Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”
Abstract
1. Introduction
2. Results
2.1. Evaluation of Stripe Rust and Leaf Rust Resistance at the Seedling Stage
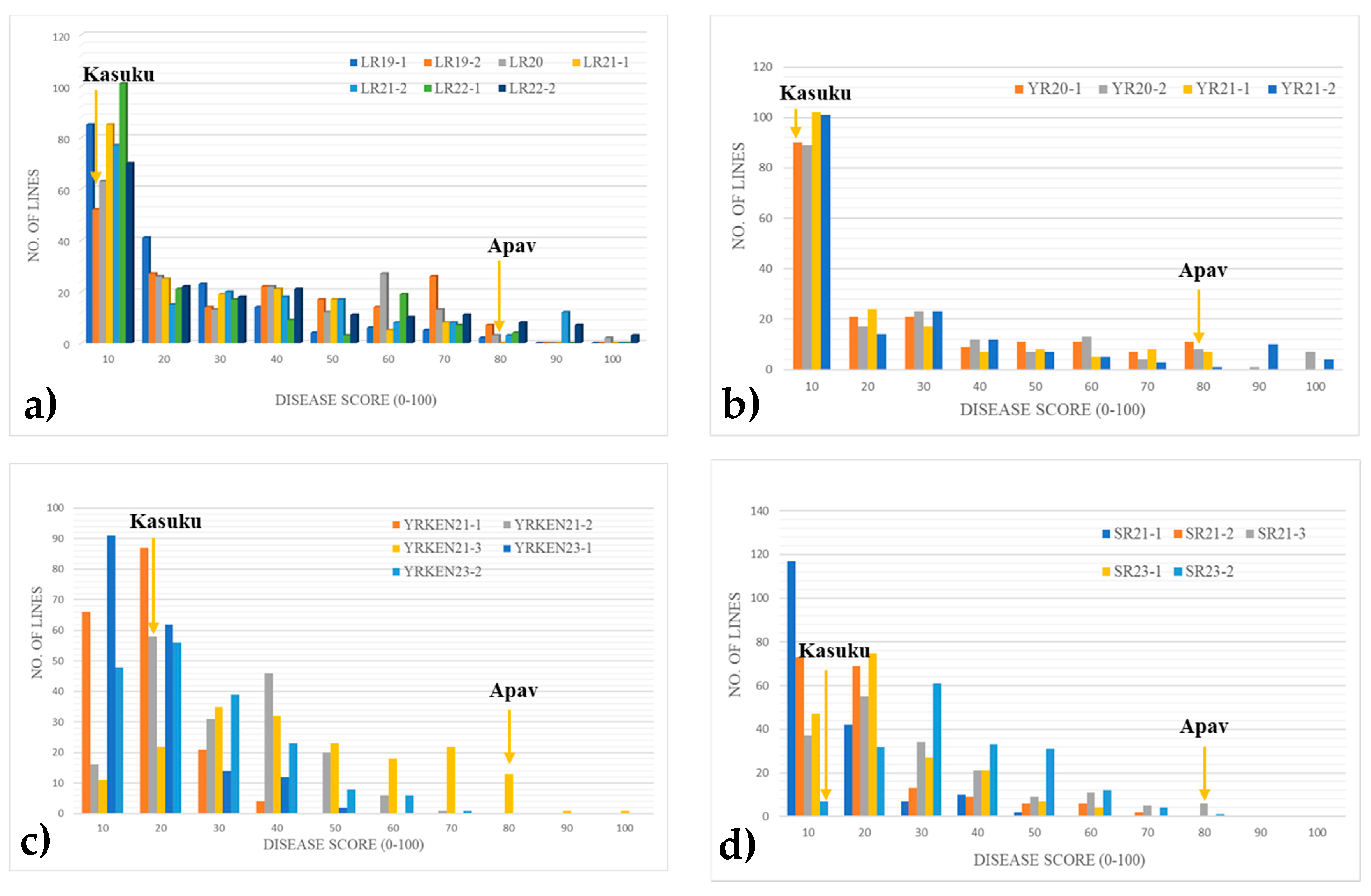
2.2. Evaluation of Stripe Rust, Leaf Rust, and Stem Resistance at the Adult Plant Stage
2.3. Environmental Influence on Disease Development
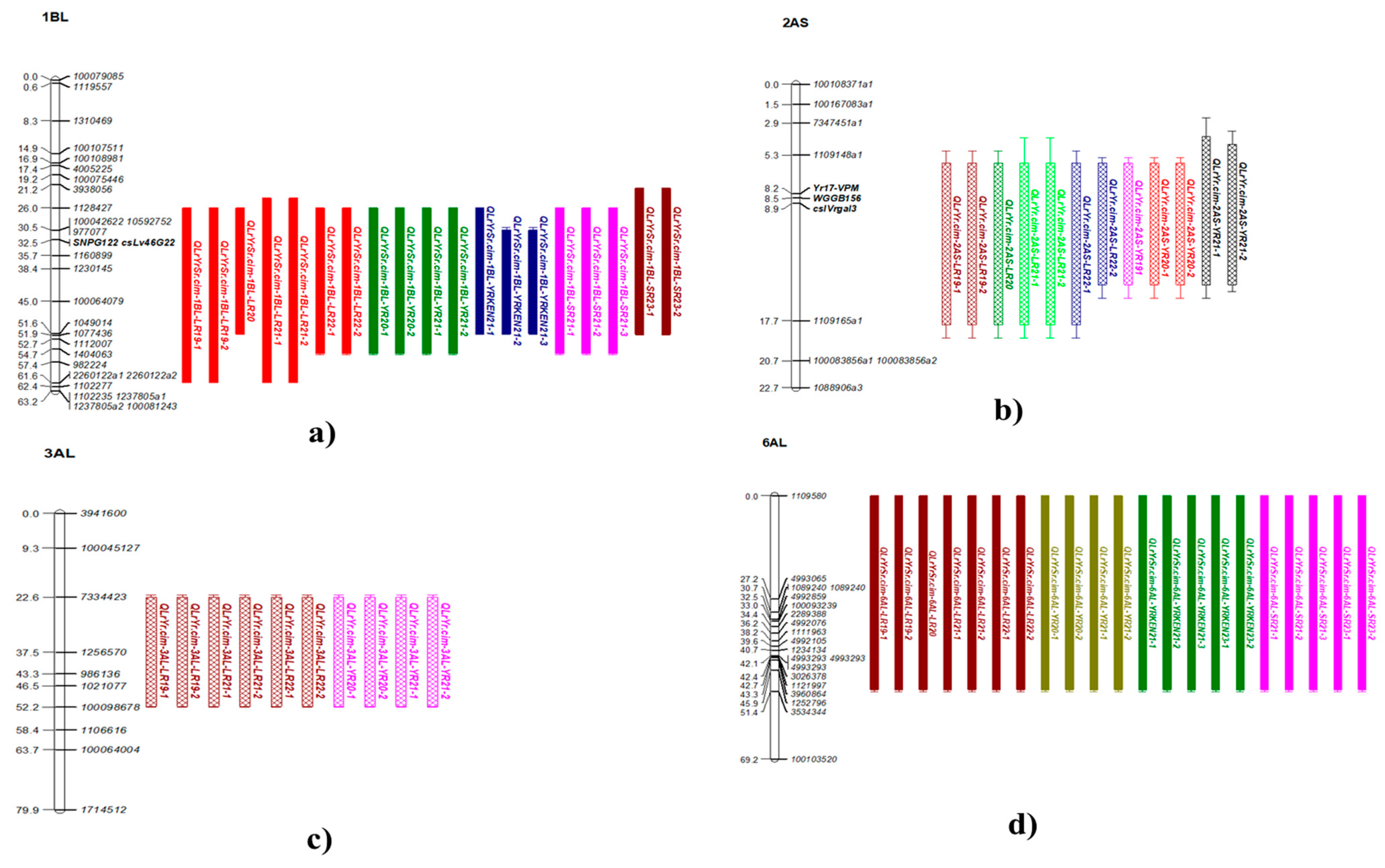
2.4. Linkage Map Construction
2.5. Significant QTL Across Environments
2.5.1. Co-Located Rust Resistance QTL
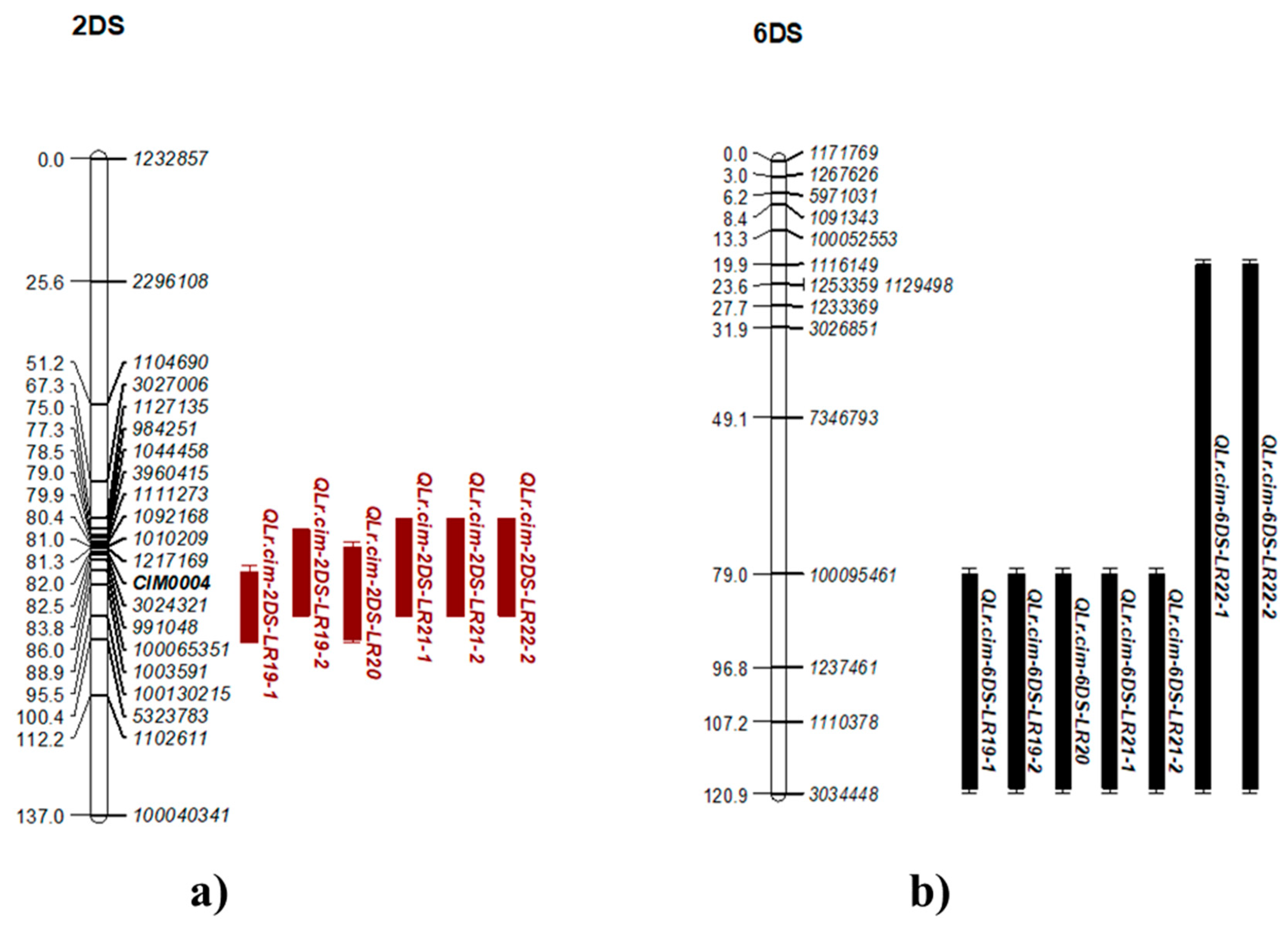
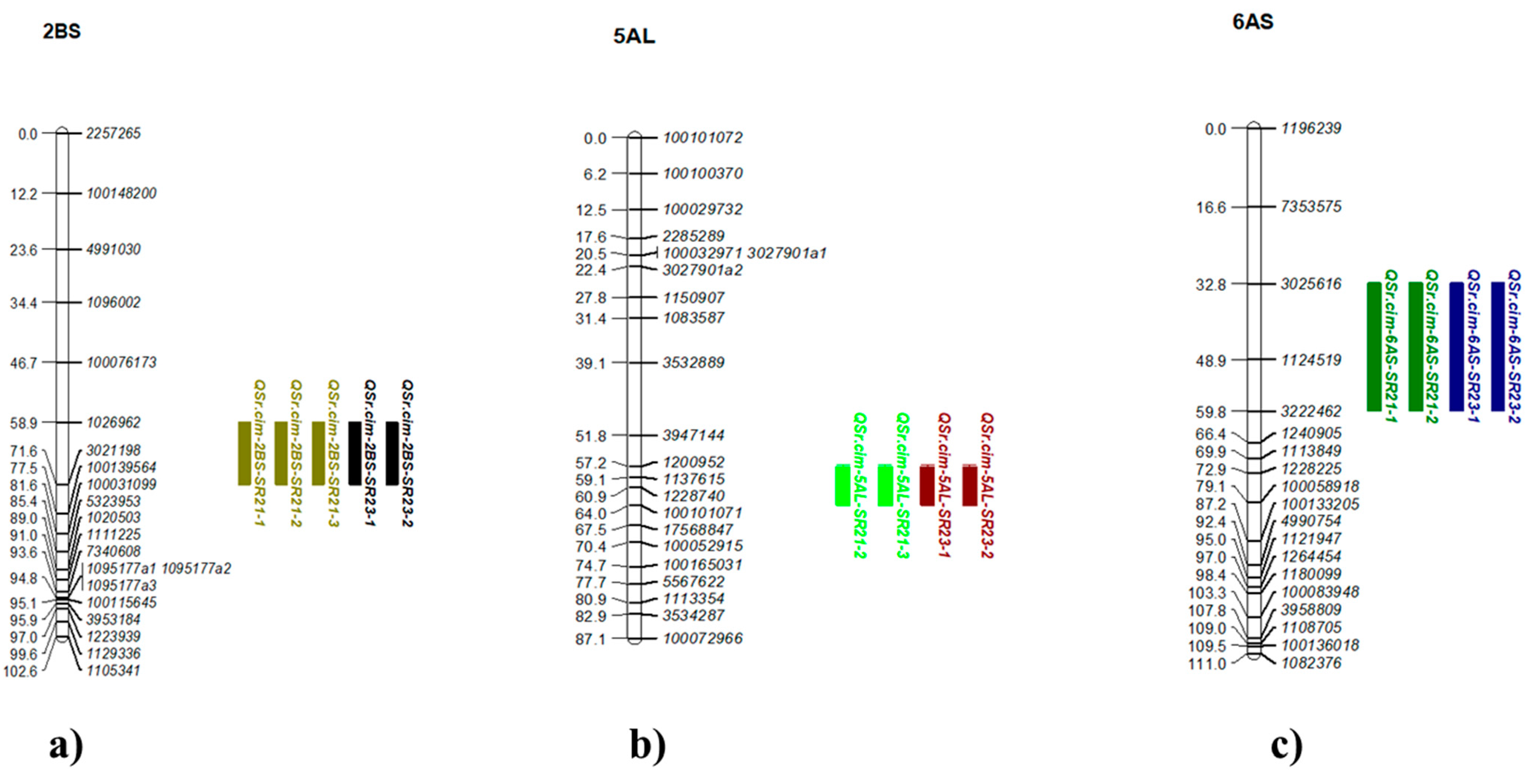
2.5.2. Other Minor QTL for Leaf Rust, Yellow Rust, and Stem Rust
2.6. Cumulative Interaction of Multiple QTL
3. Discussion
3.1. Co-Located QTL Regions
3.1.1. Genomic Regions Associated with Triple Rust Resistance
3.1.2. Genomic Regions Associated with Dual Rust Resistance
3.2. Minor QTL Regions
3.2.1. Genomic Regions Associated with LR Resistance
3.2.2. Genomic Regions Associated with YR Resistance
3.2.3. Genomic Regions Associated with SR Resistance
4. Materials and Methods
4.1. Plant and Pathogen Materials
4.2. Greenhouse Screening
4.3. Field Screening
4.4. Statistical Analysis
4.5. Genotyping and Linkage Map Construction
4.6. QTL Analysis
4.7. Evaluation of Marker Selectable Resistance Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Saari, E.E.; Prescott, J.M. World distribution in relation to economic losses. In The Cereal Rusts Volume II: Diseases, Distribution, Epidemiology and Control; Roelfs, A.P., Bushnell, W.R., Eds.; Academic Press: Orlando, FL, USA, 1985; pp. 259–298. [Google Scholar]
- Figueroa, M.; Hammond-Kosack, K.E.; Solomon, P.S. A review of wheat diseases-a field perspective. Mol. Plant Pathol. 2018, 19, 1523–1536. [Google Scholar]
- Qureshi, N. Rust Resistance in Wheat: Gene Discovery and Development of Molecular Markers Using Diverse Genomic Resources. Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2017. Available online: http://hdl.handle.net/2123/18003 (accessed on 21 August 2024).
- Oliver, R.P. A reassessment of the risk of rust fungi developing resistance to fungicides. Pest Manag. Sci. 2014, 70, 1641–1645. [Google Scholar] [CrossRef] [PubMed]
- Bariana, H.S. Breeding for Disease Resistance. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Harcourt, Academic Press: Oxford, UK, 2003; pp. 244–253. [Google Scholar]
- Chen, X.M. Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat. Can. J. Plant Pathol. 2005, 27, 314–337. [Google Scholar] [CrossRef]
- Ellis, J.G.; Lagudah, E.S.; Spielmeyer, W.; Dodds, P.N. The past, present and future of breeding rust resistant wheat. Front. Plant Sci. 2014, 5, 641. [Google Scholar] [CrossRef]
- Kolmer, J.A. Genetics of resistance to wheat leaf rust. Annu. Rev. Phytopathol. 1996, 34, 435–455. [Google Scholar] [PubMed]
- Sucher, J.; Boni, R.; Yang, P.; Rogowsky, P.; Büchner, H.; Kastner, C.; Kumlehn, J.; Krattinger, S.G.; Keller, B. The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol. J. 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Agrios, G. Plant Pathology, 5th ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; Volume 26–27, pp. 398–401. [Google Scholar]
- Kolmer, J. Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 2013, 4, 70–84. [Google Scholar] [CrossRef]
