Wheat Drought Tolerance: Unveiling a Synergistic Future with Conventional and Molecular Breeding Strategies
Abstract
1. Introduction
2. Wheat Growth Stages and Response to Drought
2.1. Drought at Seedling Stage
2.2. Drought at Tillering Stage
2.3. Drought at Grain Filling Stage
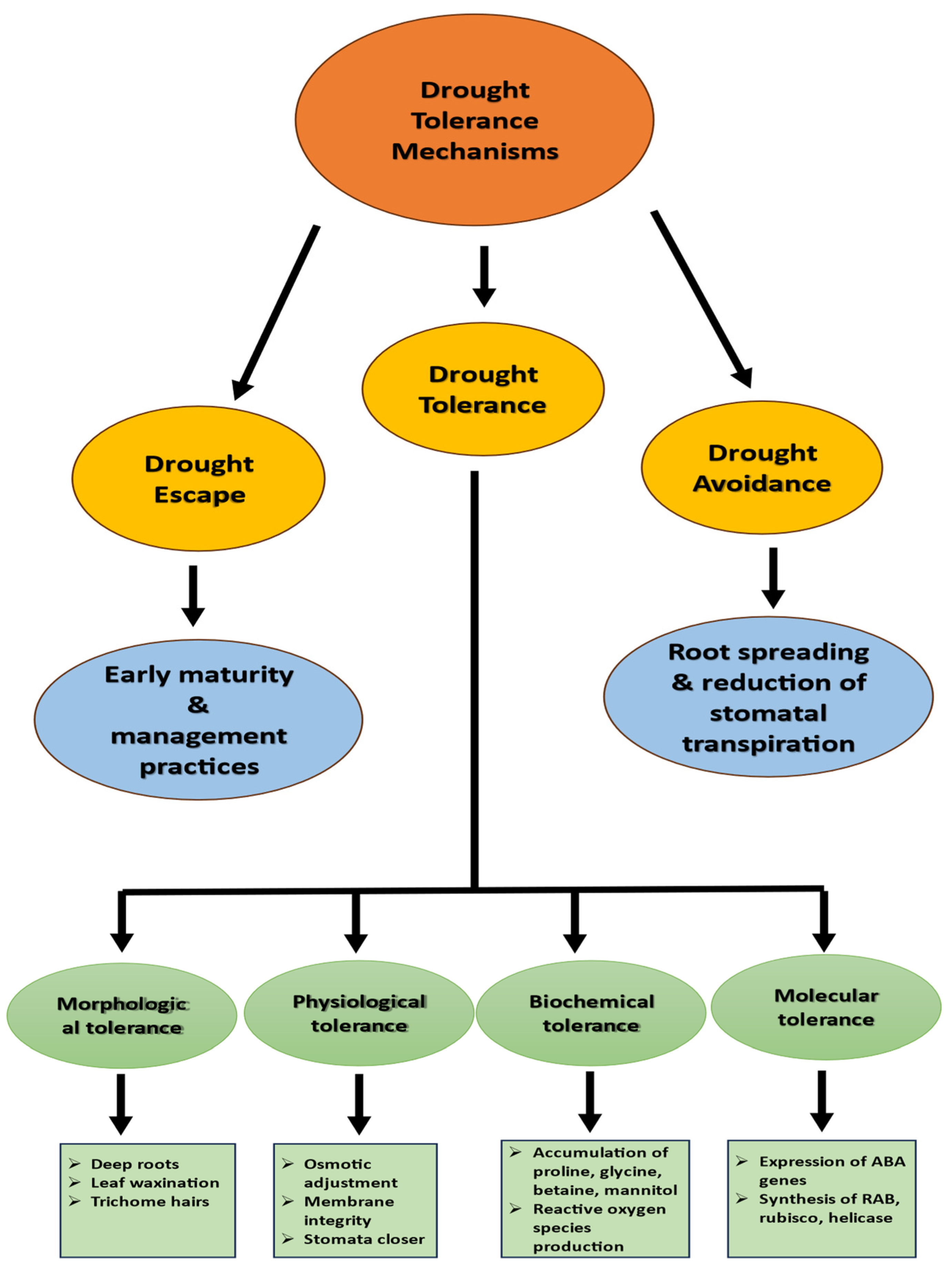
3. Adaptations for Drought Stress
3.1. Morpho-Physiological Adaptation
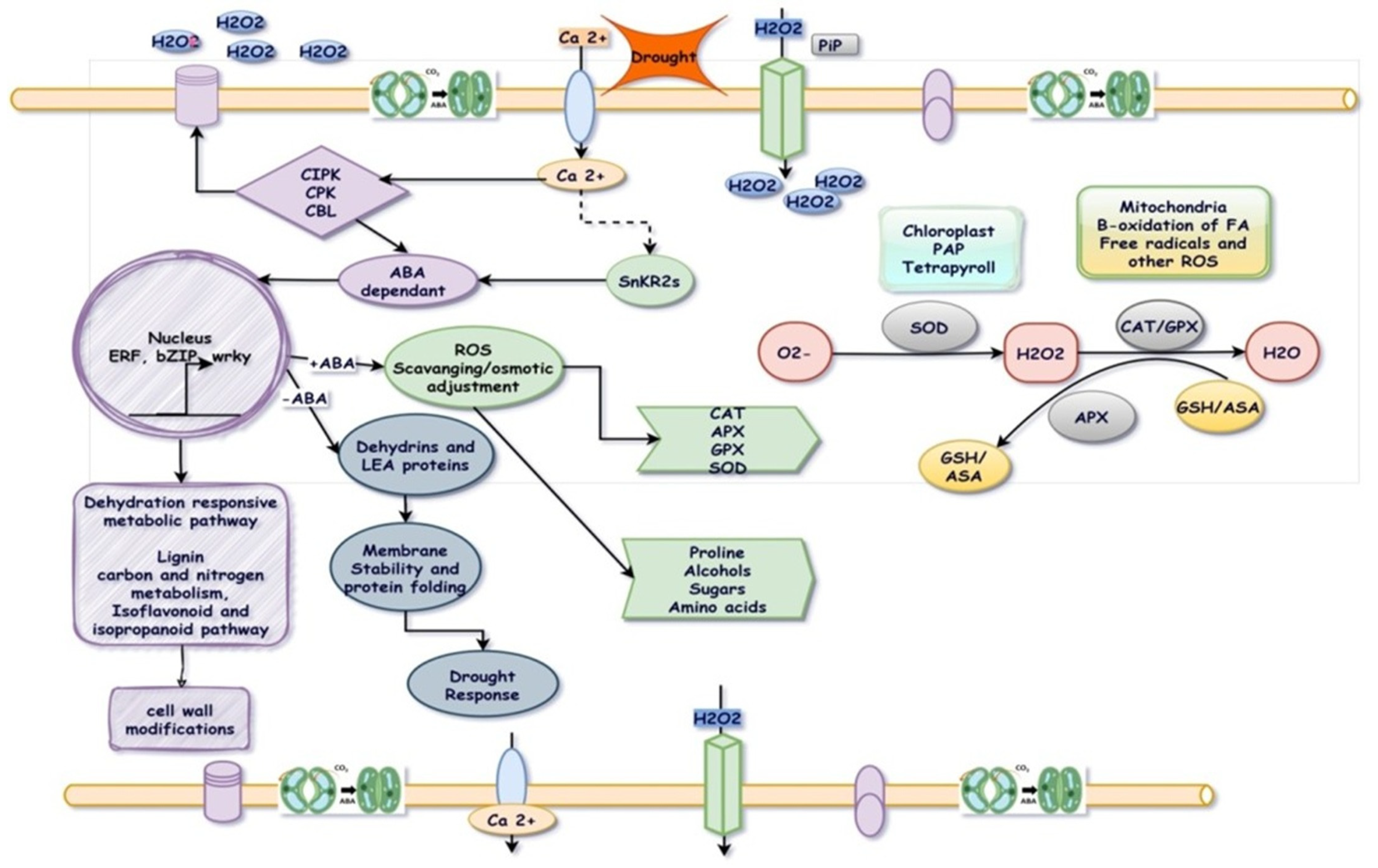
3.2. Cellular and Biochemical Adaptation
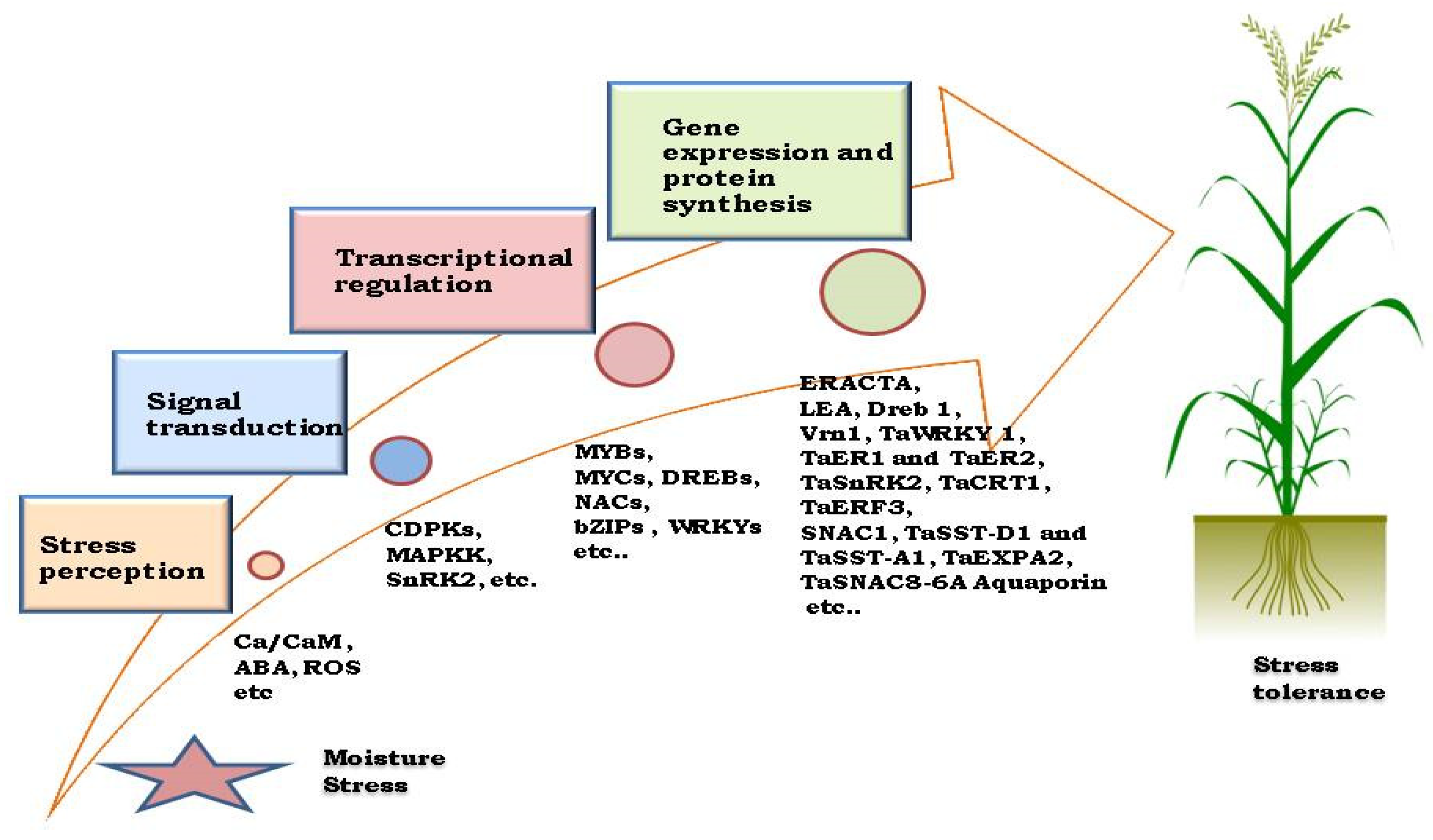
3.3. Molecular Adaptation
| Trait | Environment | Yield Factor | Techniques | Reference |
|---|---|---|---|---|
| Seedling vigor | Pot, dry | Water uptake | Visual | [61] |
