; Cardona, T.; Chaloner, E.; Engel, B.D.; Grossman, A.R.; Harris, D. Lighting the way: Compelling open questions in photosynthesis research. Plant Cell 2024, 36, 3914–3943. [Google Scholar] [CrossRef]
- González, M.-C.; Cejudo, F.J.; Sahrawy, M.; Serrato, A.J. Current knowledge on mechanisms preventing photosynthesis redox imbalance in plants. Antioxidants 2021, 10, 1789. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species generation, hazards, and defense mechanisms in plants under environmental (abiotic and biotic) stress conditions. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 617–658. [Google Scholar]
- Dharshini, L.C.P.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell Signal. 2020, 72, 109670. [Google Scholar] [CrossRef]
- Burman, N.; Khurana, J.P. Photoregulation of Chloroplast Development: Retrograde Signaling. In Plastid Development in Leaves during Growth and Senescence; Springer: Dordrecht, The Netherlands, 2013; pp. 569–588. [Google Scholar]
- Liu, W.; Zhang, Y.; Zhang, B.; Zou, H. Expression of ZmNAGK in tobacco enhances heat stress tolerance via activation of antioxidant-associated defense. Plant Physiol. Biochem. 2023, 199, 107719. [Google Scholar] [CrossRef]
- Pfalz, J.; Oelmüller, R. Plastid Retrograde Signals: More to Discover. In Sensory Biology of Plants; Springer: Singapore, 2019; pp. 477–507. [Google Scholar]
- Gollan, P.J.; Tikkanen, M.; Aro, E.-M. Photosynthetic light reactions: Integral to chloroplast retrograde signalling. Curr. Opin. Plant Biol. 2015, 27, 180–191. [Google Scholar] [CrossRef]
- Huibin, Z.; Liu, H.; Aboulnaga, E.; Liu, H.; Cheng, T.; Xian, M. Microbial production of isoprene: Opportunities and challenges. In Industrial Biotechnology: Products and Processes; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 473–504. [Google Scholar]
- Aarthy, T. Limonoid Biosynthesis in Azadirachta Indica: Characterization of Pathway Genes and Analysis of Labeled Metabolites Through Stable Isotope Feeding. Ph.D. Thesis, CSIR-National Chemical Laboratory, Pune, India, 2019. [Google Scholar]
- Dubreuil, C.; Jin, X.; Barajas-Lopez, J.d.D.; Hewitt, T.C.; Tanz, S.K.; Dobrenel, T.; Schröder, W.P.; Hanson, J.; Pesquet, E.; Grönlund, A. Establishment of photosynthesis through chloroplast development is controlled by two distinct regulatory phases. Plant Physiol. 2018, 176, 1199–1214. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Mahajan, A.; Takacs, E.M.; Stern, D.B.; Cahoon, A.B. Developmental and cell type characterization of bundle sheath and mesophyll chloroplast transcript abundance in maize. Curr. Genet. 2011, 57, 89–102. [Google Scholar]
- Durgud, M.; Gupta, S.; Ivanov, I.; Omidbakhshfard, M.A.; Benina, M.; Alseekh, S.; Staykov, N.; Hauenstein, M.; Dijkwel, P.P.; Hörtensteiner, S. Molecular mechanisms preventing senescence in response to prolonged darkness in a desiccation-tolerant plant. Plant Physiol. 2018, 177, 1319–1338. [Google Scholar] [PubMed]
- Liebers, M.; Cozzi, C.; Uecker, F.; Chambon, L.; Blanvillain, R.; Pfannschmidt, T. Biogenic signals from plastids and their role in chloroplast development. J. Exp. Bot. 2022, 73, 7105–7125. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Bourgault, R.; Mohammadi, M.; Matschi, S.; Philippe, G.; Smith, L.G.; Gore, M.A.; Molina, I.; Scanlon, M.J. Transcriptomic network analyses shed light on the regulation of cuticle development in maize leaves. Proc. Natl. Acad. Sci. USA 2020, 117, 12464–12471. [Google Scholar] [PubMed]
