Plant Nitrogen Assimilation: A Climate Change Perspective
Abstract
:1. Nitrogen Assimilation in a Changing Climate
2. Effects of Elevated Carbon Dioxide
| Environmental Factor | Common Name | Scientific Name | Growth/Yield/Biomass Production | Total N Content | N Uptake | Protein | Experimental Condition | References |
|---|---|---|---|---|---|---|---|---|
| Non-leguminous C3 | ||||||||
| e[CO2] | Wheat | Triticum aestivum L. | ↑ | ↓ | ↑ | NM | FACE | [23] |
| Wheat | Triticum aestivum L. | ↑ | ↓ | ↑ | NM | FACE | [22] | |
| Wheat | Triticum aestivum L. cv. Yipti | NM | NM | ↑ | ↓ | FACE | [24] | |
| Wheat | Triticum aestivum L. genotypes, BTS and GE | LN ↑ shoot, ↓ root HN ↑ shoot, ↓ root | LN - HN ↓ | LN ↑ HN ↓ | NM | Growth chamber | [33] | |
| Wheat | Triticum aestivum L. “Ayahikari” | ↑ | ↓ | ↑ | NM | Growth chamber | [34] | |
| Rice | Oryza sativa L. “Nipponbare” | ↑ | ↓ | ↑ | NM | Growth chamber | [34] | |
| Potato | Solanum tuberosum L. “Irish Cobbler”) | ↑ | ↓ | ↑ | NM | Growth chamber | [34] | |
| Rice | Oryza sativa L. japonica “Kitaake” Wild Type and OsCV Silenced | OsCV Silence ↑ > WT ↑ | As amino acid OsCV Silence − WT ↓ | OsCV Silence ↑ WT ↓ | OsCV Silence ↑ WT↓ | Growth chamber | [35] | |
| HT | Canola | Brassica napus L., cv. 6056 | ↓ | NM | NM | NM | Growth chamber | [36] |
| Tomato | Solanum lycopersicum L. | ↓ | ↓ | ↓ | ↓ N uptake proteins | Growth chamber | [37] | |
| Wheat | Triticum aestivum L. | ↓ | NM | NM | NM | Growth chamber | [38] | |
| WS | Barley | Hordeum vulgare L. | ↓ | ↓ | ↓ | ↓ | Greenhouse | [39] |
| Sweet potato | Ipomoea batatas (L.) Lam. cv. Xushu 32 and Ningzishu 1 | ↓ | ↑ NO3− shoots ↓ NO3− roots NH4+ - | ↓ | ↑ NR leaves ↓ NR roots | Greenhouse | [40] | |
| Non-leguminous C4 | ||||||||
| e[CO2] | Corn | Zea mays L. | ↑ | ↓ | ↑ | NM | Open-top chamber | [41] |
| Guinea grass | Panicum maximum Jacq. “Natsukaze” | ↑ | ↓ | ↑ | NM | Growth chamber | [34] | |
| Amaranth | Amaranthus spp. L. (Tusrushin seeds, Co., Ltd., Japan) | ↑ | - | ↑ | NM | Growth chamber | [34] | |
