Flowers and Leaves of Artemisia absinthium and Artemisia annua Phytochemical Characterization, Anti-Inflammatory, Antioxidant, and Anti-Proliferative Activities Evaluation
Abstract
1. Introduction
2. Results
2.1. Quantitative Phytochemical Analysis
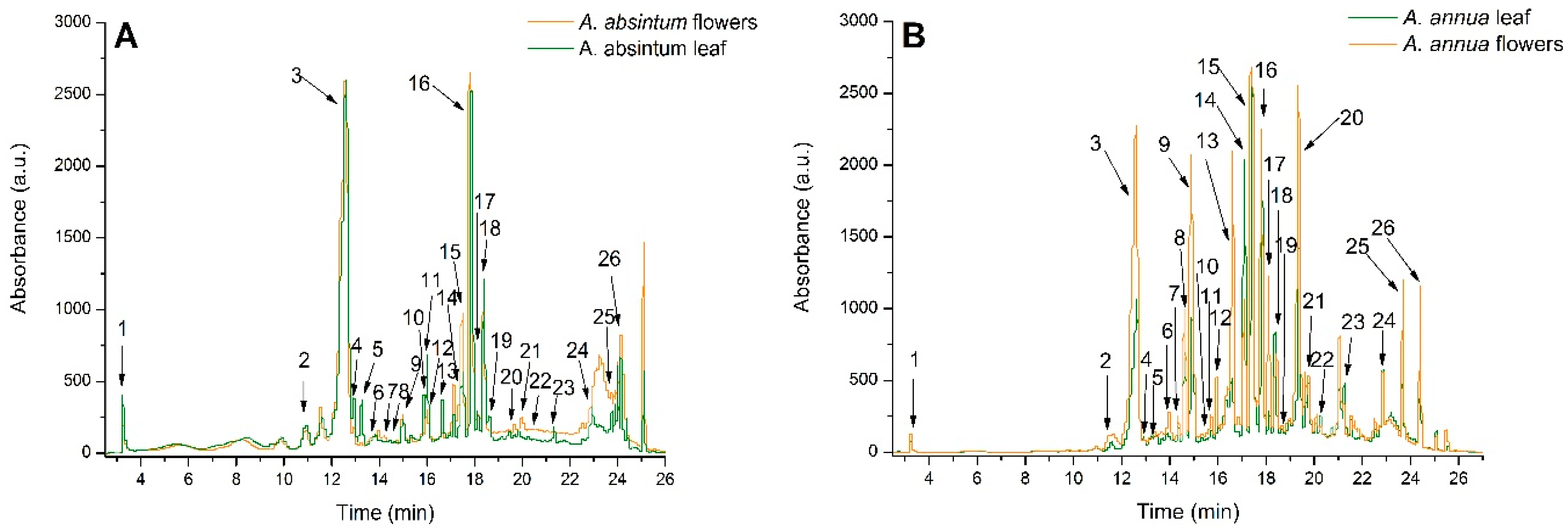
2.2. Qualitative Phytochemical Analysis
2.3. In Vitro Antioxidant Activity
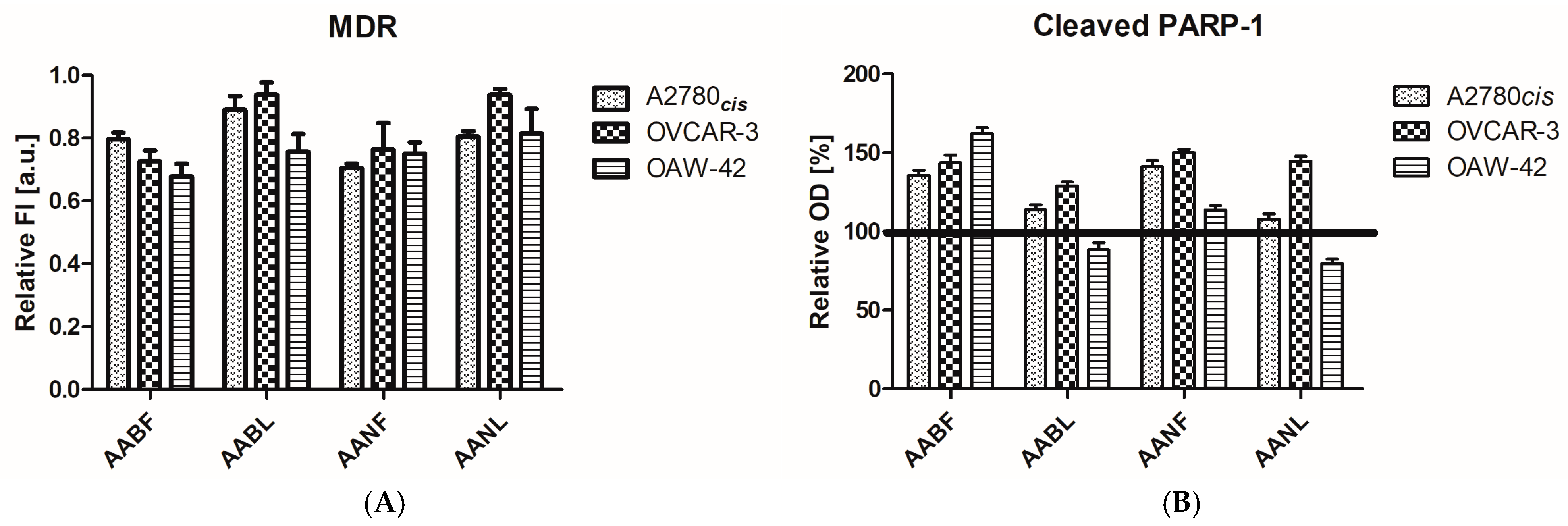
2.4. In Vitro Antiproliferative Activity
2.5. In Vivo Anti-Inflammatory Activity
2.6. In Vivo Antioxidant Activity
2.7. Liver and Renal Toxicity Assessment
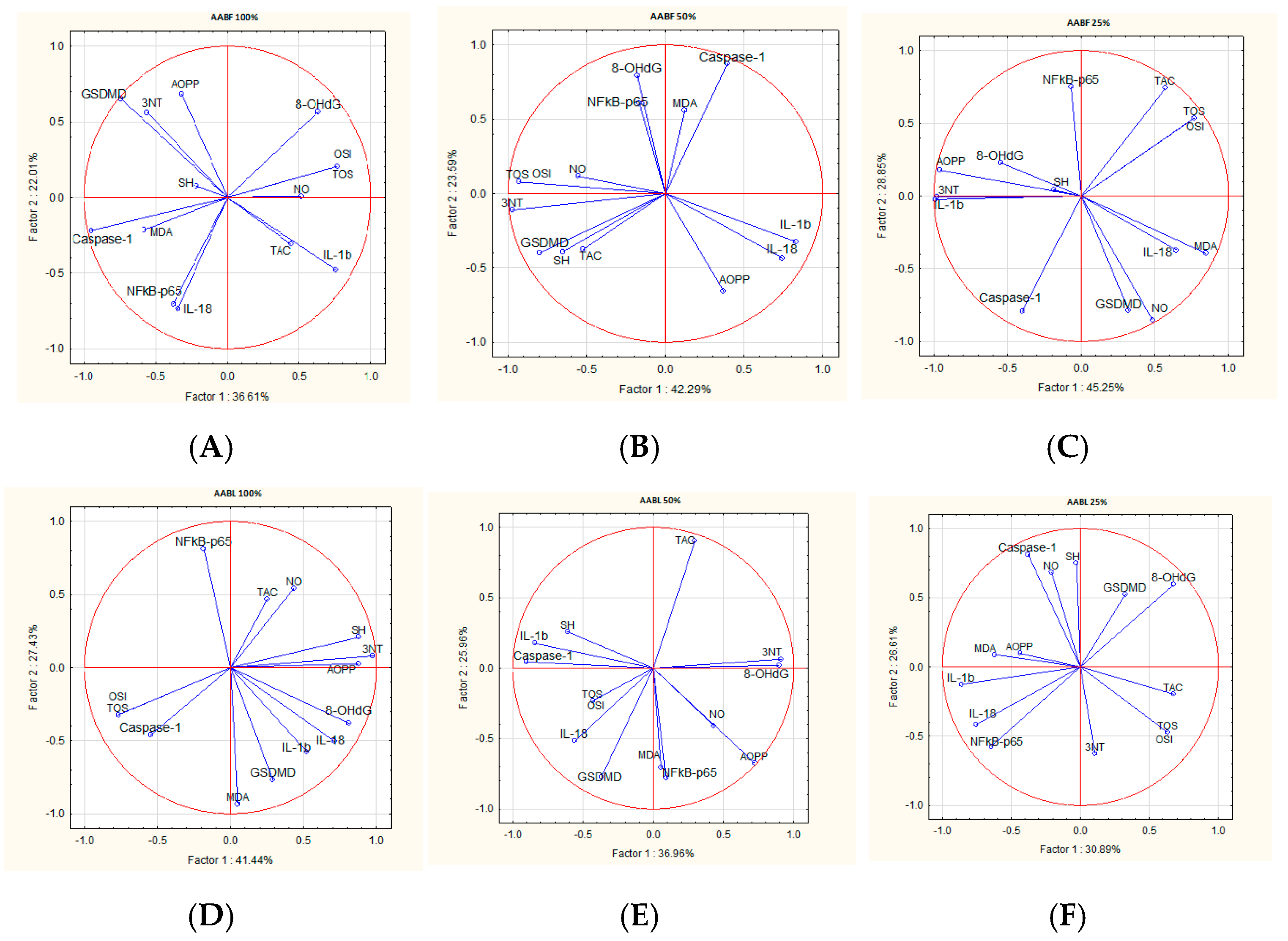
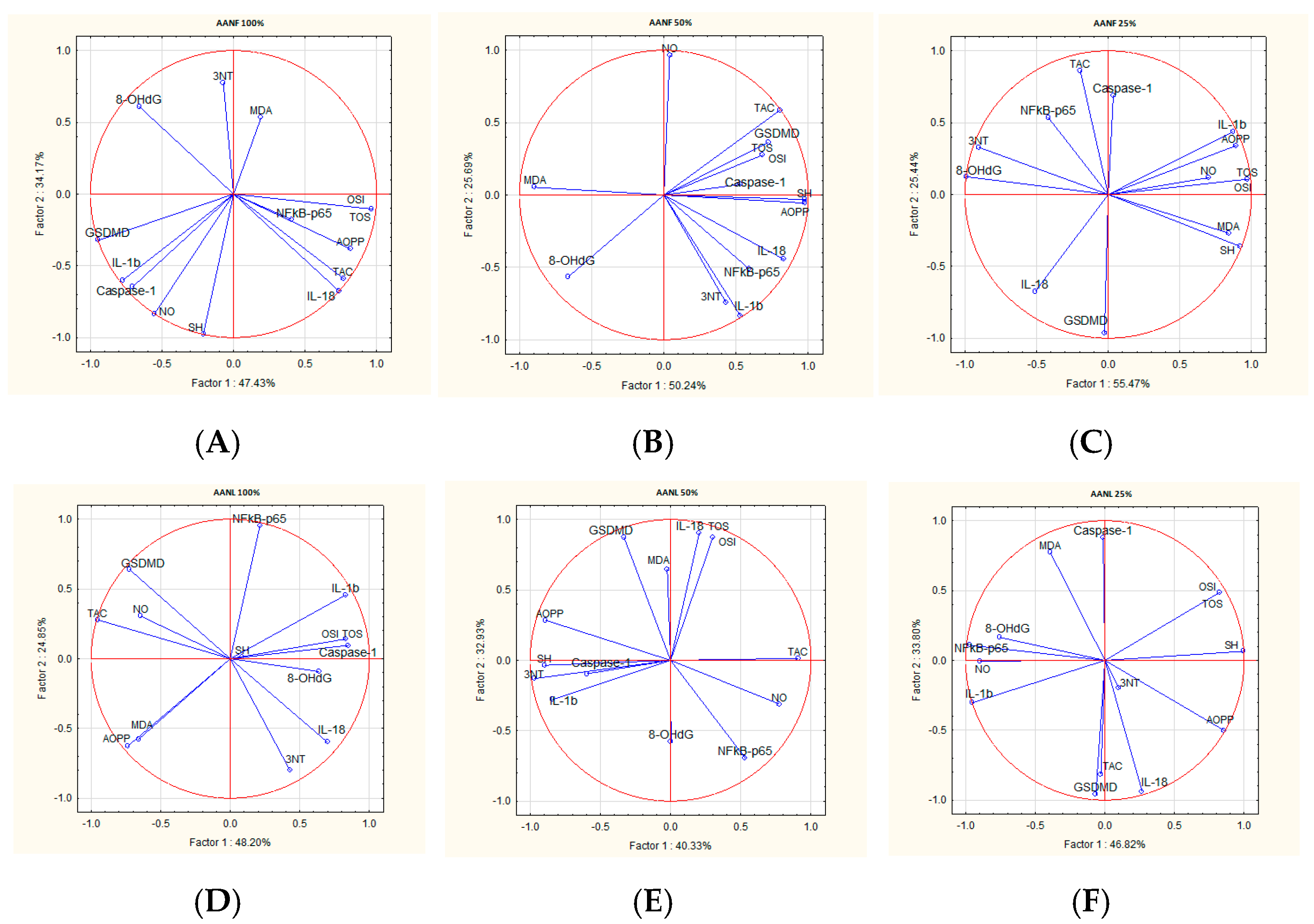
2.8. Correlations Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Plant Extract Preparation
4.3. Quantitative Phytochemical Analysis
4.4. Qualitative Phytochemical Analysis
4.5. In Vitro Antioxidant Activity Analysis
4.5.1. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Capacity
4.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.5.3. Hydrogen Peroxide (H2O2) Scavenging Activity
4.5.4. Nitric Oxide (NO) Radical Scavenging Assay
4.6. In Vitro Antiproliferative Activity
4.6.1. MTT Cytotoxicity Assay
4.6.2. PARP-1 Cleavage ELISA Assay
4.6.3. Multidrug Resistance (MDR) Assay
4.7. In Vivo Experimental Design
4.7.1. Animal Subjects
4.7.2. Experimental Protocol
4.7.3. Oxidative Stress Markers Assessment
Total Oxidative Status (TOS)
Total Antioxidant Capacity (TAC)
Oxidative Stress Index (OSI)
8-Hydroxydeoxyguanosine (8-OHdG)
Advanced Oxidation Protein Products (AOPP)
The Malondialdehyde (MDA)
Nitric Oxide Synthesis (NO)
3-Nitrotyrosine (3NT)
Total Thiols (SHs)
4.7.4. Inflammatory Markers Assessment
4.7.5. Toxicity Assessment
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Owen-Woods, C.; Joulia, R.; Barkaway, A.; Rolas, L.; Ma, B.; Nottebaum, A.F.; Arkill, K.P.; Stein, M.; Girbl, T.; Golding, M.; et al. Local Microvascular Leakage Promotes Trafficking of Activated Neutrophils to Remote Organs. J. Clin. Investig. 2020, 130, 2301–2318. [Google Scholar] [CrossRef]
- Geiger, S.S.; Essers, M.A.G. Inflammation’s Epigenetic Footprint in Hematopoietic Stem Cells. Cell Stem Cell 2020, 26, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiang, C.; Que, Z.; Li, C.; Wang, W.; Yin, L.; Chu, C.; Zhou, Y. Neutrophil Heterogeneity and Aging: Implications for COVID-19 and Wound Healing. Front. Immunol. 2023, 14, 1201651. [Google Scholar] [CrossRef] [PubMed]
- Beyrau, M.; Bodkin, J.V.; Nourshargh, S. Neutrophil Heterogeneity in Health and Disease: A Revitalized Avenue in Inflammation and Immunity. Open Biol. 2012, 2, 120134. [Google Scholar] [CrossRef]
- Colom, B.; Bodkin, J.V.; Beyrau, M.; Woodfin, A.; Ody, C.; Rourke, C.; Chavakis, T.; Brohi, K.; Imhof, B.A.; Nourshargh, S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration InVivo. Immunity 2015, 42, 1075–1086. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Liakopoulos, V.; Dounousi, E.; Mark, P.B. Oxidative Stress in End-Stage Renal Disease: Pathophysiology and Potential Interventions. Oxid. Med. Cell Longev. 2023, 2023, 9870138. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Nani, A.; Tehami, W. Targeting Inflammasome Pathway by Polyphenols as a Strategy for Pancreatitis, Gastrointestinal and Liver Diseases Management: An Updated Review. Front. Nutr. 2023, 10, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Toldo, S.; Mezzaroma, E.; Buckley, L.F.; Potere, N.; Di Nisio, M.; Biondi-Zoccai, G.; Van Tassell, B.W.; Abbate, A. Targeting the NLRP3 Inflammasome in Cardiovascular Diseases. Pharmacol. Ther. 2022, 236, 108053. [Google Scholar] [CrossRef]