- Brunner, S.; Stirnweis, D.; Diaz Quijano, C.; Buesing, G.; Herren, G.; Parlange, F.; Barret, P.; Tassy, C.; Sautter, C.; Winzeler, M.; et al. Transgenic Pm3 multilines of wheat show increased powdery mildew resistance in the field. Plant Biotechnol. J. 2012, 10, 398–409. [Google Scholar] [CrossRef]
- Dyck, P.L.; Samborsk, D.J.; Anderson, R.G. Inheritance of adult-plant leaf rust resistance derived from common wheat varieties Exchange and Frontana. Can. J. Genet. Cytol. 1966, 8, 665–671. [Google Scholar] [CrossRef]
- Singh, R.P. Genetic association of leaf rust resistance gene Lr34 with adult plant resistance to stripe rust in bread wheat. Phytopathol 1992, 82, 835–838. [Google Scholar] [CrossRef]
- Spielmeyer, W.; McIntosh, R.A.; Kolmer, J.; Lagudah, E.S. Powdery mildew resistance and Lr34/Yr18 genes for durable resistance to leaf and stripe rust cosegregate at a locus on the short arm of chromosome 7D of wheat. Theor. Appl. Genet. 2005, 111, 731–735. [Google Scholar] [CrossRef]
- Lillemo, M.; Asalf, B.; Singh, R.P.; Huerta-Espino, J.; Chen, X.M.; He, Z.H.; Bjørnstad, A. The adult plant rust resistance loci Lr34/Yr18 and Lr46/Yr29 are important determinants of partial resistance to powdery mildew in bread wheat line Saar. Theor. Appl. Genet. 2008, 116, 1155–1166. [Google Scholar] [CrossRef]
- Singh, R.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Bariana, H.S.; Bansal, U.; McCallum, B.D.; Hiebert, C.; Bhavani, S.; Singh, S.; Lan, C.X.; et al. Lr34/Yr18/Sr57/Pm38/Bdv1/Ltn1 confers slow rusting, adult plant resistance to Puccinia graminis f. sp. tritici. In Proceedings of the 13th Cereal Rust and Powdery Mildew Conference, Beijing, China, 28 August–1 September 2012; China Agricultural Science and Technology Press: Beijing, China, 2012; p. 173. [Google Scholar]
- Lillemo, M.; Joshi, A.K.; Prasad, R.; Chand, R.; Singh, R.P. QTL for spot blotch in bread wheat line Saar co-locate to the biotrophic disease resistance loci Lr34 and Lr46. Theor. Appl. Genet. 2013, 126, 711–719. [Google Scholar] [CrossRef]
- Singh, R.P.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.; Basnet, B.R.; Bhavani, S.; Lagudah, E.S. Pleiotropic gene Lr46/Yr29/Pm39/Ltn2 confers slow rusting.; adult plant resistance to wheat stem rust fungus. In Proceedings of the Borlaug Global Rust Initiative, 2013 Technical Workshop, New Delhi, India, 19–22 August 2013; p. 17.1. [Google Scholar]
- Herrera-Foessel, S.A.; Singh, R.P.; Lillemo, M.; Huerta-Espino, J.; Bhavani, S.; Singh, S.; Lan, C.; Calvo-Salazar, V.; Lagudah, E.S. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 2014, 127, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Dyck, P.L. Genetics of adult-plant leaf rust resistance in ‘Chinese spring’ and ‘sturdy’ wheats. Crop Sci. 1991, 3, 309–311. [Google Scholar] [CrossRef]
- Lagudah, E.S.; McFadden, H.; Singh, R.P.; Huerta-Espino, J.; Bariana, H.S.; Spielmeyer, W. Molecular genetic characterization of the Lr34⁄Yr18 slow rusting resistance gene region in wheat. Theor. Appl. Genet. 2006, 114, 21–30. [Google Scholar] [CrossRef]
- Shah, S.J.A.; Hussain, S.; Ahmad, M.; Farhatullah, A.I.; Ibrahim, M. Using leaf tip necrosis as a phenotypic marker to predict the presence of durable rust resistance gene pair Lr34/Yr18 in wheat. J. Gen. Plant Pathol. 2011, 77, 174–177. [Google Scholar] [CrossRef]
- Singh, R.P. Association between gene Lr34 for leaf rust resistance and leaf tip necrosis in wheat. Crop Sci. 1992, 32, 874–878. [Google Scholar] [CrossRef]