- Li, X.; Cai, C.; Wang, Z.; Fan, B.; Zhu, C.; Chen, Z. Plastid translation elongation factor Tu is prone to heat-induced aggregation despite its critical role in plant heat tolerance. Plant Physiol. 2018, 176, 3027–3045. [Google Scholar]
- Momcilovic, I. Maize Chloroplast Protein Synthesis Elongation Factor Tu (EF-Tu): Localization, Abundance and Potential Role in Heat Tolerance; University of South Dakota: Vermillion, SD, USA, 2004. [Google Scholar]
- Sharma, M.; Singh, R.K.; Sharma, C.K. Functioning of different plant heat-shock proteins in maize (Zea mays) and molecular chaperones—An insigHT. Int. J. Agric. Stat. Sci. 2018, 14, 203. [Google Scholar]
- Liu, S.; Zheng, L.; Jia, J.; Guo, J.; Zheng, M.; Zhao, J.; Shao, J.; Liu, X.; An, L.; Yu, F. Chloroplast translation elongation factor EF-Tu/SVR11 is involved in var2-mediated leaf variegation and leaf development in Arabidopsis. Front. Plant Sci. 2019, 10, 295. [Google Scholar] [CrossRef]
- Borgström, C.; Persson, V.C.; Rogova, O.; Osiro, K.O.; Lundberg, E.; Spégel, P.; Gorwa-Grauslund, M. Using phosphoglucose isomerase-deficient (pgi1 Δ) Saccharomyces cerevisiae to map the impact of sugar phosphate levels on d-glucose and d-xylose sensing. Microb. Cell Factories 2022, 21, 253. [Google Scholar] [CrossRef]
- Preiser, A.L.; Banerjee, A.; Fisher, N.; Sharkey, T.D. Supply and consumption of glucose 6-phosphate in the chloroplast stroma. bioRxiv 2018, 442434. [Google Scholar] [CrossRef]
- Preiser, A.L.; Fisher, N.; Banerjee, A.; Sharkey, T.D. Plastidic glucose-6-phosphate dehydrogenases are regulated to maintain activity in the light. Biochem. J. 2019, 476, 1539–1551. [Google Scholar]
- Preiser, A.L.; Banerjee, A.; Weise, S.E.; Renna, L.; Brandizzi, F.; Sharkey, T.D. Phosphoglucoisomerase is an important regulatory enzyme in partitioning carbon out of the Calvin-Benson cycle. Front. Plant Sci. 2020, 11, 580726. [Google Scholar]
- Vosloh, D. Subcellular Compartmentation of Primary Carbon Metabolism in Mesophyll Cells of Arabidopsis thaliana. Ph.D. Thesis, Universität Potsdam, Potsdam, Germany, 2011. [Google Scholar]
- Rajaram, H.; Chaurasia, A.K.; Apte, S.K. Cyanobacterial heat-shock response: Role and regulation of molecular chaperones. Microbiology 2014, 160, 647–658. [Google Scholar]
- Shahinnia, F.; Tula, S.; Hensel, G.; Reiahisamani, N.; Nasr, N.; Kumlehn, J.; Gómez, R.; Lodeyro, A.F.; Carrillo, N.; Hajirezaei, M.R. Plastid-targeted cyanobacterial flavodiiron proteins maintain carbohydrate turnover and enhance drought stress tolerance in barley. Front. Plant Sci. 2021, 11, 613731. [Google Scholar]
- Shahinnia, F.; Tula, S.; Hensel, G.; Reiahisamani, N.; Nasr, N.; Kumlehn, J.; Gómez, R.; Lodeyro, A.F.; Carrillo, N.; Hajirezaei, M.R. Integrating cyanobacterial flavodiiron proteins within the chloroplast photosynthetic electron transport chain maintains carbohydrate turnover and enhances drought stress tolerance in barley. bioRxiv 2020, 2020-09. [Google Scholar] [CrossRef]
- Rosas-Saavedra, C.; Quiroz, L.F.; Parra, S.; Gonzalez-Calquin, C.; Arias, D.; Ocarez, N.; Lopez, F.; Stange, C. Putative Daucus carota capsanthin-capsorubin synthase (DcCCS) possesses lycopene β-cyclase activity, boosts carotenoid levels, and increases salt tolerance in heterologous plants. Plants 2023, 12, 2788. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.; Arenas-M, A.; Flores-Ortiz, C.; Peirano, C.; Handford, M.; Stange, C. Daucus carota DcPSY2 and DcLCYB1 as tools for carotenoid metabolic engineering to improve the nutritional value of fruits. Front. Plant Sci. 2021, 12, 677553. [Google Scholar]