| HT | Corn | Zea mays L. | ↓ | ↑ | NM | ↑ | Open-top chamber | [42] |
| Waxy corn | Zea mays L. sinensis Kulesh cv. Suyunuo 5 | ↓ | NM | ↓ | ↓ | Greenhouse | [43] | |
| WS | Corn | Zea mays L. | ↓ | ↓ | ↓ | ↓ | Greenhouse | [39] |
| Corn | Zea mays L. | ↓ | ↓ | ↓ | NM | Greenhouse | [44] | |
| Corn | Zea mays L. | NM | ↑ | ↑ NH4+ | NM | Greenhouse | [9] | |
| Leguminous C3 | ||||||||
| e[CO2] | Chickpea | Cicer arietinum L. | ↑ | - | ↑ | NM | Field | [30] |
| Common bean | Phaseolus vulgaris L. | ↑ | ↑ | NM | NM | Growth room | [29] | |
| Soybean | Glycine max L. | ↑ | - | ↑ | NM | FACE | [28] | |
| HT | Common bean | Phaseolus vulgaris L. | ↓ | ↓ | NM | ↓ | Growth chamber | [45] |
| Mung bean | Vigna radiata L. | ↑ | ↑ NBI | NM | NM | Growth chamber | [46] | |
| WS | Alfalfa | Medicago sativa L. | ↓ | NM | NM | ↓ | Field | [47] |
| Soybean | Glycine max (L.) Merr. cv. Shennong17, Shennong8, Shennong12 | ↓ | ↓ | ↓ | ↓ | Field | [48] | |
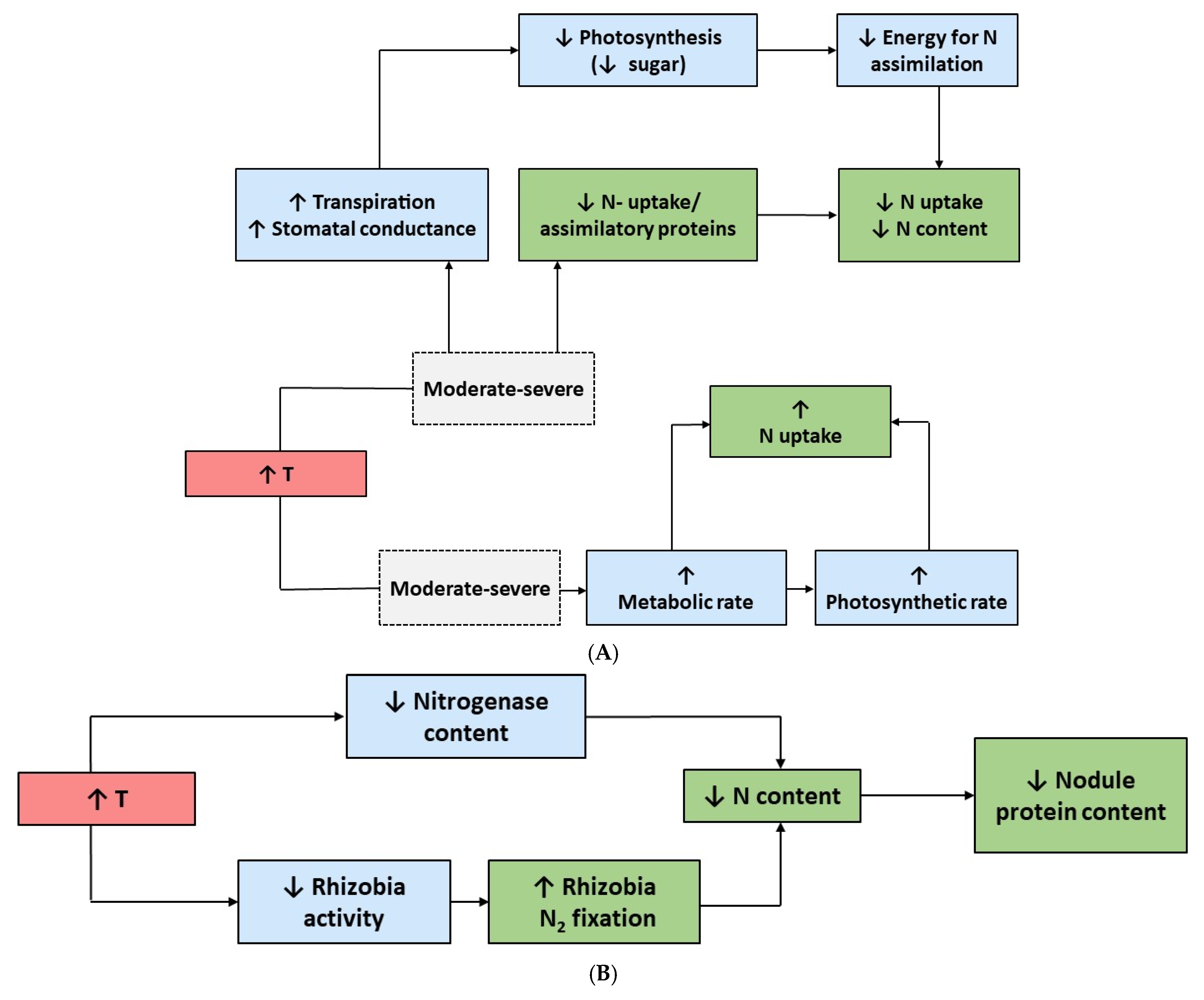
3. Effects of High Temperature
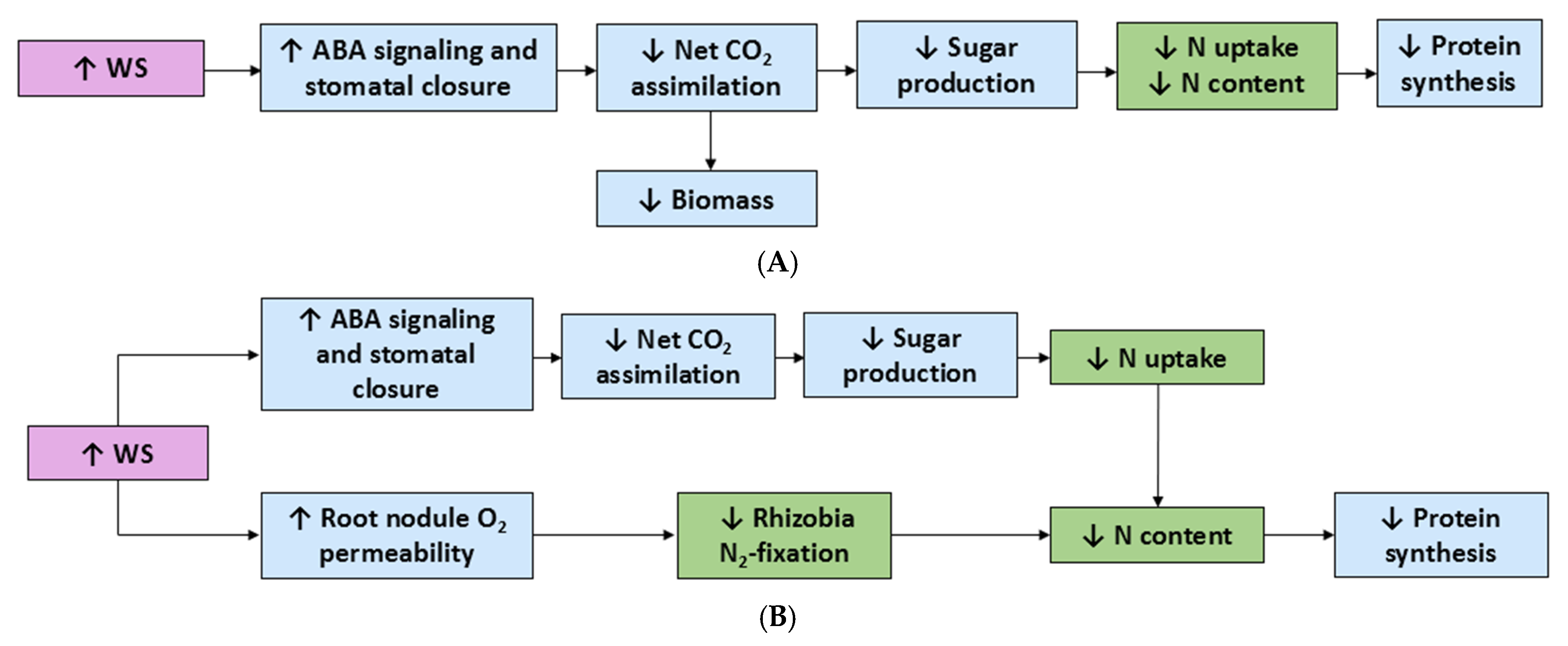
4. Effects of Water Stress
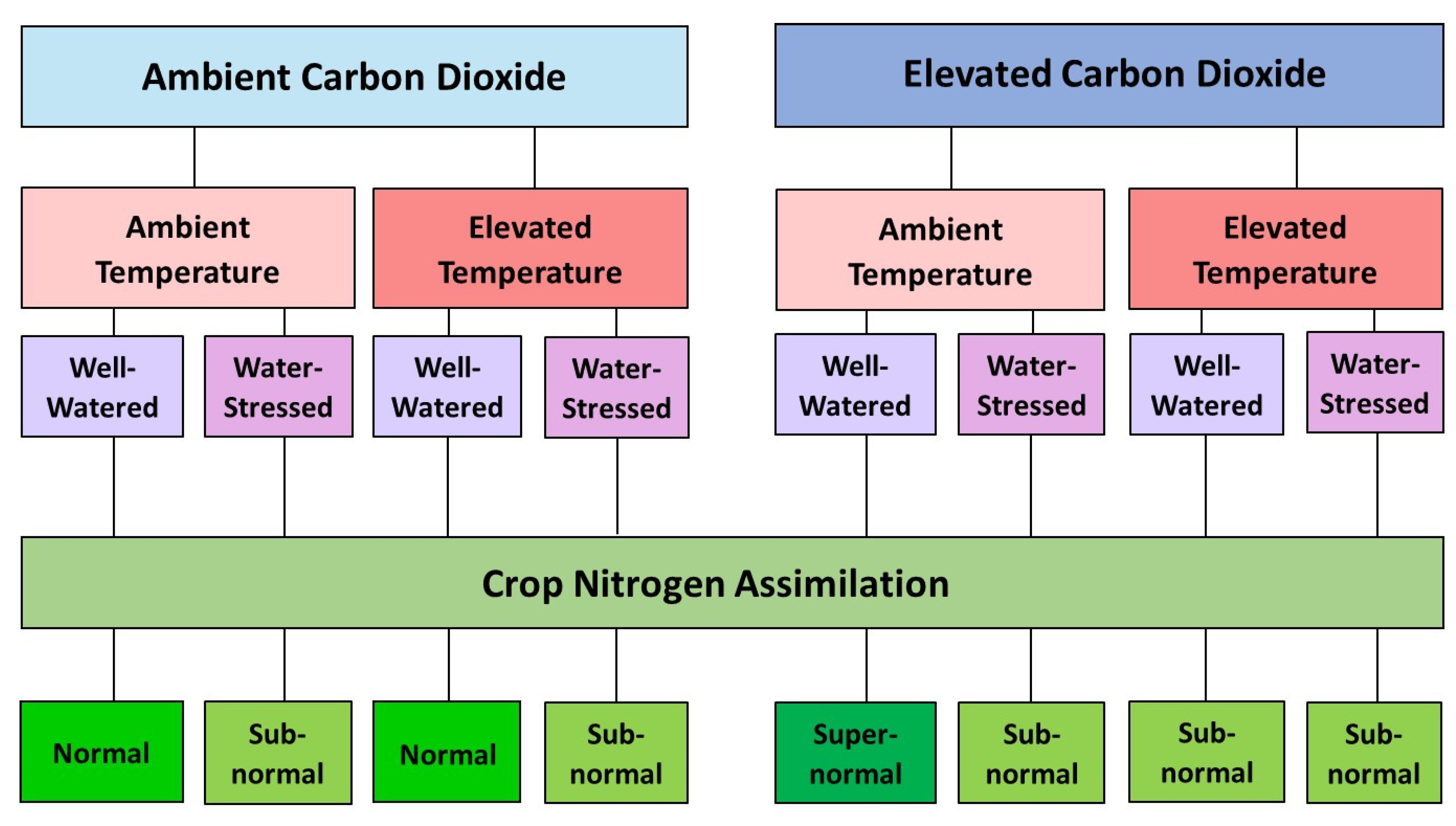
5. Interactive Effects of Elevated CO2, High Temperature, and Water Stress
5.1. Combined Effects of Elevated CO2 and High Temperature
5.2. Combined Effects of Elevated CO2 and Water Stress
5.3. Combined Effects of High Temperature and Water Stress
5.4. Combined Effects of Elevated CO2, High Temperature, and Water Stress
| Environmental Factors | Common Name | Scientific Name | Growth/Yield/Biomass Production | Total N Content | N Uptake | Amino Acids | Protein | Experimental Condition | References |
|---|---|---|---|---|---|---|---|---|---|
| Non-leguminous C3 | |||||||||
| e[CO2] × HT × WS | Canola | Brassica napus L. cv. 45H72 | e[CO2] × HT ×WS > a[CO2] × HT × WS | NM | NM | NM | NM | Growth chamber | [102] |
| Arabidopsis | Arabidopsis thaliana L. | NM | NM | NM | HT × WS > e[CO2] × WS × HT | ↓ | Climate chamber | [123] | |
| Tomato | Solanum lycopersicum L. “OuBei” and Solanum pimpinellifolium L. ‘LA2093’ | e[CO2] × HT ×WS > a[CO2] × HT × WS | NM | NM | NM | NM | Climate chamber | [122] | |