- McFadden, E.S. A successful transfer of emmer characters to vulgare wheat. J. Am. Soc. Agron. 1930, 22, 1020–1034. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Rajaram, S. Achieving near-immunity to leaf and stripe rusts in wheat by combining slow rusting resistance genes. Acta Phytopathol. Entomol. Hung. 2000, 35, 133–139. [Google Scholar]
- da Silva, G.B.P.; Zanella, C.M.; Martinelli, J.A.; Chaves, M.S.; Hiebert, C.W.; McCallum, B.D.; Boyd, L.A. Quantitative trait loci conferring leaf rust resistance in hexaploid wheat. Phytopathology 2018, 108, 1344–1354. [Google Scholar]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A.; et al. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc. Natl. Acad. Sci. USA 2013, 110, 8057–8062. [Google Scholar]
- Wang, S.; Wong, D.; Forrest, K.; Allen, A.; Chao, S.; Huang, B.E.; Maccaferri, M.; Salvi, S.; Milner, S.G.; Cattivelli, L.; et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnol. J. 2014, 12, 787–796. [Google Scholar] [CrossRef]
- Akbari, M.; Wenzl, P.; Caig, V.; Carling, J.; Xia, L.; Yang, S.; Uszynski, G.; Mohler, V.; Lehmensiek, A.; Kuchel, H.; et al. Diversity arrays technology (DArT) for high-throughput profiling of the hexaploid wheat genome. Theor. Appl. Genet. 2006, 113, 1409–1420. [Google Scholar]
- International Wheat Genome Sequencing Consortium (IWGSC). Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 2018, 361, 661. [Google Scholar]
- Qureshi, N.; Singh, R.P.; Gonzalez, B.M.; Velazquez-Miranda, H.; Bhavani, S. Genomic regions associated with resistance to three rusts in CIMMYT wheat line “Mokue#1”. Int. J. Mol. Sci. 2023, 24, 12160. [Google Scholar] [CrossRef]
- Singh, R.P.; Huerta-Espino, J.; Bhavani, S.; Herrera-Foessel, S.A.; Singh, D.; Singh, P.K.; Velu, G.; Mason, R.E.; Jin, Y.; Njau, P.; et al. Race non-specific resistance to rust diseases in CIMMYT spring wheats. Euphytica 2011, 179, 175–186. [Google Scholar]
- Kolmer, J.A. A QTL on chromosome 5BL in wheat enhances leaf rust resistance of Lr46. Mol. Breed. 2015, 35, 74–81. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Lan, C.X.; He, Z.H. Quantitative trait loci of stripe rust resistance in wheat. Theor. Appl. Genet. 2013, 126, 2427–2449. [Google Scholar]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; William, H.M.; Bouchet, S.; Cloutier, S.; McFadden, H.; Lagudah, E.S. Leaf tip necrosis, molecular markers and beta1-proteasome subunits associated with the slow rusting resistance genes Lr46/Yr29. Theor. Appl. Genet. 2006, 112, 500–508. [Google Scholar] [CrossRef]
- Marais, G.F.; Badenhorst, P.E.; Eksteen, A.; Pretorius, Z.A. Reduction of Aegilops sharonensis chromatin associated with resistance genes Lr56 and Yr38 in wheat. Euphytica 2010, 171, 15–22. [Google Scholar] [CrossRef]
- Marais, F.; Marais, A.; McCallum, B.; Pretorius, Z. Transfer of leaf rust and stripe rust resistance genes Lr62 and Yr42 from Aegilops neglecta Req. ex Bertol. to common wheat. Crop Sci. 2009, 49, 871–879. [Google Scholar] [CrossRef]
- Cao, J.; Deng, Z.Y.; Wang, M.N.; Wang, X.P.; Jing, J.X.; Zhang, X.Q.; Shang, H.S.; Li, Z.Q. Inheritance and molecular mapping of an alien stripe-rust resistance gene from a wheat-Psathyrostachys huashanica translocation line. Plant Sci. 2008, 174, 544–549. [Google Scholar]
- Kolmer, J.; Bernardo, A.; Bai, G.; Hayden, M.; Anderson, J. Thatcher wheat line RL6149 carries Lr64 and a second leaf rust resistance gene on chromosome 1DS. Theor. Appl. Genet. 2019, 132, 2809–2814. [Google Scholar]