- Bertinaria, M. Infammasome Inhibitors. Molecules 2021, 26, 6912. [Google Scholar] [CrossRef]
- Davidson, C.B.; El Sabbagh, D.E.S.; Machado, A.K.; Pappis, L.; Sagrillo, M.R.; Somacal, S.; Emanuelli, T.; Schultz, J.V.; Augusto Pereira da Rocha, J.; Santos, A.F.d.; et al. Euterpe Oleracea Mart. Bioactive Molecules: Promising Agents to Modulate the NLRP3 Inflammasome. Biology 2024, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Hasan, A.; Biswas, P.; Bondhon, T.A.; Jannat, K.; Paul, T.K.; Paul, A.K.; Jahan, R.; Nissapatorn, V.; Mahboob, T.; Wilairatana, P.; et al. Can Artemisia Herba-Alba Be Useful for Managing COVID-19 and Comorbidities? Molecules 2022, 27, 492. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Semwal, P.; Painuli, S.; Badoni, H.; Ezzat, S.M.; Farid, M.M.; Merghany, R.M.; Aborehab, N.M.; Salem, M.A.; et al. Artemisia spp.: An Update on Its Chemical Composition, Pharmacological and Toxicological Profiles. Oxid. Med. Cell Longev. 2022, 2022, 5628601. [Google Scholar] [CrossRef]
- Salehi, S.; Mirzaie, A.; Sadat Shandiz, S.A.; Noorbazargan, H.; Rahimi, A.; Yarmohammadi, S.; Ashrafi, F. Chemical Composition, Antioxidant, Antibacterial and Cytotoxic Effects of Artemisia marschalliana Sprengel Extract. Nat. Prod. Res. 2017, 31, 469–472. [Google Scholar] [CrossRef]
- Nurlybekova, A.; Kudaibergen, A.; Kazymbetova, A.; Amangeldi, M.; Baiseitova, A.; Ospanov, M.; Aisa, H.A.; Ye, Y.; Ibrahim, M.A.; Jenis, J. Traditional Use, Phytochemical Profiles and Pharmacological Properties of Artemisia Genus from Central Asia. Molecules 2022, 27, 5128. [Google Scholar] [CrossRef] [PubMed]
- Adekenov, S.M.; Shaimerdenova, Z.R.; Ermekkyzy, A. Anatomical Study and Histochemical Analysis of Artemisia leucodes Schrenk. Fitoterapia 2020, 146, 104721. [Google Scholar] [CrossRef]
- Jaradat, N.; Dwikat, M.; Amer, J.; Ghanim, M.; Hawash, M.; Hussein, F.; Issa, L.; Ishtawe, S.; Salah, S.; Nasser, S. Total Phenolic Contents, Cytotoxic, Free Radicals, Porcine Pancreatic α-Amylase, and Lipase Suppressant Activities of Artemisia Dracunculus Plant from Palestine. Front. Pharmacol. 2024, 15, 1351743. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef] [PubMed]
- Erhan, S.E.; Pârvu, A.E.; Ciorîță, A.; Putri, A.A.; Molina, A.J.V.; Pârvu, M.; Moț, A.C. Chemical Composition and Anti-Inflammatory Effect of Phellodendron Amurense Rupr. Stem Bark Extract. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13306. [Google Scholar] [CrossRef]
- Kiki, G.A.à.; Pop, R.M.; Sabin, O.; Bocsan, I.C.; Chedea, V.S.; Socaci, S.A.; Pârvu, A.E.; Finsia, E.; Francis, T.; Mathieu, Z.; et al. Polyphenols from Dichrostachys Cinerea Fruits Anti-Inflammatory, Analgesic, and Antioxidant Capacity in Freund’s Adjuvant-Induced Arthritic Rat Model. Molecules 2022, 27, 5445. [Google Scholar] [CrossRef]