| e[CO2] × HT × WS | Sorghum | Sorghum bicolor L. | e[CO2] × HT × WS > a[CO2] × HT × WS | NM | NM | NM | NM | Greenhouse | [124] |
| Leguminous C3 | |||||||||
| e[CO2] × HT × WS | Alfalfa | Medicago sativa L. | e[CO2] × WS × HT > a[CO2] × WS × HT | - | NM | NM | NM | Growth chambers | [119] |
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiz, L.; Møller, I.; Murphy, A.; Peer, W. Fundamentals of Plant Physiology, 2nd ed.; Oxford University Press: New York, NY, USA, 2025. [Google Scholar]
- Ma, C.; Ban, T.; Yu, H.; Li, Q.; Li, X.; Jiang, W.; Xie, J. Urea addition promotes the metabolism and utilization of nitrogen in cucumber. Agronomy 2019, 9, 262. [Google Scholar] [CrossRef]
- Aluko, O.O.; Liu, Z.; Sun, X. The interplay of carbon and nitrogen distribution: Prospects for improved crop yields. Mod. Agric. 2023, 1, 57–75. [Google Scholar] [CrossRef]
- Cott, G.M.; Jansen, M.A.K.; Megonigal, J.P. Uptake of organic nitrogen by coastal wetland plants under elevated CO2. Plant Soil 2020, 450, 521–535. [Google Scholar]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef]
- Kishorekumar, R.; Bulle, M.; Wany, A.; Gupta, K.J. An Overview of Important Enzymes Involved in Nitrogen Assimilation of Plants. In Nitrogen Metabolism in Plants; Gupta, K.J., Ed.; Springer: New York, NY, USA, 2020; pp. 1–13. [Google Scholar]
- Wang, H.; Yang, Z.; Yu, Y.; Chen, S.; He, Z.; Wang, Y.; Jiang, L.; Wang, G.; Yang, C.; Liu, B.; et al. Drought enhances nitrogen uptake and assimilation in maize roots. Agron. J. 2017, 109, 39–46. [Google Scholar] [CrossRef]
- Helliwell, K.E. Emerging trends in nitrogen and phosphorus signalling in photosynthetic eukaryotes. Trends Plant Sci. 2023, 28, 344–358. [Google Scholar]
- Gao, Y.; Qi, S.; Wang, Y. Nitrate signaling and use efficiency in crops. Plant Commun. 2022, 3, 500–512. [Google Scholar] [CrossRef]
- Lan, X. Trends in Atmospheric CO2—NOAA Global Monitoring Laboratory. Available online: https://gml.noaa.gov/ccgg/trends/data.html (accessed on 18 February 2025).