- Ren, Y.; Singh, R.P.; Basnet, B.R.; Lan, C.X.; Huerta-Espino, J.; Lagudah, E.S.; Ponce-Molina, L.J. Identification and mapping of adult plant resistance loci to leaf rust and stripe rust in common wheat cultivar Kundan. Plant Dis. 2017, 101, 456–463. [Google Scholar] [CrossRef]
- Beukert, U.; Liu, G.; Thorwarth, P.; Boeven, P.H.G.; Longin, C.F.H.; Zhao, Y.; Ganal, M.; Serfling, A.; Ordon, F.; Reif, J.C. The potential of hybrid breeding to enhance leaf rust and stripe rust resistance in wheat. Theor Appl Genet. 2020, 133, 2171–2181. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Dieseth, J.A.; Alsheikh, M.; Yang, E.; Holzapfel, J.; Schürmann, F.; Morales, L.; Michel, S.; Buerstmayr, H.; Bhavani, S.; et al. A major yellow rust resistance QTL on chromosome 6A shows increased frequency in recent Norwegian spring wheat cultivars and breeding lines. Theor. Appl. Genet. 2023, 136, 164. [Google Scholar] [CrossRef] [PubMed]
- Shahinnia, F.; Geyer, M.; Schürmann, F.; Rudolphi, S.; Holzapfel, J.; Kempf, H.; Stadlmeier, M.; Löschenberger, F.; Morales, L.; Buerstmayr, H.; et al. Genome-wide association study and genomic prediction of resistance to stripe rust in current Central and Northern European winter wheat germplasm. Theor. Appl. Genet. 2022, 135, 3583–3595. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.M.; Schulthess, A.W.; Padmarasu, S.; Boeven, P.H.G.; Schacht, J.; Himmelbach, A.; Steuernagel, B.; Wulff, B.B.H.; Reif, J.C.; Stein, N.; et al. Catalogue of resistance gene homologs and a chromosome-scale reference sequence support resistance gene mapping in winter wheat. Plant Biotechnol. J. 2022, 20, 1730–1742. [Google Scholar] [CrossRef]
- Bouvet, L.; Percival-Alwyn, L.; Berry, S.; Fenwick, P.; Mantello, C.C.; Sharma, R.; Holdgate, S.; Mackay, I.J.; Cockram, J. Wheat genetic loci conferring resistance to stripe rust in the face of genetically diverse races of the fungus Puccinia striiformis f. sp. tritici. Theor. Appl. Genet. 2022, 135, 301–319. [Google Scholar]
- Cheng, B.; Gao, X.; Cao, N.; Ding, Y.; Chen, T.; Zhou, Q.; Gao, Y.; Xin, Z.; Zhang, L. QTL mapping for adult plant resistance to wheat stripe rust in M96-5 × Guixie 3 wheat population. J. Appl. Genet. 2022, 63, 265–279. [Google Scholar]
- Feng, J.; Chen, G.; Wei, Y.; Liu, Y.; Jiang, Q.; Li, W.; Pu, Z.; Lan, X.; Dai, S.; Zheng, Y. Identification and genetic mapping of a recessive gene for resistance to stripe rust in wheat line LM168-1. Mol. Breed. 2014, 33, 601–609. [Google Scholar] [CrossRef]
- Bulli, P.; Zhang, J.; Chao, S.; Chen, X.; Pumphrey, M. Genetic architecture of resistance to stripe rust in a global winter wheat germplasm collection. G3 Genes Genomes Genet. 2016, 6, 2237–2253. [Google Scholar] [CrossRef]
- Dolores Vazquez, M.; James Peterson, C.; Riera-Lizarazu, O.; Chen, X.; Heesacker, A.; Ammar, K.; Crossa, J.; Mundt, C.C. Genetic analysis of adult plant.; quantitative resistance to stripe rust in wheat cultivar “Stephens” in multi-environment trials. Theor. Appl. Genet. 2012, 124, 1–11. [Google Scholar] [CrossRef]
- William, H.M.; Singh, R.P.; Huerta-Espino, J.; Palacios, G.; Suenaga, K. Characterization of genetic loci conferring adult plant resistance to leaf rust and stripe rust in spring wheat. Genome 2006, 49, 977–990. [Google Scholar]