- Moreno, J.C.; Martinez-Jaime, S.; Kosmacz, M.; Sokolowska, E.M.; Schulz, P.; Fischer, A.; Luzarowska, U.; Havaux, M.; Skirycz, A. A multi-OMICs approach sheds light on the higher yield phenotype and enhanced abiotic stress tolerance in tobacco lines expressing the carrot lycopene β-cyclase1 gene. Front. Plant Sci. 2021, 12, 624365. [Google Scholar]
- Moreno, J.C.; Mi, J.; Agrawal, S.; Kössler, S.; Turečková, V.; Tarkowská, D.; Thiele, W.; Al-Babili, S.; Bock, R.; Schöttler, M.A. Expression of a carotenogenic gene allows faster biomass production by redesigning plant architecture and improving photosynthetic efficiency in tobacco. Plant J. 2020, 103, 1967–1984. [Google Scholar]
- Saikia, B.; Singh, S.; Debbarma, J.; Velmurugan, N.; Dekaboruah, H.; Arunkumar, K.P.; Chikkaputtaiah, C. Multigene CRISPR/Cas9 genome editing of hybrid proline rich proteins (HyPRPs) for sustainable multi-stress tolerance in crops: The review of a promising approach. Physiol. Mol. Biol. Plants 2020, 26, 857–869. [Google Scholar]
- Singh, S.; Selvakumar, R.; Mangal, M.; Kalia, P. Breeding and genomic investigations for quality and nutraceutical traits in vegetable crops—A review. Indian J. Hortic. 2020, 77, 1–40. [Google Scholar]
- Stange, C. Daucus carota DcPSY2 and DcLCYB1 as Tools for Carotenoid Metabolic Engineering to Improve the Nutritional Value of Fruits. Chloroplast Biotechnol. Crop Improv. 2021. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [PubMed]
- Arcos, Y.; Godoy, F.; Flores-Ortiz, C.; Arenas-M, A.; Stange, C. Boosting carotenoid content in Malus domestica var. Fuji By Expressing AtDXR Through Agrobacterium-Mediat. Transform. Method. Biotechnol. Bioeng. 2020, 117, 2209–2222. [Google Scholar] [PubMed]
- Pogson, B.J.; Ganguly, D.; Albrecht-Borth, V. Insights into chloroplast biogenesis and development. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 1017–1024. [Google Scholar]
- Hung, C.-Y.; Zhang, J.; Bhattacharya, C.; Li, H.; Kittur, F.S.; Oldham, C.E.; Wei, X.; Burkey, K.O.; Chen, J.; Xie, J. Transformation of long-lived albino Epipremnum aureum ‘Golden Pothos’ and restoring chloroplast development. Front. Plant Sci. 2021, 12, 647507. [Google Scholar]
- Hu, J.; Liu, M.; Wang, D.; Liang, Y.; Zong, Y.; Li, Y.; Cao, D.; Liu, B. Transcriptional and genetic characteristic of chimera pea generation via double ethyl methanesulfonate-induced mutation revealed by transcription analysis. Front. Plant Sci. 2024, 15, 1439547. [Google Scholar]
- Schröder, P.M. The Evolution of B-Family GATA Transcription Factors in the Plant Lineage. Ph.D. Thesis, Technische Universität of München, München, Germany, 2023. [Google Scholar]
- Rae, B.D.; Long, B.M.; Förster, B.; Nguyen, N.D.; Velanis, C.N.; Atkinson, N.; Hee, W.Y.; Mukherjee, B.; Price, G.D.; McCormick, A.J. Progress and challenges of engineering a biophysical CO2-concentrating mechanism into higher plants. J. Exp. Bot. 2017, 68, 3717–3737. [Google Scholar]
- Adler, L.; Díaz-Ramos, A.; Mao, Y.; Pukacz, K.R.; Fei, C.; McCormick, A.J. New horizons for building pyrenoid-based CO2-concentrating mechanisms in plants to improve yields. Plant Physiol. 2022, 190, 1609–1627. [Google Scholar]