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Wang, J.; Vanga, S.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of climate change on the yield of cereal crops: A review. Climate 2018, 6, 41. [Google Scholar] [CrossRef]
- Irigoyen, J.J.; Goicoechea, N.; Antolín, M.C.; Pascual, I.; Sánchez-Díaz, M.; Aguirreolea, J.; Morales, F. Growth, photosynthetic acclimation and yield quality in legumes under climate change simulations: An updated survey. Plant Sci. 2014, 226, 22–29. [Google Scholar] [PubMed]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosynth. Res. 2015, 123, 117–128. [Google Scholar] [PubMed]
- Singer, S.D.; Chatterton, S.; Soolanayakanahally, R.Y.; Subedi, U.; Chen, G.; Acharya, S.N. Potential effects of a high CO2 future on leguminous species. Plant-Environ. Interact. 2020, 1, 67–94. [Google Scholar]
- Jones, H.G. Plants and Microclimate: A Quantitative Approach to Environmental Plant Physiology, 3rd ed.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Tausz-Posch, S.; Tausz, M.; Bourgault, M. Elevated [CO2] effects on crops: Advances in understanding acclimation, nitrogen dynamics and interactions with drought and other organisms. Plant Biol. 2020, 22, 38–51. [Google Scholar]
- Asensio, J.S.R.; Rachmilevitch, S.; Bloom, A.J. Responses of Arabidopsis and wheat to rising CO2 depend on nitrogen source and nighttime CO2 levels. Plant Physiol. 2015, 168, 156–163. [Google Scholar]
- Bahrami, H.; De Kok, L.J.; Armstrong, R.; Fitzgerald, G.J.; Bourgault, M.; Henty, S.; Tausz, M.; Tausz-Posch, S. The proportion of nitrate in leaf nitrogen, but not changes in root growth, are associated with decreased grain protein in wheat under elevated [CO2]. J. Plant Physiol. 2017, 216, 44–51. [Google Scholar]
- Dier, M.; Meinen, R.; Erbs, M.; Kollhorst, L.; Baillie, C.; Kaufholdt, D.; Kücke, M.; Weigel, H.; Zörb, C.; Hänsch, R.; et al. Effects of free air carbon dioxide enrichment (FACE) on nitrogen assimilation and growth of winter wheat under nitrate and ammonium fertilization. Glob. Change Biol. 2018, 24, e40–e54. [Google Scholar]
- Han, X.; Hao, X.; Lam, S.K.; Wang, H.; Li, Y.; Wheeler, T.; Ju, H.; Lin, E. Yield and nitrogen accumulation and partitioning in winter wheat under elevated CO2: A 3-year free-air CO2 enrichment experiment. Agric. Ecosyst. Environ. 2015, 209, 132–137. [Google Scholar]
- Fernando, N.; Panozzo, J.; Tausz, M.; Norton, R.; Fitzgerald, G.; Seneweera, S. Rising atmospheric CO2 concentration affects mineral nutrient and protein concentration of wheat grain. Food Chem. 2012, 133, 1307–1311. [Google Scholar]
- Krämer, K.; Brock, J.; Heyer, A.G. Interaction of nitrate assimilation and photorespiration at elevated CO2. Front. Plant Sci. 2022, 13, 897924. [Google Scholar]
- Wang, J.; Liu, X.; Zhang, X.; Li, L.; Lam, S.K.; Pan, G. Changes in plant C, N and P ratios under elevated [CO2] and canopy warming in a rice-winter wheat rotation system. Sci. Rep. 2019, 9, 5424. [Google Scholar]
- Feng, Z.; Rütting, T.; Pleijel, H.; Wallin, G.; Reich, P.B.; Kammann, C.I.; Newton, P.C.D.; Kobayashi, K.; Luo, Y.; Uddling, J. Constraints to nitrogen acquisition of terrestrial plants under elevated CO2. Glob. Change Biol. 2015, 21, 3152–3168. [Google Scholar]
- Hao, X.; Li, P.; Han, X.; Norton, R.M.; Lam, S.K.; Zong, Y.; Sun, M.; Lin, E.; Gao, Z. Effects of free-air CO2 enrichment (FACE) on N, P and K uptake of soybean in northern China. Agric. For. Meteorol. 2016, 218–219, 261–266. [Google Scholar]
- Andrews, M.; Condron, L.M.; Kemp, P.D.; Topping, J.F.; Lindsey, K.; Hodge, S.; Raven, J.A. Elevated CO2 effects on nitrogen assimilation and growth of C3 vascular plants are similar regardless of N-form assimilated. J. Exp. Bot. 2019, 70, 683–690. [Google Scholar]
- Chakrabarti, B.; Singh, S.D.; Bhatia, A.; Kumar, V.; Harit, R.C. Yield and nitrogen uptake in wheat and chickpea grown under elevated carbon dioxide level. Natl. Acad. Sci. Lett. 2020, 43, 109–113. [Google Scholar]
- Nunes-Nesi, A.; Fernie, A.R.; Stitt, M. Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant 2010, 3, 973–996. [Google Scholar]
- Liu, Y.; Duan, X.; Zhao, X.; Ding, W.; Wang, Y.; Xiong, Y. Diverse nitrogen signals activate convergent ROP2-TOR signaling in Arabidopsis. Dev. Cell 2021, 56, 1283–1295. [Google Scholar]
- Adavi, S.B.; Sathee, L. Elevated CO2 alters tissue balance of nitrogen metabolism and downregulates nitrogen assimilation and signalling gene expression in wheat seedlings receiving high nitrate supply. Protoplasma 2021, 258, 219–233. [Google Scholar]
- Igarashi, M.; Yi, Y.; Yano, K. Revisiting why plants become N deficient under elevated CO2: Importance to meet N demand regardless of the fed-form. Front. Plant Sci. 2021, 12, 726186. [Google Scholar]
- Umnajkitikorn, K.; Sade, N.; Rubio Wilhelmi, M.D.M.; Gilbert, M.E.; Blumwald, E. Silencing of OsCV (chloroplast vesiculation) maintained photorespiration and N assimilation in rice plants grown under elevated CO2. Plant Cell Environ. 2020, 43, 920–933. [Google Scholar]