- Chera, E.I.; Pop, R.M.; Pârvu, M.; Sorițău, O.; Uifălean, A.; Cătoi, F.A.; Cecan, A.; Negoescu, A.G.; Achimaș-Cadariu, P.; Pârvu, A.E. Flaxseed Ethanol Extracts’ Antitumor, Antioxidant, and Anti-Inflammatory Potential. Antioxidants 2022, 11, 892. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds from Plants Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef]
- Miklášová, N.; Fischer-Fodor, E.; Lönnecke, P.; Schrepler, M.P.; Virag, P.; Tatomir, C.; Cernea, V.I.; Hey-Hawkins, E.; Silaghi-Dumitrescu, L. Antiproliferative Effect and Genotoxicity of Novel Synthesized Palladium Complexes with Organoarsenic Ligands. J. Inorg. Biochem. 2009, 103, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Francischi, J.N.; Frade, T.I.C.; Almeida, M.P.A.d.; Queiroz, B.F.G.d.; Bakhle, Y.S. Ketamine-Xylazine Anaesthesia and Orofacial Administration of Substance P: A Lethal Combination in Rats. Neuropeptides 2017, 62, 21–26. [Google Scholar] [CrossRef]
- Erel, O. A New Automated Colorimetric Method for Measuring Total Oxidant Status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A Novel Automated Method to Measure Total Antioxidant Response against Potent Free Radical Reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Harma, M.; Harma, M.; Erel, O. Increased Oxidative Stress in Patients with Hydatidiform Mole. Swiss Med. Wkly. 2003, 133, 563–566. [Google Scholar] [CrossRef]
- Nandakumar, A.; Nataraj, P.; James, A.; Krishnan, R.; Mahesh, K.M. Estimation of Salivary 8-Hydroxydeoxyguanosine (8-OHdG) as a Potential Biomarker in Assessing Progression towards Malignancy: A Case-Control Studyoxidative. Asian Pac. J. Cancer Prev. 2020, 21, 2325–2329. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Mitev, D.; Gradeva, H.; Stoyanova, Z.; Petrova, N.; Karova, N.; Dimov, D.; Iliev, V.; Koychev, A.; Prakova, G.; Vlaykova, T. Evaluation of Thiol Compounds and Lipid Peroxidative Products in Plasma of Patients with Copd. Trakia J. Sci. 2010, 8, 306–314. [Google Scholar]
- Ghasemi, A.; Hedayati, M.I.; Biabani III, H. Protein Precipitation Methods Evaluated for Determination of Serum Nitric Oxide End Products by the Griess Assay. J. Med. Sci. Res. 2007, 15, 29–32. [Google Scholar]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Ahsan, H. 3-Nitrotyrosine: A Biomarker of Nitrogen Free Radical Species Modified Proteins in Systemic Autoimmunogenic Conditions. Hum. Immunol. 2013, 74, 1392–1399. [Google Scholar] [CrossRef]
- Erel, O.; Neselioglu, S. A Novel and Automated Assay for Thiol/Disulphide Homeostasis. Clin. Biochem. 2014, 47, 326–332. [Google Scholar] [CrossRef]
- Țicolea, M.; Pop, R.M.; Pârvu, M.; Usatiuc, L.-O.; Uifălean, A.; Ranga, F.; Pârvu, A.E. Phytochemical Composition Antioxidant and Anti-Inflammatory Activity of Artemisia dracunculus and Artemisia abrotanum. Antioxidants 2024, 13, 1016. [Google Scholar] [CrossRef]
- Taukoorah, U.; Mahomoodally, M.F. Crude Aloe Vera Gel Shows Antioxidant Propensities and Inhibits Pancreatic Lipase and Glucose Movement in Vitro. Adv. Pharmacol. Sci. 2016, 2016, 3720850. [Google Scholar] [CrossRef]
- Saunoriute, S.S.; Ragažinskiene, O.; Ivanauskas, L.; Marksa, M.; Laužike, K.; Lina, R. Separations Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages. Separations 2023, 10, 545. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.Z.; Ali, A.; Ahmad, A.; Saeed, A.; Malik, S.A. Antioxidant and Phytochemical Analysis of Ranunculus Arvensis L. Extracts. BMC Res. Notes 2015, 8, 279. [Google Scholar] [CrossRef]