- Vazquez, M.D.; Zemetra, R.; Peterson, C.J.; Chen, X.M.; Heesacker, A.; Mundt, C.C. Multi-location wheat stripe rust QTL analysis: Genetic background and epistatic interactions. Theor. Appl. Genet. 2015, 128, 1307–1318. [Google Scholar]
- Zhang, L.; Li, Z.; Lillemo, M.; Xia, X.; Liu, D.; Yang, W.; Luo, J.; Wang, H. QTL mapping for adult-plant resistance to leaf rust in CIMMYT wheat cultivar Saar. Agric. Food Sci. 2009, 42, 388–397. [Google Scholar]
- Kumar, D.; Kumar, A.; Chhokar, V.; Gangwar, O.P.; Bhardwaj, S.C.; Sivasamy, M.; Prasad, S.V.S.; Prakasha, T.L.; Khan, H.; Singh, R.; et al. Genome-wide association studies in diverse spring wheat panel for stripe, stem, and leaf rust resistance. Front. Plant Sci. 2020, 11, 748. [Google Scholar]
- Sharma, J.S.; Zhang, Q.; Rouse, M.N.; Klindworth, D.L.; Friesen, T.L.; Long, Y.; Olivera, P.D.; Jin, Y.; McClean, P.E.; Xu, S.S.; et al. Mapping and characterization of two stem rust resistance genes derived from cultivated emmer wheat accession PI 193883. Theor. Appl. Genet. 2019, 132, 3177–3189. [Google Scholar] [CrossRef]
- Sharma, J.S.; Che, M.; Fetch, T.; McCallum, B.D.; Xu, S.S.; Hiebert, C.W. Identification of Sr67, a new gene for stem rust resistance in KU168-2 located close to the Sr13 locus in wheat. Theor. Appl. Genet. 2024, 137, 30. [Google Scholar] [CrossRef] [PubMed]
- Megerssa, S.H.; Ammar, K.; Acevedo, M.; Brown-Guedira, G.; Ward, B.; Degete, A.G.; Randhawa, M.S.; Sorrells, M.E. Multiple-race stem rust resistance loci identified in durum wheat using genome-wide association mapping. Front. Plant Sci. 2020, 11, 598509. [Google Scholar] [CrossRef] [PubMed]
- Marone, D.; Mazzucotelli, E.; Matny, O.; Desiderio, F.; Sciara, G.; Maccaferri, M.; Marcotuli, I.; Gadaleta, A.; Steffenson, B.; Mastrangelo, A.M. QTL mapping of stem rust resistance in populations of durum wheat. Genes 2022, 13, 1793. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Koo, D.H.; Juliana, P.; Rife, T.; Singh, D.; Lemes da Silva, C.; Lux, T.; Dorn, K.M.; Clinesmith, M.; Silva, P.; et al. The Aegilops ventricosa 2NvS segment in bread wheat: Cytology, genomics and breeding. Theor. Appl. Genet. 2021, 134, 529–542. [Google Scholar] [CrossRef]
- Helguera, M.; Khan, I.A.; Kolmer, J.; Lijavetzky, D.; Zhong-Qi, L.; Dubcovsky, J. PCR assays for the Lr37-Yr17-Sr38 cluster of rust resistance genes and their use to develop isogenic hard red spring wheat lines. Crop Sci. 2003, 43, 1839–1847. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, C.; Singh, R.P.; Randhawa, M.S.; Bhavani, S.; Kumar, U.; Huerta-Espino, J.; Lagudah, E.; Lan, C. Stripe rust and leaf rust resistance in CIMMYT wheat line “Mucuy” is conferred by combinations of race-specific and adult-plant resistance loci. Front. Plant Sci. 2022, 13, 880138. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Chen, X.M.; Wan, A.M.; Zhan, G.M.; Kang, Z.S.; Cao, S.Q.; Jin, S.L.; Morgounov, A.; Akin, B.; Mert, Z.; et al. Virulence characterization of international collections of the wheat stripe rust pathogen, Puccinia striiformis f. sp. tritici. Plant Dis. 2013, 97, 379–386. [Google Scholar] [CrossRef]
- Hovmøller, M.S. Report for Puccinia striiformis Race Analysis 2013, Global Rust Reference Center (GRRC), Aarhus University, Flakkebjerg, DK-4200 Slagelse, Denmark. 2014. Available online: https://agro.au.dk/fileadmin/Summary_of_Puccinia_striiformis_race_analyses_2013.pdf (accessed on 1 May 2024).