- Evans, C.C.; Qaderi, M.M. Supplemental nitrogen alleviates the negative effects of higher temperature on the vegetative growth of canola regardless of carbon dioxide concentration. Plant Stress 2024, 13, 100521. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar]
- Bista, D.; Heckathorn, S.; Jayawardena, D.; Mishra, S.; Boldt, J. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and -tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef]
- Xia, H.; Xu, T.; Zhang, J.; Shen, K.; Li, Z.; Liu, J. Drought-induced responses of nitrogen metabolism in Ipomoea batatas. Plants 2020, 9, 1341. [Google Scholar] [CrossRef]
- Xie, X.; Li, R.; Zhang, Y.; Shen, S.; Bao, Y. Effect of elevated [CO2] on assimilation, allocation of nitrogen and phosphorus by maize (Zea mays L.). Commun. Soil Sci. Plant Anal. 2018, 49, 1032–1044. [Google Scholar]
- Abebe, A.; Pathak, H.; Singh, S.D.; Bhatia, A.; Harit, R.C.; Kumar, V. Growth, yield and quality of maize with elevated atmospheric carbon dioxide and temperature in north–west India. Agric. Ecosyst. Environ. 2016, 218, 66–72. [Google Scholar]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Weakened carbon and nitrogen metabolisms under post-silking heat stress reduce the yield and dry matter accumulation in waxy maize. J. Integr. Agric. 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Hungria, M.; Kaschuk, G. Regulation of N2 fixation and NO3−/NH4+ assimilation in nodulated and N-fertilized Phaseolus vulgaris L. exposed to high temperature stress. Environ. Exp. Bot. 2014, 98, 32–39. [Google Scholar]
- Reardon, M.E.; Qaderi, M.M. Individual and interactive effects of temperature, carbon dioxide and abscisic acid on mung bean (Vigna radiata) plants. J. Plant Interact. 2017, 12, 295–303. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Q.; Ge, G.; Han, G.; Jia, Y. Influence of drought stress on alfalfa yields and nutritional composition. BMC Plant Biol. 2018, 18, 13. [Google Scholar]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of drought stress at reproductive stages on growth and nitrogen metabolism in soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Gojon, A.; Cassan, O.; Bach, L.; Lejay, L.; Martin, A. The decline of plant mineral nutrition under rising CO2: Physiological and molecular aspects of a bad deal. Trends Plant Sci. 2023, 28, 185–198. [Google Scholar]
- Wang, F.; Gao, J.; Yong, J.W.H.; Wang, Q.; Ma, J.; He, X. Higher atmospheric CO2 levels favor C3 plants over C4 plants in utilizing ammonium as a nitrogen source. Front. Plant Sci. 2020, 11, 537443. [Google Scholar]
- Rogers, A.; Gibon, Y.; Stitt, M.; Morgan, P.B.; Bernacchi, C.J.; Ort, D.R.; Long, S.P. Increased C availability at elevated carbon dioxide concentration improves N assimilation in a legume. Plant Cell Environ. 2006, 29, 1651–1658. [Google Scholar] [CrossRef]
- Rogers, A.; Ainsworth, E.A.; Leakey, A.D.B. Will elevated carbon dioxide concentration amplify the benefits of nitrogen fixation in legumes? Plant Physiol. 2009, 151, 1009–1016. [Google Scholar] [CrossRef]
- Vara Prasad, P.V.; Allen, L.H.; Boote, K.J. Crop responses to elevated carbon dioxide and interaction with temperature: Grain legumes. J. Crop Improv. 2005, 13, 113–155. [Google Scholar]
- Britto De Assis Prado, C.H.; Haik Guedes De Camargo-Bortolin, L.; Castro, É.; Martinez, C.A. Leaf Dynamics of Panicum maximum under future climatic changes. PLoS ONE 2016, 11, e0149620. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Amador, A.; Araus, J.L. Interactive effects of elevated [CO2] and water stress on physiological traits and gene expression during vegetative growth in four durum wheat genotypes. Front. Plant Sci. 2016, 7, 1738. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [PubMed]
- Taniguchi, M.; Miyake, H. Redox-shuttling between chloroplast and cytosol: Integration of intra-chloroplast and extra-chloroplast metabolism. Curr. Opin. Plant Biol. 2012, 15, 252–260. [Google Scholar] [PubMed]
- Jauregui, I.; Aroca, R.; Garnica, M.; Zamarreño, Á.M.; García-Mina, J.M.; Serret, M.D.; Parry, M.; Irigoyen, J.J.; Aranjuelo, I. Nitrogen assimilation and transpiration: Key processes conditioning responsiveness of wheat to elevated [CO2] and temperature. Physiol. Plant. 2015, 155, 338–354. [Google Scholar] [PubMed]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Change 2014, 4, 477–480. [Google Scholar]
- Bloom, A.J.; Kasemsap, P.; Rubio-Asensio, J.S. Rising atmospheric CO2 concentration inhibits nitrate assimilation in shoots but enhances it in roots of C3 plants. Physiol. Plant. 2020, 168, 963–972. [Google Scholar]
- Krämer, K.; Kepp, G.; Brock, J.; Stutz, S.; Heyer, A.G. Acclimation to elevated CO2 affects the C/N balance by reducing de novo N-assimilation. Physiol. Plant. 2022, 174, e13615. [Google Scholar]
- Schlüter, U.; Weber, A.P.M. Regulation and evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 2020, 71, 183–215. [Google Scholar]
- Cabeza, R.A.; Schulze, J.; Salinas-Roco, S.; Morales-González, A.; Amigo, R.; Pérez-Díaz, R.; Carrasco, B.; Contreras-Soto, R.; Maldonado, C.; Pedreschi, R.; et al. The inhibition of N2 fixation by nitrogen is attenuated by the P supply, altering the plant metabolism. Environ. Exp. Bot. 2024, 222, 105762. [Google Scholar]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar]