- Kumarappan, C.T.; Thilagam, E.; Mandal, S.C. Antioxidant Activity of Polyphenolic Extracts of Ichnocarpus Frutescens. Saudi J. Biol. Sci. 2012, 19, 349–355. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 77–106. [Google Scholar]
- Makhija, I.K.; Aswatha Ram, H.N.; Shreedhara, C.S.; Vijay Kumar, S.; Devkar, R. In Vitro Antioxidant Studies of Sitopaladi Churna, a Polyherbal Ayurvedic Formulation. Free Radic. Antioxid. 2011, 1, 37–41. [Google Scholar] [CrossRef]
- Albensi, B.C. What Is Nuclear Factor Kappa B (NF-ΚB) Doing in and to the Mitochondrion? Front. Cell Dev. Biol. 2019, 7, 154. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome Signaling and Regulation of Interleukin-1 Family Cytokines. J. Exp. Med. 2020, 217, e20190314. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Kang, N.; Yu, Y.; Mi, Y.; Guo, J.; Wu, J.; Weng, C.F. Polyphenols, Flavonoids and Inflammasomes: The Role of Cigarette Smoke in COPD. Eur. Respir. Rev. 2022, 31, 220028. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, M.E.; Dubyak, G.R.; Abbott, D.W. Post-Translational Control of NLRP3 inflammasome Signaling. J. Biol. Chem. 2024, 300, 107386. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Neutrophil in Vascular Inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Elgendy, S.A.; Baloza, S.H.; Mohammed, L.A.; Nasr, H.E.; Osama El-Shaer, N.; Ghamry, H.I.; Althobaiti, S.A.; Shukry, M.; Soliman, M.M.; Elnoury, H.A. Ameliorative Impacts of Wheat Germ Oil against Ethanol-Induced Hepatic and Renal Dysfunction in Rats: Involvement of Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Signaling Pathways. Life 2022, 12, 1671. [Google Scholar] [CrossRef]
- Rasool, R.; Ullah, I.; Shahid, S.; Mubeen, B.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Murtaza, B.N.; Nadeem, M.S.; et al. In Vivo Assessment of the Ameliorative Impact of Some Medicinal Plant Extracts on Lipopolysaccharide-Induced Multiple Sclerosis in Wistar Rats. Molecules 2022, 27, 1608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Ouyang, X.; Jiang, Z.; Xie, Z.; Fan, L.; Zhu, D.; Li, L. Advanced Oxidation Protein Products Play Critical Roles in Liver Diseases. Eur. J. Clin. Investig. 2019, 49, e13098. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological Markers of Oxidative Stress: Applications to Cardiovascular Research and Practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef]
- Schmölz, L.; Wallert, M.; Lorkowski, S. Optimized Incubation Regime for Nitric Oxide Measurements in Murine Macrophages Using the Griess Assay. J. Immunol. Methods 2017, 449, 68–70. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Shah, N.; Choudhary, A.K.; Jha, U.K.; Yadav, U.C.; Gupta, P.K.; Pakuwal, U. Oxidative Stress and Antioxidants in Disease and Cancer: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwar, R.; Arivuchelvan, A.; Mekala, P.; Imayarasi, K. Sex-Specific Reference Intervals for Wistar Albino Rats: Hematology and Clinical Biochemistry. Indian J. Anim. Health 2021, 60, 58–65. [Google Scholar] [CrossRef]