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Rosewarne, G.M.; Periyannan, S.K.; Viccars, L.; Calvo-Salazar, V.; Lan, C.; Lagudah, E.S. Lr68: A new gene conferring slow rusting resistance to leaf rust in wheat. Theor. Appl. Genet. 2012, 124, 1475–1486. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Friebe, B.; Jiang, J.; The, D.; Gill, B.S. Cytogenetical studies in wheat XVI. Chromosome location of a new gene for resistance to leaf rust in a Japanese wheat-rye translocation line. Euphytica 1995, 82, 141–147. [Google Scholar] [CrossRef]
- Xue, S.; Kolmer, J.A.; Wang, S.; Yan, L. Mapping of leaf rust resistance genes and molecular characterization of the 2NS/2AS translocation in the wheat cultivar Jagger. G3 Genes Genomes Genet. 2018, 8, 2059–2065. [Google Scholar] [CrossRef] [PubMed]
- Rauf, Y.; Lan, C.; Randhawa, M.; Singh, R.P.; Huerta-Espino, J.; Anderson, J.A. Quantitative trait loci mapping reveals the complexity of adult plant resistance to leaf rust in spring wheat ‘Copio’. Crop Sci. 2022, 62, 1037–1050. [Google Scholar] [CrossRef]
- Chu, C.G.; Friesen, T.L.; Xu, S.S.; Faris, J.D.; Kolmer, J.A. Identification of novel QTLs for seedling and adult plant leaf rust resistance in a wheat doubled haploid population. Theor. Appl. Genet. 2009, 119, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Gebrewahid, T.W.; Zhang, P.; Zhou, Y.; Yan, X.; Xia, X.; He, Z.; Liu, D.; Li, Z. QTL mapping of adult plant resistance to stripe rust and leaf rust in a Fuyu 3/Zhengzhou 5389 wheat population. Crop J. 2020, 8, 655–665. [Google Scholar] [CrossRef]
- Zhang, P.; Yan, X.; Gebrewahid, T.W.; Zhou, Y.; Yang, E.; Xia, X.; He, Z.; Li, Z.; Liu, D. Genome-wide association mapping of leaf rust and stripe rust resistance in wheat accessions using the 90K SNP array. Theor. Appl. Genet. 2021, 134, 1233–1251. [Google Scholar]
- Tehseen, M.M.; Tonk, F.A.; Tosun, M.; Randhawa, H.S.; Kurtulus, E.; Ozseven, I.; Akin, B.; Nur Zulfuagaoglu, O.; Nazari, K. QTL Mapping of adult plant resistance to stripe rust in a doubled haploid wheat population. Front. Genet. 2022, 13, 900558. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Welling, C.R.; Park, R.F. Wheat Rusts, An Atlas of Resistance Genes; CSIRO: Melbourne, Australia, 1995; p. 200. [Google Scholar]
- McIntosh, R.A.; Yamazaki, Y.; Dubcovsky, J.; Rogers, J.; Morris, C.; Somers, D.J.; Appels, R.; Devos, K.M. Catalogue of Gene Symbols for Wheat, National BioResource Project, Komugi-Wheat Genetic Resources Database. 2008. Available online: http://shigen.nig.ac.jp/wheat/komugi/genes/download.jsp (accessed on 1 January 2024).
- Lan, C.; Hale, I.L.; Herrera-Foessel, S.A.; Basnet, B.R.; Randhawa, M.S.; Huerta-Espino, J.; Dubcovsky, J.; Singh, R.P. Characterization and mapping of leaf rust and stripe rust resistance loci in hexaploid wheat lines UC1110 and PI610750 under Mexican environments. Front. Plant Sci. 2017, 8, 1450. [Google Scholar]
- Zhang, R.; Singh, R.P.; Lillemo, M.; He, X.; Randhawa, M.S.; Huerta-Espino, J.; Singh, P.K.; Li, Z.; Lan, C. Two main stripe rust resistance genes identified in synthetic-derived wheat line Soru# 1. Phytopathology 2019, 109, 120–126. [Google Scholar]
- Cobo, N.; Pflüger, L.; Chen, X.M.; Dubcovsky, J. Mapping QTL for resistance to new virulent races of wheat stripe rust from two Argentinean wheat cultivars. Crop Sci. 2018, 58, 2470–2483. [Google Scholar] [CrossRef]
- Basnet, B.R.; Singh, R.P.; Ibrahim, A.M.H.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Lan, C.; Rudd, J.C. Characterization of Yr54 and other genes associated with adult plant resistance to yellow rust and leaf rust in common wheat Quaiu 3. Mol. Breed. 2014, 33, 385–399. [Google Scholar] [CrossRef]
- Yang, E.N.; Rosewarne, G.M.; Herrera-Foessel, S.A.; Huerta-Espino, J.; Tang, Z.X.; Sun, C.F.; Ren, Z.L.; Singh, R.P. QTL analysis of the spring wheat “Chapio” identifies stable stripe rust resistance despite inter-continental genotype × environment interactions. Theor. Appl. Genet. 2013, 126, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Dedryver, F.; Paillard, S.; Mallard, S.; Robert, O.; Trottet, M.; Nègre, S.; Verplancke, G.; Jahier, J. Characterization of genetic components involved in durable resistance to stripe rust in the bread wheat ‘Renan’. Phytopathology 2009, 99, 968–973. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Liu, C.; Shi, Q.; Huang, Y.; Han, F. Centromere satellite repeats have undergone rapid changes in polyploid wheat subgenomes. Plant Cell 2019, 31, 2035–2051. [Google Scholar] [CrossRef]
- Somers, D.J.; Isaac, P.; Edwards, K. A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2004, 109, 1105–1114. [Google Scholar] [CrossRef]
- Bariana, H.S.; Kant, L.; Qureshi, N.; Forrest, K.; Miah, H.; Bansal, U. Identification and characterisation of stripe rust resistance genes Yr66 and Yr67 in wheat cultivar VL Gehun 892. Agronomy 2022, 12, 318. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Lang, J.; Ma, S.Q.; Zhang, B.S. Genetic analysis and SSR mapping on a new stem stripe rust resistance gene YrY206 in Aegilops tauschii. Chin. J. Biotechnol. 2008, 24, 1475–1479. [Google Scholar]
- Sun, C.; Zhang, P.; Fang, Z.W.; Zhang, X.; Yin, J.L.; Ma, D.F.; Zhu, Y. Genetic analysis and molecular mapping of stripe rust resistance in an excellent wheat line Sanshumai1. J. Plant Pathol. 2019, 101, 235–241. [Google Scholar] [CrossRef]
- Huang, S.; Liu, S.J.; Zhang, Y.B.; Xie, Y.Z.; Wang, X.T.; Jiao, H.X.; Wu, S.; Zeng, Q.; Wang, Q.; Singh, R.P.; et al. Genome-wide wheat 55K SNP-based mapping of stripe rust resistance loci in wheat cultivar Shaannong 33 and their alleles frequencies in current Chinese wheat cultivars and breeding lines. Plant Dis. 2021, 105, 1048–1056. [Google Scholar] [CrossRef]