- Pérez-Jiménez, M.; Piñero, M.C.; Del Amor, F.M. Heat shock, high CO2 and nitrogen fertilization effects in pepper plants submitted to elevated temperatures. Sci. Hortic. 2019, 244, 322–329. [Google Scholar]
- Abo Gamar, M.I.; Kisiala, A.; Emery, R.J.N.; Yeung, E.C.; Stone, S.L.; Qaderi, M.M. Elevated carbon dioxide decreases the adverse effects of higher temperature and drought stress by mitigating oxidative stress and improving water status in Arabidopsis thaliana. Planta 2019, 250, 1191–1214. [Google Scholar] [PubMed]
- Goel, P.; Singh, A.K. Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. PLoS ONE 2015, 10, e0143645. [Google Scholar]
- Bian, Z.; Wang, Y.; Zhang, X.; Li, T.; Grundy, S.; Yang, Q.; Cheng, R. A review of environment effects on nitrate accumulation in leafy vegetables grown in controlled environments. Foods 2020, 9, 732. [Google Scholar] [CrossRef]
- Sewelam, N.; Brilhaus, D.; Bräutigam, A.; Alseekh, S.; Fernie, A.R.; Maurino, V.G. Molecular plant responses to combined abiotic stresses put a spotlight on unknown and abundant genes. J. Exp. Bot. 2020, 71, 5098–5112. [Google Scholar]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar]
- Jayawardena, D.M.; Heckathorn, S.A.; Bista, D.R.; Mishra, S.; Boldt, J.K.; Krause, C.R. Elevated CO2 plus chronic warming reduce nitrogen uptake and levels or activities of nitrogen-uptake and -assimilatory proteins in tomato roots. Physiol. Plant. 2017, 159, 354–365. [Google Scholar]
- Sita, K.; Sehgal, A.; HanumanthaRao, B.; Nair, R.M.; Vara Prasad, P.V.; Kumar, S.; Gaur, P.M.; Farooq, M.; Siddique, K.H.M.; Varshney, R.K.; et al. Food legumes and rising temperatures: Effects, adaptive functional mechanisms specific to reproductive growth stage and strategies to improve heat tolerance. Front. Plant Sci. 2017, 8, 1658. [Google Scholar]
- Zahran, H.H. Rhizobium -legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar]
- Huang, L.; Li, M.; Zhou, K.; Sun, T.; Hu, L.; Li, C.; Ma, F. Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol. Biochem. 2018, 127, 185–193. [Google Scholar]
- Ye, J.Y.; Tian, W.H.; Jin, C.W. Nitrogen in plants: From nutrition to the modulation of abiotic stress adaptation. Stress Biol. 2022, 2, 4. [Google Scholar]
- Han, M.-L.; Lv, Q.-Y.; Zhang, J.; Wang, T.; Zhang, C.-X.; Tan, R.-J.; Wang, Y.-L.; Zhong, L.-Y.; Gao, Y.-Q.; Chao, Z.-F.; et al. Decreasing nitrogen assimilation under drought stress by suppressing DST-mediated activation of Nitrate Reductase 1.2 in rice. Mol. Plant 2022, 15, 167–178. [Google Scholar] [PubMed]
- Schwember, A.R.; Schulze, J.; Del Pozo, A.; Cabeza, R.A. Regulation of symbiotic nitrogen fixation in legume root nodules. Plants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar]
- Meng, S.; Zhang, C.; Su, L.; Li, Y.; Zhao, Z. Nitrogen uptake and metabolism of Populus simonii in response to PEG-induced drought stress. Environ. Exp. Bot. 2016, 123, 78–87. [Google Scholar]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.D.M.; Ríos, J.J.; Blasco, B.; Rosales, M.Á.; Melgarejo, R.; Romero, L.; Ruiz, J.M. Ammonia production and assimilation: Its importance as a tolerance mechanism during moderate water deficit in tomato plants. J. Plant Physiol. 2011, 168, 816–823. [Google Scholar]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen metabolism in adaptation of photosynthesis to water stress in rice grown under different nitrogen levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell. Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar]
- Zhong, C.; Jian, S.-F.; Huang, J.; Jin, Q.-Y.; Cao, X.-C. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol. Biochem. 2019, 135, 41–50. [Google Scholar]
- Díaz, P.; Borsani, O.; Márquez, A.; Monza, J. Nitrogen metabolism in relation to drought stress responses in cultivated and model lotus species. Lotus Newsletter 2005, 35, 83–92. [Google Scholar]
- Hare, P.D.; Cress, W.A.; van Staden, J. Proline synthesis and degradation: A model system for elucidating stress-related signal transduction. J. Exp. Bot. 1998, 50, 413–434. [Google Scholar]
- Pratelli, R.; Pilot, G. Regulation of amino acid metabolic enzymes and transporters in plants. J. Exp. Bot. 2014, 65, 5535–5556. [Google Scholar] [CrossRef] [PubMed]
- Bandurska, H.; Niedziela, J.; Pietrowska-Borek, M.; Nuc, K.; Chadzinikolau, T.; Radzikowska, D. Regulation of proline biosynthesis and resistance to drought stress in two barley (Hordeum vulgare L.) genotypes of different origin. Plant Physiol. Biochem. 2017, 118, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, D.M.; Heckathorn, S.A.; Rajanayake, K.K.; Boldt, J.K.; Isailovic, D. Elevated carbon dioxide and chronic warming together decrease nitrogen uptake Rate, net translocation, and assimilation in tomato. Plants 2021, 10, 722. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M.; Shah, D.; Maurya, S.; Jagadish, K.S.V. Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crops Res. 2017, 206, 149–157. [Google Scholar]