- Andrade-Oliveira, V.; Foresto-Neto, O.; Watanabe, I.K.M.; Zatz, R.; Câmara, N.O.S. Inflammation in Renal Diseases: New and Old Players. Front. Pharmacol. 2019, 10, 1192. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
| Total Polyphenols Content mgGAE/g d.w. Plant Material | Total Flavonoid Content mg QE/100 g d.w. Plant Material | |
|---|---|---|
| A. absinthium flowers (1 gr/1 mL) | 1.67 ± 0.08 | 139.94 ± 10.87 |
| A. absinthium leaves (1 gr/1.2 mL) | 1.49 ± 0.02 | 84.94 ± 6.92 |
| A. annua flowers (1 gr/1.2 mL) | 2.16 ± 0.04 | 214.23 ± 32.07 |
| A. annua leaves (1 gr/1.2 mL) | 1.24 ± 0.01 | 120.21 ± 9.45 |
| Peak No. | Rt (min) | Compound | Concentration (μg/g) | |||
|---|---|---|---|---|---|---|
| A. absinthium Flowers | A. absinthium Leaves | A. annua Flowers | A. annua Leaves | |||
| 1 | 3.22 | 3,5-Dihydroxybenzoic acid | 49.91 ± 2.83 | 77.87 ± 4.24 | 26.43 ± 1.41 | 20.24 ± 1.50 |
| 2 | 11.04 | 3-Caffeoylquinic acid (Neochlorogenic acid) | 185.42 ± 5.66 | 236.10 ± 11.24 | 55.45 ± 3.54 | 57.90 ± 3.50 |
| 3 | 12.57 | 5-Caffeoylquinic acid (Chlorogenic acid) | 2659.23 ± 88.39 | 2823.06 ± 79.90 | 2232.88 ± 89.8 | 1064.44 ± 63.50 |
| 4 | 12.95 | 4-Caffeoylquinic acid (Criptochlorogenic acid) | 88.67 ± 4.24 | 182.43 ± 6.29 | 46.31 ± 1.63 | 55.72 ± 2.25 |
| 5 | 13.21 | Caffeoyl acid-glucoside | 44.13 ± 1.34 | 191.64 ± 6.72 | 96.58 ± 3.54 | 82.71 ± 4.80 |
| 6 | 13.72 | Iso-Ferulic acid | 76.89 ± 5.66 | 130.95 ± 4.17 | 246.52 ± 15.4 | 110.12 ± 3.95 |
| 7 | 14.24 | Caffeoyl tartaric acid | 71.71 ± 5.02 | n.d. | 116.55 ± 4.95 | 85.84 ± 5.48 |
| 8 | 14.64 | Apigenin-arabinosyl-glucoside | 13.72 ± 1.34 | 13.97 ± 1.27 | 168.29 ± 7.07 | 118.22 ± 5.00 |
| 9 | 15.02 | 3-Feruloylquinic acid | 166.47 ± 4.95 | 163.05 ± 3.54 | 1201.73 ± 70.71 | 546.98 ± 32.50 |
| 10 | 15.47 | 4-Feruloylquinic acid | 64.54 ± 4.23 | 67.29 ± 4.95 | 98.49 ± 2.83 | 70.06 ± 6.50 |
| 11 | 15.88 | 5-Feruloylquinic acid | 126.35 ± 9.90 | 175.81 ± 5.66 | 155.29 ± 16.26 | 109.01 ± 3.45 |
| 12 | 16.03 | Quercetin-rutinoside (Rutin) | 149.05 ± 11.31 | 220.72 ± 11.31 | 187.90 ± 12.02 | 89.04 ± 6.55 |
| 13 | 16.66 | Apigenin-glucosyl-arabinoside | 30.54 ± 2.12 | 83.69 ± 4.24 | 297.29 ± 7.78 | 113.69 ± 6.50 |
| 14 | 17.13 | Isorhamnetin-rutinoside | 234.74 ± 17.68 | 102.70 ± 4.95 | 429.73 ± 19.80 | 790.47 ± 13.45 |
| 15 | 17.52 | 3,4-Dicaffeoylquinic acid | 467.33 ± 15.56 | 344.13 ± 11.31 | 1652.25 ± 67.18 | 1302.00 ± 80.50 |
| 16 | 17.86 | 3,5-Dicaffeoylquinic acid | 1450.86 ± 74.25 | 1097.25 ± 62.93 | 1006.37 ± 48.79 | 805.10 ± 21.50 |
| 17 | 18.11 | Quinic acid derivative | 115.28 ± 5.66 | 141.81 ± 5.66 | 485.51 ± 6.36 | 149.02 ± 12.50 |
| 18 | 18.35 | 4,5-Dicaffeoylquinic acid | 512.49 ± 21.21 | 458.49 ± 19.09 | 425.25 ± 16.26 | 369.09 ± 8.50 |
| 19 | 18.73 | 3-Feruloyl-4-caffeoylquinic acid | n.d | 187.16 ± 12.02 | 107.04 ± 6.36 | 122.08 ± 6.50 |
| 20 | 19.42 | 4-Feruloyl-5-caffeoylquinic acid | 125.82 ± 4.24 | 130.41 ± 4.24 | 1461.61 ± 60.81 | 794.42 ± 29.50 |
| 21 | 19.84 | 3,4-Diferuloylquinic acid | 193.70 ± 5.66 | 152.57 ± 5.66 | 284.51 ± 13.44 | 252.47 ± 25.75 |
| 22 | 20.44 | 3,5-Diferuloylquinic acid | n.d | 59.45 ± 6.36 | 170.22 ± 3.54 | 126.01 ± 8.50 |
| 23 | 21.32 | 5-Caffeoyl-4-feruloyl-quinic acid | 117.41 ± 4.95 | 93.11 ± 4.24 | 436.15 ± 17.68 | 218.89 ± 17.65 |