- Rollar, S.; Geyer, M.; Hartl, L.; Mohler, V.; Ordon, F.; Serfling, A. Quantitative trait loci mapping of adult plant and seedling resistance to stripe rust (Puccinia striiformis Westend.) in a multiparent advanced generation intercross wheat population. Front. Plant Sci. 2021, 12, 684671. [Google Scholar]
- Marais, G.F.; Pretorius, Z.A.; Marais, A.S.; Wellings, C.R. Transfer of rust resistance genes from Triticum species to common wheat. S. Afr. J. Plant Soil 2003, 20, 193–198. [Google Scholar] [CrossRef]
- Uauy, C.; Brevis, J.C.; Chen, X.M.; Khan, I.; Jackson, L.; Chicaiza, O.; Distelfeld, A.; Fahima, T.; Dubcovsky, J. High-temperature adult-plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1. Theor. Appl. Genet. 2005, 112, 97–105. [Google Scholar] [PubMed]
- Dong, Z.Z.; Hegarty, J.M.; Zhang, J.L.; Zhang, W.J.; Chao, S.M.; Chen, X.M.; Zhou, Y.; Dubcovsky, J. Validation and characterization of a QTL for adult plant resistance to stripe rust on wheat chromosome arm 6BS (Yr78). Theor. Appl. Genet. 2017, 130, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Santra, D.K.; Chen, X.M.; Santra, M.; Campbell, K.G.; Kidwell, K.K. Identification and mapping QTL for high-temperature adult-plant resistance to stripe rust in winter wheat (Triticum aestivium L.) cultivar Stephens. Theor. Appl. Genet. 2008, 117, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Bariana, H.S.; Bansal, U.K.; Schmidt, A.; Lehmensiek, A.; Kaur, K.; Miah, H.; Howes, N.; Mcintyre, C.L. Molecular mapping of adult plant stripe rust resistance in wheat and identification of pyramided QTL genotypes. Euphytica 2010, 176, 251–260. [Google Scholar] [CrossRef]
- Liu, L.; Wang, M.N.; Feng, J.Y.; See, D.R.; Chao, S.M.; Chen, X.M. Combination of all-stage and high-temperature adult-plant resistance QTL confers high-level, durable resistance to stripe rust in winter wheat cultivar Madsen. Theor. Appl. Genet. 2018, 131, 1835–1849. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Gong, F.; Jin, Y.; Xia, Y.; He, Y.; Jiang, Y.; Zhou, Q.; He, J.; Feng, L.; et al. Identification and mapping of QTL for stripe rust resistance in the Chinese wheat cultivar Shumai126. Plant Dis. 2022, 106, 1278–1285. [Google Scholar] [CrossRef]
- Niu, Z.; Klindworth, D.L.; Friesen, T.L.; Chao, S.; Jin, Y.; Cai, X.; Xu, S.S. Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics 2011, 187, 1011–1021. [Google Scholar] [CrossRef]
- Wu, S.; Pumphrey, M.O.; Bai, G. Molecular mapping of stem rust resistance gene Sr40 in wheat. Crop Sci. 2009, 49, 1681–1686. [Google Scholar] [CrossRef]
- Kosgey, Z.C.; Edae, E.A.; Dill-Macky, R.; Jin, Y.; Bulbula, W.D.; Gemechu, A.; Macharia, G.; Bhavani, S.; Randhawa, M.S.; Rouse, M.N. Mapping and validation of stem rust resistance loci in spring wheat line CI 14275. Front. Plant Sci. 2021, 11, 609659. [Google Scholar] [CrossRef]
- Bajgain, P.; Rouse, M.N.; Bulli, P.; Bhavani, S.; Gordon, T.; Wanyera, R.; Njau, P.N.; Legesse, W.; Anderson, J.A.; Pumphrey, M.O. Association mapping of North American spring wheat breeding germplasm reveals loci conferring resistance to Ug99 and other African stem rust races. BMC Plant Biol. 2015, 15, 249. [Google Scholar]
- Rouse, M.N.; Talbert, L.E.; Singh, D.; Sherman, J.D. Complementary epistasis involving Sr12 explains adult plant resistance to stem rust in Thatcher wheat (Triticum aestivum L.). Theor. Appl. Genet. 2014, 127, 1549–1559. [Google Scholar] [CrossRef]
- Prins, R.; Dreisigacker, S.; Pretorius, Z.; van Schalkwyk, H.; Wessels, E.; Smit, C.; Bender, C.; Singh, D.; Boyd, L.A. Stem rust resistance in a geographically diverse collection of spring wheat lines collected from across Africa. Front. Plant Sci. 2016, 7, 973. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.S.; Overlander, M.; Faris, J.D.; Klindworth, D.L.; Rouse, M.N.; Kang, H.; Long, Y.; Jin, Y.; Lagudah, E.S.; Xu, S.S. Characterization of synthetic wheat line Largo for resistance to stem rust. G3 Genes Genomes Genet. 2021, 11, jkab193. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Szabo, L.J.; Rouse, M.N.; Fetch, T., Jr.; Pretorius, Z.A.; Wanyera, R.; Njau, P. Detection of virulence to resistance gene Sr36 within the TTKS race lineage of Puccinia graminis f. sp. tritici. Plant Dis. 2009, 93, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Bhavani, S.; Singh, R.P.; Argillier, O.; Huerta-Espino, J.; Singh, S.; Njau, P.; Brun, S.; Lacam, S.; Desmouceaux, N. Mapping durable adult plant stem rust resistance to the race Ug99 group in six CIMMYT wheats. In Proceedings of the Borlaug Global Rust Initiative 2011 Technical Workshop, Ithaca, NY, USA, 13–16 June 2011; McIntosh, R.A., Ed.; Borlaug Global Rust Initiative: St. Paul, MN, USA, 2011; pp. 43–53. [Google Scholar]
- Njau, P.N.; Bhavani, S.; Huerta-Espino, J.; Keller, B.; Singh, R.P. Identification of QTL associated with durable adult plant resistance to stem rust race Ug99 in wheat cultivar ‘Pavon 76’. Euphytica 2013, 190, 33–44. [Google Scholar] [CrossRef]
- Quraishi, U.M.; Pont, C.; Ain, Q.U.; Flores, R.; Burlot, L.; Alaux, M.; Quesneville, H.; Salse, J. Combined Genomic and Genetic Data Integration of Major Agronomical Traits in Bread Wheat (Triticum aestivum L.). Front. Plant Sci. 2017, 8, 1843. [Google Scholar] [CrossRef]
- Letta, T.; Maccaferri, M.; Badebo, A.; Ammar, K.; Ricci, A.; Crossa, J.; Tuberosa, R. Searching for novel sources of field resistance to Ug99 and Ethiopian stem rust races in durum wheat via association mapping. Theor. Appl. Genet. 2013, 126, 1237–1256. [Google Scholar] [CrossRef]
- Genievskaya, Y.; Abugalieva, S.; Rsaliyev, A.; Yskakova, G.; Turuspekov, Y. QTL mapping for seedling and adult plant resistance to leaf and stem rusts in Pamyati Azieva × Paragon mapping population of bread wheat. Agronomy 2020, 10, 1285. [Google Scholar] [CrossRef]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef]
- Singh, R.P.; McIntosh, R.A. Cytogenetical studies in wheat. XIV. Sr8b for resistance to Puccinia graminis f. sp. tritici. Can. J. Genet. Cytol. 1986, 28, 189–197. [Google Scholar] [CrossRef]