- Kupriyanova, E.V.; Pronina, N.A.; Los, D.A. Adapting from low to high: An update to CO2-concentrating mechanisms of cyanobacteria and microalgae. Plants 2023, 12, 1569. [Google Scholar] [CrossRef]
- Hines, K.M. The Role of Chloroplast Carbonic Anhydrases in the Development of C3 Photosynthesizing Plants. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 2019. [Google Scholar]
- Inckemann, R. Advancing Chloroplast Synthetic Biology by Developing High Throughput Prototyping Capabilities and Novel Genetic Tools; Philipps-Universität Marburg: Marburg, Germany, 2024. [Google Scholar]
- Yu, Y.; Yu, P.-C.; Chang, W.-J.; Yu, K.; Lin, C.-S. Plastid transformation: How does it work? Can it be applied to crops? What can it offer? Int. J. Mol. Sci. 2020, 21, 4854. [Google Scholar] [CrossRef]
- Ozyigit, I.I.; Yucebilgili Kurtoglu, K. Particle bombardment technology and its applications in plants. Mol. Biol. Rep. 2020, 47, 9831–9847. [Google Scholar] [PubMed]
- Liu, Y.; Zhang, S.; Zhang, S.; Zhang, H.; Li, G.; Sun, R.; Li, F. Efficient transformation of the isolated microspores of Chinese cabbage (Brassica rapa L. ssp. pekinensis) by particle bombardment. Plant Methods 2024, 20, 17. [Google Scholar] [PubMed]
- Kumar, A.U.; Ling, A.P.K. Gene introduction approaches in chloroplast transformation and its applications. J. Genet. Eng. Biotechnol. 2021, 19, 148. [Google Scholar]
- Ortiz-Matamoros, M.F.; Villanueva, M.A.; Islas-Flores, T. Genetic transformation of cell-walled plant and algae cells: Delivering DNA through the cell wall. Brief. Funct. Genom. 2018, 17, 26–33. [Google Scholar]
- Elhefnawy, S.M.; Elsheery, N.I. Use of nanoparticles in improving photosynthesis in crop plants under stress. In Photosynthesis; Elsevier: Amsterdam, The Netherlands, 2023; pp. 105–135. [Google Scholar]
- Santana, I.; Jeon, S.-J.; Kim, H.-I.; Islam, M.R.; Castillo, C.; Garcia, G.F.; Newkirk, G.M.; Giraldo, J.P. Targeted carbon nanostructures for chemical and gene delivery to plant chloroplasts. ACS Nano 2022, 16, 12156–12173. [Google Scholar]
- Chandrasekaran, R.; Rajiv, P.; Abd-Elsalam, K.A. Carbon nanotubes: Plant gene delivery and genome editing. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–296. [Google Scholar]
- Liu, Y.-X.; Fan, L.; Liang, G.; Tu, Z.-L.; Fei, Z.; Lin, Y.-J. Advancing approach and toolbox in optimization of chloroplast genetic transformation technology. J. Integr. Agric. 2023, 22, 1951–1966. [Google Scholar]
- Santana, I. Impact of Targeted Nanomaterials for Chloroplast Bioengineering on Arabidopsis thaliana; University of California: Riverside, CA, USA, 2022. [Google Scholar]
- Zhang, A.; Wang, T.; Yuan, L.; Shen, Y.; Liu, K.; Liu, B.; Xu, K.; Elsadek, M.A.; Wang, Y.; Wu, L. Horizontal transfer of plasmid-like extrachromosomal circular DNAs across graft junctions in Solanaceae. Mol. Hortic. 2024, 4, 41. [Google Scholar] [PubMed]
- Sidorov, V.; Armstrong, C.; Ream, T.; Ye, X.; Saltarikos, A. “Cell grafting”: A new approach for transferring cytoplasmic or nuclear genome between plants. Plant Cell Rep. 2018, 37, 1077–1089. [Google Scholar]
- Arya, S.S.; Tanwar, N.; Lenka, S.K. Prospects of nano-and peptide-carriers to deliver CRISPR cargos in plants to edit across and beyond central dogma. Nanotechnol. Environ. Eng. 2021, 6, 22. [Google Scholar]
- Tanihara, F.; Hirata, M.; Otoi, T. Current status of the application of gene editing in pigs. J. Reprod. Dev. 2021, 67, 177–187. [Google Scholar] [CrossRef]