| 24 | 22.91 | 3,4,5-Tricaffeoylquinic acid | 498.85 ± 22.63 | 259.34 ± 5.66 | 233.76 ± 20.51 | 292.95 ± 14.00 |
| 25 | 23.68 | 3,5-Dihydroxy-6,7,4′-trimethoxyflavone | 41.17 ± 3.54 | 63.02 ± 2.83 | 117.02 ± 7.78 | 71.59 ± 5.50 |
| 26 | 24.39 | 3,5-Dihydroxy-6,7,3′,4′-tetramethoxyflavone | 113.29 ± 4.24 | 20.33 ± 3.54 | 108.81 ± 2.83 | 82.77 ± 9.50 |
| Total Phenolics | 7597.65 ± 354.26 | 7476.46 ± 361.33 | 11,848.05 ± 502.05 | 7900.94 ± 428.13 | ||
| DPPH μg TE/g d.w. Plant Material | FRAP μg TE/g d.w. Plant Material | H2O2 mg TE/g d.w. Plant Material | NO Scavenging Activity mg QE/g d.w. Plant Material | |
|---|---|---|---|---|
| A. absinthium flowers (1 gr/1 mL) | 87.54 ± 7.96 | 62.45 ± 4.33 | 85.18 ± 9.07 | 66.45 ± 5.16 |
| A. absinthium leaves (1 gr/1.2 mL) | 189.64 ± 16.09 | 46.45 ± 2.18 | 87.42 ± 6.24 | 145.12 ± 11.06 |
| A. annua flowers (1 gr/1.2 mL) | 73.08 ± 4.51 | 30.74 ± 1.25 | 70.59 ± 4.09 | 68.23 ± 3.92 |
| A.annua leaves (1 gr/1.2 mL) | 77.28 ± 4.71 | 51.43 ± 2.56 | 84.51 ± 4.75 | 64.93 ± 3.84 |
| TROLOX (mg) | 12.03 ± 0.44 | 11.26 ± 1.09 | 22.48 ± 1.73 | |
| Quercitin (mg) | 22.34 ± 2.48 |
| Cell Line/ Plant Extract | A2780cis | OVCAR-3 | OAW-42 | HaCaT |
|---|---|---|---|---|
| A. absinthium flowers | 13.96 ± 0.08 | 23.73 ± 1.14 | 21.82 ± 2.11 | 33.20 ± 0.86 |
| A. absinthium leaf | 18.48 ± 1.23 | 59.80 ± 3.85 | 28.06 ± 1.25 | 47.22 ± 0.30 |
| A. annua flowers | 9.29 ± 0.62 | 38.30 ± 2.99 | 16.24 ± 0.19 | 51.09 ± 1.88 |
| A.annua leaf | 15.70 ± 2.67 | 34.77 ± 0.81 | 18.45 ± 1.44 | 63.34 ± 1.76 |
| Groups | NfkB-p65 (ng/mL) | IL-1b (pg/mL) | IL-18 (pg/mL) | GSDMD (ng/mL) | Caspase-1 (pg/mL) |
|---|---|---|---|---|---|
| CONTROL | 138.47 ± 8.52 | 22.36 ± 2.71 | 18.81 ± 7.12 | 4.91 ± 0.84 | 14.26 ± 1.34 |
| INFL | 382.05 ± 19.37 aaa | 58.25 ± 55.61 aaa | 60.09 ± 5.46 aaa | 10.14 ± 1.24 aaa | 139.08 ± 5.08 aaa |
| AABF 100% | 236.95 ± 12.88 b,cc,dd | 29.11 ± 5.50 bbb | 18.28 ± 1.93 bbb,cc | 5.08 ± 0.87 bbb | 19.08 ± 2.29 bbb,cc,dd |
| AABF 50% | 234.52 ± 25.73 b,cc,dd | 24.99 ± 2.55 bbb | 17.08 ± 1.06 bbb,cc | 6.00 ± 0.88 bbb | 32.23 ± 2.76 bbb |
| AABF 25% | 305.58 ± 20.42 ccc,ddd | 30.09 ± 6.31 bbb,ddd | 22.46 ± 2.70 bbb,c | 8.98 ± 0.74 | 39.89 ± 2.39 bbb |
| AABL 100% | 233.40 ± 43.43 b,cc,dd | 21.46 ± 3.76 bbb | 20.34 ± 4.99 bbb,c | 3.89 ± 0.64 bbb | 24.81 ± 3.71 bbb,cc,dd |
| AABL 50% | 269.33 ± 18.65 b,cc,dd | 26.02 ± 5.10 bbb | 22.91 ± 3.67 bbb,c | 4.87 ± 0.82 bbb | 36.30 ± 4.31 bbb |
| AABL 25% | 332.33 ± 18.31 ccc,ddd | 22.94 ± 2.56 bbb,ddd | 22.26 ± 4.23 bbb,c | 6.63 ± 0.12 b | 36.76 ± 3.00 bbb |
| AANF 100% | 256.51 ± 14.96 b,cc,dd | 27.35 ± 3.72 bbb | 18.51 ± 1.59 bbb,cc | 4.19 ± 0.61 bbb | 29.16 ± 4.89 bbb,cc,dd |
| AANF 50% | 241.39 ± 12.2 b,cc,dd | 27.61 ± 1.78 bbb | 18.54 ± 1.41 bbb,cc | 5.55 ± 0.49 bbb | 35.50 ± 4.05 bbb |
| AANF 25% | 239.89 ± 15.62 b,cc,dd | 24.22 ± 2.53 bbb | 22.23 ± 4.73 bbb,c | 9.15 ± 0.65 | 71.47 ± 7.24 |
| AANL 100% | 244.11 ± 20.12 b,cc,dd | 26.17 ± 4.18 | 16.47 ± 1.58 bbb,cc | 3.88 ± 0.44 bbb | 27.03 ± 3.86 bbb,cc,dd |
| AANL 50% | 253.23 ± 13.75 b,cc,dd | 24.46 ± 2.04 bbb | 18.74 ± 1.34 bbb,cc | 4.93 ± 0.42 bbb | 34.39 ± 3.54 bbb |
| AANL 25% | 260.91 ± 19.00 b,cc,dd | 28.13 ± 3.65 bbb | 24.51 ± 2.51 bbb,cc | 5.45 ± 0.26 bb | 73.26 ± 5.61 |