- Hailu, E.; Woldaeb, G.; Denbel, W.; Alemu, W.; Abebe, T. Distribution of stem rust (Puccinia graminis f. sp. tritici) races in Ethiopia. Adv. Crop Sci. Tech. 2015, 3, 173. [Google Scholar] [CrossRef][Green Version]
- Randhawa, M.S.; Singh, R.P.; Dreisigacker, S.; Bhavani, S.; Huerta-Espino, J.; Rouse, M.N.; Nirmala, J.; Sandoval-Sanchez, M. Identification and validation of a common stem rust resistance locus in two bi-parental populations. Front. Plant Sci. 2018, 9, 1788. [Google Scholar]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E. Rust Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Texcoco, Mexico, 1992. [Google Scholar]
- Bansal, U.K.; Kazi, A.G.; Singh, B.; Hare, R.A.; Bariana, H.S. Mapping of durable stripe rust resistance in a durum wheat cultivar Wollaroi. Mol. Breed. 2014, 33, 51–59. [Google Scholar]
- Taylor, J.; Butler, D. R package AS Map, efficient genetic linkage map construction and diagnosis. J. Statist. Soft. 2017, 79, 1–29. [Google Scholar]
- Hussain, W.; Baenziger, P.S.; Belamkar, V.; Guttieri, M.J.; Venegas, J.P.; Easterly, A.; Sallam, A.; Poland, J. Genotyping-by-Sequencing derived high-density linkage map and its application to QTL mapping of flag leaf traits in bread wheat. Sci. Rep. 2017, 7, 16394. [Google Scholar]
- Manly, K.F.; Cudmore, R.H., Jr.; Meer, J.M. Map Manager QTX.; cross-platform software for genetic mapping. Mamm. Genome 2001, 12, 930–932. [Google Scholar]
- Wang, S.; Basten, C.J.; Zeng, Z.B. Windows QTL Cartographer 2.5. Department of Statistics; North Carolina State University: Raleigh, NC, USA, 2011. [Google Scholar]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar]
- Toth, J.; Pandurangan, S.; Burt, A.J.; Fetch, J.M.; Kumar, S. Marker-assisted breeding of hexaploidy spring wheat in the Canadian Prairies. Can. J. Plant Sci. 2019, 99, 111–127. [Google Scholar]
- Wang, Y.; Zhang, H.; Xie, J.; Guo, B.; Chen, Y.; Zhang, H.; Lu, P.; Wu, Q.; Li, M.; Zhang, D.; et al. Mapping stripe rust resistance genes by BSR-Seq: YrMM58 and YrHY1 on chromosome 2AS in Chinese wheat lines Mengmai 58 and Huaiyang 1 are Yr17. Crop J. 2018, 6, 91–98. [Google Scholar] [CrossRef]
- He, X.; Kabir, M.R.; Roy, K.K.; Marza, F.; Chawade, A.; Duveiller, E.; Saint-Pierre, C.; Singh, P.K. Genetic dissection for head blast resistance in wheat using two mapping populations. Heredity 2022, 128, 402–410. [Google Scholar] [CrossRef] [PubMed]




| LR19-1 | LR19-2 | LR20 | LR21-1 | LR21-2 | LR22-1 | LR22-2 | YR20-1 | YR20-2 | YR21-1 | YR21-2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LR19-1 | 1.00 | - | - | - | - | ||||||
| LR19-2 | 0.81 | 1.00 | - | - | - | - | |||||
| LR20 | 0.76 | 0.85 | 1.00 | - | - | - | - | ||||
| LR21-1 | 0.78 | 0.84 | 0.79 | 1.00 | - | - | - | - | |||
| LR21-2 | 0.78 | 0.81 | 0.81 | 0.91 | 1.00 | - | - | - | - | ||
| LR22-1 | 0.76 | 0.76 | 0.77 | 0.75 | 0.71 | 1.00 | - | - | - | - | |
| LR22-2 | 0.80 | 0.85 | 0.86 | 0.81 | 0.78 | 0.94 | 1.00 | - | - | - | - |
| YR20-1 | - | - | - | - | - | - | - | 1.00 | |||
| YR20-2 | - | - | - | - | - | - | - | 0.91 | 1.00 | ||
| YR21-1 | - | - | - | - | - | - | - | 0.88 | 0.87 | 1.00 | |
| YR21-2 | - | - | - | - | - | - | - | 0.86 | 0.85 | 0.96 | 1.00 |
| YRKEN21-1 | YRKEN21-2 | YRKEN21-3 | YRKEN23-1 | YRKEN23-2 | SR21-1 | SR21-2 | SR21-3 | SR23-1 | SR23-2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| YRKEN21-1 | 1.00 | - | - | - | - | - | ||||
| YRKEN21-2 | 0.78 | 1.00 | - | - | - | - | - | |||
| YRKEN21-3 | 0.75 | 0.94 | 1.00 | - | - | - | - | - | ||
| YRKEN23-1 | 0.75 | 0.77 | 0.70 | 1.00 | - | - | - | - | - | |
| YRKEN23-2 | 0.76 | 0.75 | 0.75 | 0.85 | 1.00 | - | - | - | - | - |
| SR21-1 | - | - | - | - | - | 1.00 | ||||
| SR21-2 | - | - | - | - | - | 0.94 | 1.00 | |||
| SR21-3 | - | - | - | - | - | 0.88 | 0.94 | 1.00 | ||
| SR23-1 | - | - | - | - | - | 0.70 | 0.74 | 0.72 | 1.00 | |
| SR23-2 | - | - | - | - | - | 0.72 | 0.75 | 0.75 | 0.95 | 1.00 |
| QTL | Years QTL Identified | Environment | Chromosomal Location | QTL Associations with Rust Diseases |
|---|---|---|---|---|
| QLrYrSr.cim-1BL | 2019, 2020, 2021, 2022 | Obregon, El-Batan, Toluca, Njoro | 1BL (678–685 Mb) | Co-located QTL for LR, YR, and SR |
| QLrYr.cim-2AS | 2019, 2020, 2021, 2022 | Obregon, El-Batan, Toluca | 2AS (~8 Mb) | Co-located QTL for LR and YR |
| QLrYr.cim-3AL | 2019, 2020, 2021, 2022 | Obregon, El-Batan, Toluca | 3AL (~747 Mb) | Co-located QTL for LR and YR |
| QLrYrSr.cim-6AL | 2019, 2020, 2021, 2022, 2023 | Obregon, El-Batan, Toluca, Njoro | 6AL (609–614 Mb) | Co-located QTL for LR, YR, and SR |
| QLr.cim-2DS | 2019, 2020, 2021, 2022 | Obregon, El-Batan | 2DS (12–17 Mb) | QTL for LR |
| QLr.cim-6DS | 2019, 2020, 2021, 2022 | Obregon, El-Batan | 6DS (7–19 Mb) | QTL for LR |
| QYrKen.cim-3DS | 2021, 2023 | Njoro | 3DS (~150 Mb) | QTL for YR |
| QYrKen.cim-6BS | 2021, 2023 | Njoro | 6BS (87–120 Mb) | QTL for YR |
| QSr.cim-2BS | 2021, 2023 | Njoro | 2BS (68–76 Mb) | QTL for SR |
| QSr.cim-5AL | 2021, 2023 | Njoro | 5AL (682 Mb) | QTL for SR |
| QSr.cim-6AS | 2021, 2023 | Njoro | 6AS (9–10 Mb) | QTL for SR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, N.; Singh, R.P.; Bhavani, S. Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”. Plants 2025, 14, 1007. https://doi.org/10.3390/plants14071007
Qureshi N, Singh RP, Bhavani S. Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”. Plants. 2025; 14(7):1007. https://doi.org/10.3390/plants14071007
Chicago/Turabian StyleQureshi, Naeela, Ravi Prakash Singh, and Sridhar Bhavani. 2025. "Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”" Plants 14, no. 7: 1007. https://doi.org/10.3390/plants14071007
APA StyleQureshi, N., Singh, R. P., & Bhavani, S. (2025). Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”. Plants, 14(7), 1007. https://doi.org/10.3390/plants14071007