- Nakazato, I.; Arimura, S.i. Genome editing in angiosperm chloroplasts: Targeted DNA double-strand break and base editing. Plant J. 2024, 120, 872–880. [Google Scholar] [CrossRef]
- Silva-Pinheiro, P.; Minczuk, M. The potential of mitochondrial genome engineering. Nat. Rev. Genet. 2022, 23, 199–214. [Google Scholar] [CrossRef]
- Patel, V.K.; Das, A.; Kumari, R.; Kajla, S. Recent progress and challenges in CRISPR-Cas9 engineered algae and cyanobacteria. Algal Res. 2023, 71, 103068. [Google Scholar] [CrossRef]
- Fidan, O.; Secgin, Z. Molecular Farming for the Production of Recombinant Pharmaceutical Proteins in Plants. In Applications of Plant Molecular Farming; Springer: Berlin/Heidelberg, Germany, 2024; pp. 235–258. [Google Scholar]
- Kurup, V.M.; Thomas, J. Edible vaccines: Promises and challenges. Mol. Biotechnol. 2020, 62, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-H.; Zhu, C.-C.; Zhang, N.-S.; Li, D.-W.; Yang, W.-D.; Liu, J.-S.; Sathishkumar, R.; Li, H.-Y. Construction of novel chloroplast expression vector and development of an efficient transformation system for the diatom Phaeodactylum tricornutum. Mar. Biotechnol. 2014, 16, 538–546. [Google Scholar] [CrossRef]
- Gaobotse, G.; Trémouillaux-Guiller, J.; Venkataraman, S.; Sherif, M.K.; Makhzoum, A.; Hefferon, K. Plant molecular pharming of biologics to combat HIV. In Applications in Plant Biotechnology; CRC Press: Boca Raton, FL, USA, 2022; pp. 296–348. [Google Scholar]
- Fang, H.; Liu, J.; Ma, R.; Zou, Y.; Ho, S.-H.; Chen, J.; Xie, Y. Functional Characterization of Lycopene β-and ε-Cyclases from a Lutein-Enriched Green Microalga Chlorella sorokiniana FZU60. Mar. Drugs 2023, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wu, H.; Liu, J.; Chen, L.; Jiang, Z.; Zhang, Y.; Tian, X.; Li, R.; Yang, Y.; Ren, X. Lycopene β-cyclase plays a critical role in carotenoid biosynthesis during persimmon fruit development and postharvest ripening. Sci. Hortic. 2021, 287, 110265. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional Benefits of Lycopene and Beta-Carotene: A Comprehensive Overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar] [CrossRef]
- Liu, L.; Shao, Z.; Zhang, M.; Liu, T.; Liu, H.; Li, S.; Liu, Y.; Wang, Q. Improvement of Carotenoid Accumulation in Tomato Fruit. In Phytonutritional Improvement of Crops; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 309–338. [Google Scholar]
- Che, Y.; Zhang, N.; Zhu, X.; Li, S.; Wang, S.; Si, H. Enhanced tolerance of the transgenic potato plants overexpressing Cu/Zn superoxide dismutase to low temperature. Sci. Hortic. 2020, 261, 108949. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail, I.; Babar, M. Role of sugars, amino acids and organic acids in improving plant abiotic stress tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar]
- Hu, X.; Wu, Z.-D.; Luo, Z.-Y.; Burner, D.M.; Pan, Y.-B.; Wu, C.-W. Genome-wide analysis of the trehalose-6-phosphate synthase (TPS) gene family and expression profiling of ScTPS genes in sugarcane. Agronomy 2020, 10, 969. [Google Scholar] [CrossRef]
- Berestovoy, M.; Pavlenko, O.; Tyurin, A.; Gorshkova, E.; Goldenkova-Pavlova, I. Altered fatty acid composition of Nicotiana benthamiana and Nicotiana excelsior leaves under transient overexpression of the cyanobacterial desC gene. Biol. Plant. 2020, 64, 167–177. [Google Scholar] [CrossRef]