| TX | 149.51 ± 12.04 bbb | 25.49 ± 3.07 bbb | 31.65 ± 4.61 bbb | 5.98 ± 2.43 b | 38.31 ± 2.51 bbb |
| DICLO | 140.83 ± 11.65 bbb | 28.62 ± 2.53 bbb | 59.71 ± 5.16 | 5.42 ± 2.24 bb | 40.26 ± 2.82 bbb |
| Groups | TOS (µmol H2O2E/L) | TAC (mmol TE/L) | OSI | AOPP (µmol/L) | MDA (nmol/L) | NO (µmol/L) | 3-NT (ng/mL) | 8-OhdG (ng/mL) | SH (µmol/L) |
|---|---|---|---|---|---|---|---|---|---|
| CONTROL | 16.22 ± 4.16 | 1.08 ± 0.00 | 16.70 ± 3.46 | 26.93 ± 10.70 | 2.05 ± 0.14 | 19.22 ± 5.71 | 20.98 ± 2.75 | 21.07 ± 2.94 | 344.65 ± 30.18 |
| INFL | 47.81 ± 11.08 aaa | 1.06 ± 0.00 aaa | 43.09 ± 9.00 aaa | 68.28 ± 6.19 aaa | 5.55 ± 0.90 aaa | 67.00 ± 5.71 aaa | 63.22 ± 9.11 aaa | 87.64 ± 11.77 aaa | 220.78 ± 53.55 aaa |
| AABF 100% | 15.80 ± 2.45 bbb | 1.08 ± 0.00 bb,cc,dd | 18.19 ± 6.34 bbb | 21.42 ± 10.63 bbb | 3.00 ± 0.31 bb | 39.23 ± 17.22 bbb | 31.22 ± 7.19 bbb,cc | 22.89 ± 5.28 bbb,cc,dd | 653.25 ± 53.79 bbb,ccc,ddd |
| AABF 50% | 15.31 ± 4.90 bbb | 1.08 ± 0.00 bb,cc,dd | 12.13 ± 3.29 bbb | 31.03 ± 6.82 bbb | 3.09 ± 0.31 bb | 50.26 ± 5.60 bb,c,dd | 34.84 ± 1.43 bbb,cc | 27.57 ± 5.69 bbb,cc,dd | 524.75 ± 74.12 bbb,ccc,ddd |
| AABF 25% | 16.66 ± 4.41 bbb | 1.08 ± 0.00 bb,cc,dd | 16.85 ± 5.73 bbb | 35.01 ± 15.54 bbb | 3.32 ± 0.46 bb | 55.25 ± 5.88 bb,c,dd | 38.94 ± 3.33 bbb,c | 28.95 ± 9.42 bbb,cc,dd | 521.00 ± 66.57 bbb,ccc,ddd |
| AABL 100% | 19.67 ± 6.86 bbb | 1.08 ± 0.00 bb,cc,dd | 14.61 ± 2.27 bbb | 27.87 ± 6.62 bbb | 2.53 ± 0.12 bbb | 44.84 ± 7.88 bb,c,dd | 15.48 ± 8.72 bbb,dd | 21.50 ± 5.09 bbb,cc,dd | 642.71 ± 67.61 bbb,ccc,ddd |
| AABL 50% | 13.12 ± 3.56 bbb | 1.08 ± 0.00 bb,cc,dd | 14.16 ± 4.54 bbb | 22.83 ± 5.94 bbb | 2.54 ± 0.21 bbb | 44.43 ± 8.52 bb,c,dd | 31.47 ± 1.95 bbb,cc | 26.50 ± 8.62 bbb,cc,dd | 679.25 ± 85.87 bbb,ccc,ddd |
| AABL 25% | 18.22 ± 6.19 bbb | 1.08 ± 0.00 bb,cc,dd | 15.42 ± 4.08 bbb | 32.95 ± 18.11 bbb | 3.28 ± 0.80 bb | 47.57 ± 6.91 bb,c,dd | 32.75 ± 7.85 bb,cc | 37.40 ± 8.86 bbb,c,d | 571.25 ± 77.49 bbb,ccc,ddd |
| AANF 100% | 18.22 ± 2.57 bbb | 1.08 ± 0.00 bb,cc,dd | 9.27 ± 1.40 bbb | 27.24 ± 7.22 bbb | 2.67 ± 0.21 bbb | 34.61 ± 2.52 bbb,d | 12.48 ± 2.92 bbb,dd | 23.28 ± 1.26 bbb,cc,dd | 337.00 ± 56.10 bbb |
| AANF 50% | 23.60 ± 9.27 bbb | 1.08 ± 0.00 bb,cc,dd | 10.25 ± 2.28 bbb | 33.54 ± 14.62 bbb | 2.94 ± 0.25 bb | 34.16 ± 9.59 bbb,d | 14.99 ± 7.56 bbb,dd | 30.94 ± 7.05 bbb,c,d | 325.80 ± 68.45 bbb |
| AANF 25% | 21.11 ± 8.23 bbb | 1.08 ± 0.00 bb,cc,dd | 13.88 ± 4.13 bbb | 40.43 ± 13.83 bbb,cc,dd | 2.91 ± 0.25 bb | 35.96 ± 10.72 bbb,d | 29.03 ± 3.64 bbb,c | 34.05 ± 7.19 bbb,c,d | 328.60 ± 64.15 bbb |
| AANL 100% | 10.03 ± 1.51 bbb | 1.08 ± 0.00 bb,cc,dd | 16.85 ± 2.38 bbb | 20.71 ± 7.71 bbb | 3.15 ± 0.34 bb | 40.03 ± 18.42 bb,c,d | 18.14 ± 5.68 bbb,dd | 18.10 ± 2.01 bbb,ccc,ddd | 329.75 ± 41.60 bbb,c,d |
| AANL 50% | 11.02 ± 2.31 bbb | 1.08 ± 0.00 bb,cc,dd | 21.83 ± 8.57 bbb | 20.94 ± 7.11 bbb | 3.17 ± 0.32 bb | 60.60 ± 4.85 ccc,ddd | 24.95 ± 6.90 bbb,c | 20.40 ± 6.62 bbb,cc,dd | 281.00 ± 128.78 bb |
| AANL 25% | 15.00 ± 4.47 bbb | 1.08 ± 0.00 bb,cc,dd | 19.53 ± 7.61 bbb | 25.49 ± 6.63 bbb | 3.26 ± 0.41 bb | 64.95 ± 4.66 ccc,ddd | 27.30 ± 4.02 bbb,dd | 29.96 ± 6.32 bbb,cc,dd | 306.25 ± 73.26 bb |