- Roy, K.S.; Bhattacharyya, P.; Neogi, S.; Rao, K.S.; Adhya, T.K. Combined effect of elevated CO2 and temperature on dry matter production, net assimilation rate, C and N allocations in tropical rice (Oryza sativa L.). Field Crops Res. 2012, 139, 71–79. [Google Scholar] [CrossRef]
- Jayawardena, D.M.; Heckathorn, S.A.; Boldt, J.K. Effects of elevated carbon dioxide and chronic warming on nitrogen (N)-uptake rate, -assimilation, and -concentration of wheat. Plants 2020, 9, 1689. [Google Scholar] [CrossRef]
- Chavan, S.G.; Duursma, R.A.; Tausz, M.; Ghannoum, O. Moderate heat stress prevented the observed biomass and yield stimulation caused by elevated CO2 in two well-watered wheat cultivars. Plant Mol. Biol. 2022, 110, 365–384. [Google Scholar]
- Serret, M.D.; Yousfi, S.; Vicente, R.; Piñero, M.C.; Otálora-Alcón, G.; Del Amor, F.M.; Araus, J.L. Interactive effects of CO2 concentration and water regime on stable isotope signatures, nitrogen assimilation and growth in sweet pepper. Front. Plant Sci. 2018, 8, 2180. [Google Scholar]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Boldt, J.K. Effect of drought and carbon dioxide on nutrient uptake and levels of nutrient-uptake proteins in roots of barley. Am. J. Bot. 2020, 107, 1401–1409. [Google Scholar]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass Leymus chinensis. Planta 2006, 224, 1080–1090. [Google Scholar] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J.; Song, T. Individual and combined effects of heat and drought and subsequent recovery on winter wheat (Triticum aestivum L.) photosynthesis, nitrogen metabolism, cell osmoregulation, and yield formation. Plant Physiol. Biochem. 2023, 196, 222–235. [Google Scholar]
- Olivera Viciedo, D.; De Mello Prado, R.; Martínez, C.A.; Habermann, E.; De Cássia Piccolo, M. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.J.; Grandis, A.; Carvalho, V.J.; Salatino, A.; Buckeridge, M.S. Isolated and combined effects of elevated CO2 and high temperature on the whole-plant biomass and the chemical composition of soybean seeds. Food Chem. 2019, 275, 610–617. [Google Scholar] [PubMed]
- Bencke-Malato, M.; De Souza, A.P.; Ribeiro-Alves, M.; Schmitz, J.F.; Buckeridge, M.S.; Alves-Ferreira, M. Short-term responses of soybean roots to individual and combinatorial effects of elevated [CO2] and water deficit. Plant Sci. 2019, 280, 283–296. [Google Scholar]
- Jumrani, K.; Bhatia, V.S. Interactive effect of temperature and water stress on physiological and biochemical processes in soybean. Physiol. Mol. Biol. Plants 2019, 25, 667–681. [Google Scholar]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Chavan, S.G.; Duursma, R.A.; Tausz, M.; Ghannoum, O. Elevated CO2 alleviates the negative impact of heat stress on wheat physiology but not on grain yield. J. Exp. Bot. 2019, 70, 6447–6459. [Google Scholar]
- Dixon, S.L.; Qaderi, M.M. Canola responds differently to nitrogen forms under temperature and carbon dioxide conditions. Theor. Exp. Plant Physiol. 2025, 37, 12. [Google Scholar]
- Xu, G.; Singh, S.; Barnaby, J.; Buyer, J.; Reddy, V.; Sicher, R. Effects of growth temperature and carbon dioxide enrichment on soybean seed components at different stages of development. Plant Physiol. Biochem. 2016, 108, 313–322. [Google Scholar]
- Shanker, A.K.; Gunnapaneni, D.; Bhanu, D.; Vanaja, M.; Lakshmi, N.J.; Yadav, S.K.; Prabhakar, M.; Singh, V.K. Elevated CO2 and water stress in combination in plants: Brothers in arms or partners in crime? Biology 2022, 11, 1330. [Google Scholar] [CrossRef] [PubMed]
- Barnaby, J.Y.; Fleisher, D.H.; Singh, S.K.; Sicher, R.C.; Reddy, V.R. Combined effects of drought and CO2 enrichment on foliar metabolites of potato (Solanum tuberosum L.) cultivars. J. Plant Interact. 2019, 14, 110–118. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Allen, L.H. Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. J. Plant Physiol. 2009, 166, 107–116. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 71, 399–408. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Kumar, A.; Nayak, A.K.; Das, B.S.; Panigrahi, N.; Dasgupta, P.; Mohanty, S.; Kumar, U.; Panneerselvam, P.; Pathak, H. Effects of water deficit stress on agronomic and physiological responses of rice and greenhouse gas emission from rice soil under elevated atmospheric CO2. Sci. Total Environ. 2019, 650, 2032–2050. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Tan, D.K.Y. Impact of high temperature and drought stresses on chickpea production. Agronomy 2018, 8, 145. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Song, T.; Wang, W.; Lv, M.; Hansen, N.C. Nitrogen modulates the effects of short-term heat, drought and combined stresses after anthesis on photosynthesis, nitrogen metabolism, yield, and water and nitrogen use efficiency of wheat. Water 2022, 14, 1407. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Reid, D.M. Methane emissions from six crop species exposed to three components of global climate change: Temperature, ultraviolet-B radiation and water stress. Physiol. Plant. 2009, 137, 139–147. [Google Scholar] [PubMed]