- Yang, W.; Wen, D.; Yang, Y.; Li, H.; Yang, C.; Yu, J.; Xiang, H. Metabolomics and transcriptomics combined with physiology reveal key metabolic pathway responses in tobacco roots exposed to NaHS. BMC Plant Biol. 2024, 24, 680. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; Huang, Y.; Pu, T.; Duan, L.; Pi, K.; Luo, J.; Long, B.; Lu, A.; Liu, R. Genome-wide identification and characterization of Glutathione S-Transferases (GSTs) and their expression profile under abiotic stresses in tobacco (Nicotiana tabacum L.). BMC Genom. 2023, 24, 341. [Google Scholar]
- Gómez, R.; Figueroa, N.; Melzer, M.; Hajirezaei, M.-R.; Carrillo, N.; Lodeyro, A.F. Photosynthetic characterization of flavodoxin-expressing tobacco plants reveals a high light acclimation-like phenotype. Biochim. Biophys. Acta-Bioenerg. 2020, 1861, 148211. [Google Scholar] [CrossRef]
- Unkel, K.; Krause, D.; Sprink, T.; Hartung, F.; Wilhelm, R. Mapping of plant SynBio developments in the agri-food sector. EFSA Support. Publ. 2020, 17, 1687E. [Google Scholar] [CrossRef]
- Szymonik, K.; Klimek-Chodacka, M.; Lukasiewicz, A.; Macko-Podgórni, A.; Grzebelus, D.; Baranski, R. Comparative analysis of the carrot miRNAome in response to salt stress. Sci. Rep. 2023, 13, 21506. [Google Scholar]
- Pandey, A.; Yadav, R.; Sanyal, I. Evaluating the pesticidal impact of plant protease inhibitors: Lethal weaponry in the co-evolutionary battle. Pest Manag. Sci. 2022, 78, 855–868. [Google Scholar]
- Kamarajugadda, S.; Daniell, H. Chloroplast-derived anthrax and other vaccine antigens: Their immunogenic and immunoprotective properties. Expert Rev. Vaccines 2006, 5, 839–849. [Google Scholar]
- Daniell, H.; Jin, S.; Zhu, X.G.; Gitzendanner, M.A.; Soltis, D.E.; Soltis, P.S. Green giant—A tiny chloroplast genome with mighty power to produce high-value proteins: History and phylogeny. Plant Biotechnol. J. 2021, 19, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Maliga, P. Plastid transformation in higher plants. Annu. Rev. Plant Biol. 2004, 55, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Birchler, J.A.; Yao, H.; Chudalayandi, S. Unraveling the genetic basis of hybrid vigor. Proc. Natl. Acad. Sci. USA 2006, 103, 12957–12958. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xiao, K.; Pan, H.; Liu, J. Chloroplast: The emerging battlefield in plant–microbe interactions. Front. Plant Sci. 2021, 12, 637853. [Google Scholar]
- Sperschneider, J.; Catanzariti, A.; DeBoer, K.; Petre, B.; Gardiner, D.; Singh, K.; Dodds, P.; Taylor, J. LOCALIZER: Subcellular localization prediction of both plant and effector proteins in the plant cell. Sci Rep. Nat. Publ. Group 2017, 7, 44598. [Google Scholar] [CrossRef]


| Plant Species | Chloroplast Genome Size (bp) | Inverted Repeat (IR) Size (bp) | Single-Copy Regions (LSCs and SSCs) | Number of Genes | Reference |
|---|---|---|---|---|---|
| Tobacco (Nicotiana tabacum) | 120,000 247,000 | IR: 25,342 | LSCs: 86,686; SSCs: 18,572 | 110–150 genes | [46] |
| Asarum minus | 15,553 | IR: 51,767 | LSCs: 88,643; SSCs: 19,504 | 20–30 genes (reduced) | [47] |
| Floydiella terrestris | 521,168 | IR: 521,168 | Not Annotated | ~200 genes (largest cpDNA) | [48] |
| Arabidopsis thaliana | ~154,000 | IR: 26,263 | LSCs: 84,197; SSCs: 17,780 | 131 genes (97 protein-coding) | [25] |
| Lamiaceae species (average) | 149,081–152,312 | 2 IRs (with average 27%) | LSCs: ~82,000; SSCs: ~17,000 | 131–133 genes (86–88 protein-coding) | [32] |