| TX | 16.21 ± 4.82 bbb | 1.08 ± 0.00 bb | 15.24 ± 1.41 bbb | 26.22 ± 4.32 bbb | 2.28 ± 0.24 bbb | 35.66 ± 4.21 bbb | 31.24 ± 4.37 bbb | 40.06 ± 4.91 bbb | 301.08 ± 28.22 bb |
| DICLO | 18.27 ± 3.12 bbb | 1.08 ± 0.00 bb | 15.28 ± 1.32 bbb | 25.54 ± 3.65 bbb | 3.09 ± 0.95 bb | 22.09 ± 2.72 bbb | 34.41 ± 2.26 bbb | 48.12 ± 5.04 bbb | 297.13 ± 19.34 |
| Groups | ALT | AST | Creatinine mg/dL | Ureea mg/dL |
|---|---|---|---|---|
| CONTROL | 45.08 ± 9.17 | 48.62 ± 3.11 | 0.74 ± 0.02 | 32.22 ± 2.32 |
| INFL | 50.59 ± 4.82 | 49.21 ± 3.46 | 1.06 ± 0.03 aa | 54.43 ± 4.43 |
| AABF 100% | 48.04 ± 5.38 | 43.32 ± 3.65 | 0.93 ± 0.01 | 53.87 ± 6.46 |
| AABF 50% | 48.25 ± 4.90 | 43.19 ± 6.84 | 0.93 ± 0.06 | 52.08 ± 6.84 |
| AABF 25% | 47.90 ± 5.63 | 45.11 ± 2.31 | 0.94 ± 0.01 | 56.20 ± 6.69 |
| AABL 100% | 41.63 ± 4.33 | 45.43 ± 4.87 | 0.95 ± 0.09 | 50.29 ± 4.12 |
| AABL 50% | 46.08 ± 6.82 | 49.23 ± 4.05 | 0.92 ± 0.06 | 52.25 ± 3.84 |
| AABL 25% | 40.82 ± 3.02 | 49.71 ± 5.44 | 0.95 ± 0.08 | 51.60 ± 2.68 |
| AANF 100% | 49.55 ± 9.64 | 40.16 ± 2.35 | 0.97 ± 0.05 | 51.17 ± 6.80 |
| AANF 50% | 46.77 ± 4.15 | 49.74 ± 3.84 | 0.92 ± 0.08 | 41.49 ± 3.89 |
| AANF 25% | 46.55 ± 3.79 | 46.48 ± 5.64 | 0.87 ± 0.01 | 50.16 ± 8.91 |
| AANL 100% | 43.24 ± 2.02 | 40.10 ± 4.64 | 0.70 ± 0.02 bb | 32.98 ± 3.18 bb |
| AANL 50% | 39.59 ± 2.24 | 40.09 ± 4.95 | 0.75 ± 0.01 bb | 27.25 ± 3.45 bb |
| AANL 25% | 43.13 ± 4.98 | 47.20 ± 5.50 | 0.73 ± 0.02 bb | 34.06 ± 2.90 bb |
| TX | 32.13 ± 5.77 | 32.86 ± 2.74 | 0.74 ± 0.02 bb | 30.42 ± 2.22 bb |
| DICLO | 37.23 ± 2.61 | 35.80 ± 2.59 | 0.81 ± 0.02 bb | 47.48 ± 5.08 bb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Țicolea, M.; Pop, R.M.; Pârvu, M.; Usatiuc, L.-O.; Uifălean, A.; Pop, D.D.; Fischer-Fodor, E.; Ranga, F.; Rusu, C.C.; Cătoi, A.F.; et al. Flowers and Leaves of Artemisia absinthium and Artemisia annua Phytochemical Characterization, Anti-Inflammatory, Antioxidant, and Anti-Proliferative Activities Evaluation. Plants 2025, 14, 1029. https://doi.org/10.3390/plants14071029
Țicolea M, Pop RM, Pârvu M, Usatiuc L-O, Uifălean A, Pop DD, Fischer-Fodor E, Ranga F, Rusu CC, Cătoi AF, et al. Flowers and Leaves of Artemisia absinthium and Artemisia annua Phytochemical Characterization, Anti-Inflammatory, Antioxidant, and Anti-Proliferative Activities Evaluation. Plants. 2025; 14(7):1029. https://doi.org/10.3390/plants14071029
Chicago/Turabian StyleȚicolea, Mădălina, Raluca Maria Pop, Marcel Pârvu, Lia-Oxana Usatiuc, Ana Uifălean, Dalina Diana Pop, Eva Fischer-Fodor, Floricuța Ranga, Crina Claudia Rusu, Adriana Florinela Cătoi, and et al. 2025. "Flowers and Leaves of Artemisia absinthium and Artemisia annua Phytochemical Characterization, Anti-Inflammatory, Antioxidant, and Anti-Proliferative Activities Evaluation" Plants 14, no. 7: 1029. https://doi.org/10.3390/plants14071029
APA StyleȚicolea, M., Pop, R. M., Pârvu, M., Usatiuc, L.-O., Uifălean, A., Pop, D. D., Fischer-Fodor, E., Ranga, F., Rusu, C. C., Cătoi, A. F., Palma-Garcia, F., Gherman, L.-M., & Pârvu, A. E. (2025). Flowers and Leaves of Artemisia absinthium and Artemisia annua Phytochemical Characterization, Anti-Inflammatory, Antioxidant, and Anti-Proliferative Activities Evaluation. Plants, 14(7), 1029. https://doi.org/10.3390/plants14071029