- Aubert, L.; Quinet, M. Comparison of heat and drought stress responses among twelve Tartary buckwheat (Fagopyrum tataricum) varieties. Plants 2022, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sumner, E.E.; Williamson, V.G.; Gleadow, R.M.; Wevill, T.; Venn, S.E. Acclimation to water stress improves tolerance to heat and freezing in a common alpine grass. Oecologia 2022, 199, 831–843. [Google Scholar] [PubMed]
- Aranjuelo, I.; Irigoyen, J.J.; Sánchez-Díaz, M. Effect of elevated temperature and water availability on CO2 exchange and nitrogen fixation of nodulated alfalfa plants. Environ. Exp. Bot. 2007, 59, 99–108. [Google Scholar]
- Dias De Oliveira, E.; Bramley, H.; Siddique, K.H.M.; Henty, S.; Berger, J.; Palta, J.A. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct. Plant Biol. 2013, 40, 160. [Google Scholar]
- AbdElgawad, H.; Farfan-Vignolo, E.R.; Vos, D.D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar]
- Zhou, R.; Yu, X.; Wen, J.; Jensen, N.B.; dos Santos, T.M.; Wu, Z.; Rosenqvist, E.; Ottosen, C.-O. Interactive effects of elevated CO2 concentration and combined heat and drought stress on tomato photosynthesis. BMC Plant Biol. 2020, 20, 260. [Google Scholar]
- Zinta, G.; AbdElgawad, H.; Peshev, D.; Weedon, J.T.; Van Den Ende, W.; Nijs, I.; Janssens, I.A.; Beemster, G.T.S.; Asard, H. Dynamics of metabolic responses to periods of combined heat and drought in Arabidopsis thaliana under ambient and elevated atmospheric CO2. J. Exp. Bot. 2018, 69, 2159–2170. [Google Scholar]
- Al-Salman, Y.; Ghannoum, O.; Cano, F.J. Elevated [CO2] negatively impacts C4 photosynthesis under heat and water stress without penalizing biomass. J. Exp. Bot. 2023, 74, 2875–2890. [Google Scholar]
- Lebedev, V.G.; Popova, A.A.; Shestibratov, K.A. Genetic engineering and genome editing for improving nitrogen use efficiency in plants. Cells 2021, 10, 3303. [Google Scholar] [CrossRef]

| Environmental Factor | Common Name | Scientific Name | Growth/Yield/Biomass Production | Total N Content | N Uptake | Protein | Experimental Condition | References |
|---|---|---|---|---|---|---|---|---|
| Non-leguminous C3 | ||||||||
| e[CO2] × HT | Sula wheat | Triticum durum Desf. cv. Sula | e[CO2] × HT > HT | HT > e[CO2] × HT | NM | HT > e[CO2] × HT | Greenhouse | [58] |
| Tomato | Solanum lycopersicum L. | HT > e[CO2] × HT | HT > e[CO2] HT | HT > e[CO2] × HT | HT > e[CO2] × HT | Greenhouse | [72] | |
| Tomato | S. lycopersicum L. cv. Big Boy | HT > e[CO2] × HT | HT > e[CO2] × HT | HT > e[CO2] × HT | HT > e[CO2] × HT | Greenhouse | [89] | |
| Rice | Oryza sativa L. var. Nerica-L-44 and Pusa 1121 | e[CO2] > e[CO2] × HT | NM | NM | e[CO2] > e[CO2] × HT | Open-top chamber | [90] | |
| Rice | Oryza sativa L., cv. Naveen | e[CO2] × HT > e[CO2] - | a[CO2] > e[CO2] × HT | e[CO2] × HT > a[CO2] | NM | Open-top chamber | [91] | |
| Wheat | Triticum aestivum L. | e[CO2] × HT = e[CO2] | e[CO2] > e[CO2] × HT | e[CO2] × HT - | e[CO2] > e[CO2] × HT | Greenhouse | [92] | |
| Wheat | Triticum aestivum L. cv Scout and Yipti | e[CO2] > e[CO2] × HT a[CO2] × HT - | e[CO2] < a[CO2] Yipti | NM | e[CO2] < a[CO2] | Greenhouse | [93] | |
| Canola | Brassica napus L., cv. 6056 | e[CO2] × HT × LN > e[CO2] × HT × ZN | NM | NM | NM | Growth chamber | [36] | |
| e[CO2] × WS | Sweet pepper | Capsicum annuum L. | e[CO2] × WS > WS | WS > e[CO2] × WS | NM | NM | Greenhouse | [94] |
| Barley | Hordeum vulgare L. | e[CO2] > e[CO2] × WS > WS | e[CO2] > WS > e[CO2] × WS | e[CO2] > e[CO2] × WS > WS | e[CO2] > e[CO2] × WS > WS | Greenhouse | [95] | |
| HT × WS | Chinese rye grass | Leymus chinensis Trin. | HT, WS > HT × WS | HT, WS > HT × WS | NM | HT, WS > HT × WS | Greenhouse | [96] |
| Wheat | Triticum aestivum L. cultivar Xiaoyan 22′ | HT, WS > HT × WS | HT, WS > HT × WS | HT, WS > HT × WS | HT, WS > HT × WS NR levels | Growth chamber | [97] | |
| Non-leguminous C4 | ||||||||
| HT × WS | Corn | Zea mays L. | HT, WS > HT × WS | HT, WS > HT × WS | HT, WS > HT × WS | WS > HT, HT × WS | Greenhouse | [44] |
| Guinea grass | Panicum maximum Jacq. | HT × WS > HT, WS | HT, WS > HT × WS | NM | NM | T-FACE | [98] | |
| Leguminous C3 | ||||||||
| e[CO2] × HT | Soybean | Glycine max L. | e[CO2] > e[CO2] × HT | a[CO2] > e[CO2] × HT | NM | - | Open-top chamber | [99] |
| e[CO2] × WS | Soybean | Glycine max L. | e[CO2] × WS > WS | NM | NM | NM | Open-top chamber | [100] |
| HT × WS | Soybean | Glycine max L. | HT, WS > HT × WS | NM | NM | NM | Greenhouse | [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qaderi, M.M.; Evans, C.C.; Spicer, M.D. Plant Nitrogen Assimilation: A Climate Change Perspective. Plants 2025, 14, 1025. https://doi.org/10.3390/plants14071025
Qaderi MM, Evans CC, Spicer MD. Plant Nitrogen Assimilation: A Climate Change Perspective. Plants. 2025; 14(7):1025. https://doi.org/10.3390/plants14071025
Chicago/Turabian StyleQaderi, Mirwais M., Cameryn C. Evans, and Madeleine D. Spicer. 2025. "Plant Nitrogen Assimilation: A Climate Change Perspective" Plants 14, no. 7: 1025. https://doi.org/10.3390/plants14071025
APA StyleQaderi, M. M., Evans, C. C., & Spicer, M. D. (2025). Plant Nitrogen Assimilation: A Climate Change Perspective. Plants, 14(7), 1025. https://doi.org/10.3390/plants14071025