| Maize (Zea mays) | 140,387 | IRa and IRb | LSCs: 82,355; SSCs: 12,536 | 120–130 genes (in some species) | [49] |
| Lima bean | 150,902 | 26,543 | LSCs: 80,218; SSCs: 17,598 | 130–140 genes | [34] |
| Cuscuta (parasitic plant) | 96,292 | None | None | <50 genes (photosynthetic loss) | [50] |
| Zonal geranium | 217,942 | 26,000 | LSCs: 130,000; SSCs: 25,000 | 200–210 genes | [51] |
| Quercus ningangensis | 160,736 | 26,000 | LSCs: 88,000; SSCs: 22,000 | 130–140 genes | [52] |
| Sicyos angulatus | 154,986 | IR: 26,276 | LSCs: 84,355; SSCs: 18,079 | 130–140 genes | [38] |
| Pistacia vera | 160,589 | 26,547 | LSCs: 88,174; SSCs: 19,330 | 130–140 genes | [39] |
| Sphaeropteris lepifera | 162,114 | IRa, IRb: 24,028 | LSCs: 86,327; SSCs: 27,731 | 140–150 genes | [40] |
| Datura and Brugmansia | 154,686–155,979 | 26,500 | LSCs: 82,000; SSCs: 17,000 | 130–150 genes | [41] |
| Xanthium spinosum | 152,422 | 25,000 | LSCs: 84,000; SSCs: 18,000 | 120–130 genes | [42] |
| Plant Species | Transgene | Type of Stress | Tolerated Stress Treatment | References |
|---|---|---|---|---|
| Nicotiana tabacum (tobacco) | TPS1 (trehalose phosphate synthase) | Drought and osmotic stress | 24 days of drought, 6% PEG | [217] |
| Nicotiana tabacum (tobacco) | merAB operon | Heavy metal stress (phytoremediation) | 400 μM phenyl-mercuric acetate, 300 μM HgCl2 | [22] |
| Nicotiana tabacum (tobacco) | Des (fatty acid desaturase) | Chilling and cold stress | Leaf discs at 4 °C for 72 h, seedlings at 2 °C for 9 days | [218] |
| Nicotiana tabacum (tobacco) | CMO (choline monooxygenase) | Toxic levels of choline, salt, and drought stress | 30 mM choline, 150 mM NaCl, 300 mM mannitol | [16] |
| Nicotiana tabacum (tobacco) | panD (aspartate decarboxylase) | High-temperature stress | 40 °C for 10 h/day for 1 week, or 45 °C for 4 h | [219] |
| Nicotiana tabacum (tobacco) | DHAR, GST, DHAR, gor, and GST gor (dehydroascorbate reductase) | Salt, chilling, and oxidative stress | 200 mM NaCl, 12 days of germination at 15 °C, leaf discs at 8 °C for 8 h | [220] |
| Nicotiana tabacum (tobacco) | Fld (flavodoxin) | Oxidative stress | 100 μM paraquat up to 24 h | [221] |
| Nicotiana tabacum (tobacco) | HPT, TCY, TMT (tocopherol biosynthesis pathway) | Oxidative and cold stress | 1 month at 4 °C | [222] |
| Daucus carota (carrot) | Badh (betaine-aldehyde dehydrogenase) | Salt stress | Maximum of 400 mM NaCl for 4 weeks | [223] |
| Nicotiana benthamiana | sporamin, CeCPI, chitinase | Salt, osmotic, and oxidative stress | Maximum of 400 mM NaCl, 3% PEG, 10 μM paraquat | [224] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, W.; Wu, Y.; Li, S.; Hua, B.; Sun, H. Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective. Plants 2025, 14, 978. https://doi.org/10.3390/plants14060978
Zhang C, Li W, Wu Y, Li S, Hua B, Sun H. Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective. Plants. 2025; 14(6):978. https://doi.org/10.3390/plants14060978
Chicago/Turabian StyleZhang, Chunhua, Wenting Li, Yahan Wu, Shengli Li, Bao Hua, and Haizhou Sun. 2025. "Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective" Plants 14, no. 6: 978. https://doi.org/10.3390/plants14060978
APA StyleZhang, C., Li, W., Wu, Y., Li, S., Hua, B., & Sun, H. (2025). Chloroplast Functionality at the Interface of Growth, Defense, and Genetic Innovation: A Multi-Omics and Technological Perspective. Plants, 14(6), 978. https://doi.org/10.3390/plants14060978