| Cooler canopy | Drought, field | Deep root | Visual | [62] |
| Leaf architecture | Any, dry | Water use efficiency | Visual/metric | [61] |
| Leaf rolling | Drought, field | Transpiration and water loss | Visual | [62] |
| Canopy green area | drought | vegetation index | Red, green, blue imaging | [63] |
| Leaf area index | drought | Normalized difference vegetation index | Red, green, blue imaging | [64] |
| Normalized difference vegetation index | Drought, field | Canopy temp | Red, green, blue imaging | [65] |
| Stay green traits | drought | - | Red, green, blue imaging | [66] |
| Semi-dwarf habit | Any | Harvest index | Visual/molecular markers | [67] |
| Root diameter | Laboratory, field | Associated with seed yield | Wax-layer screen | [68] |
| Deep roots | Field, dry | Water uptake | Infrared thermometry | [69] |
| Deep root | Laboratory, field | Greatest number of shallow roots | Wax-layer screen | [68] |
| Root architecture | Laboratory, field | Nitrogen uptake efficiency | High throughput laboratory screens | [70] |
| Growth rate/biomass | Any, Dry | Water uptake, water use efficiency | Metric/spectral reflectance | [71] |
| Biomass, leaf area index | Field | Green area indexes | Conventional digital cameras | [72] |
4. Breeding Strategies for Developing Drought Stress Tolerance in Wheat
4.1. Convetional Approaches
4.1.1. Utilization of Wild Species for Trait Manipulation
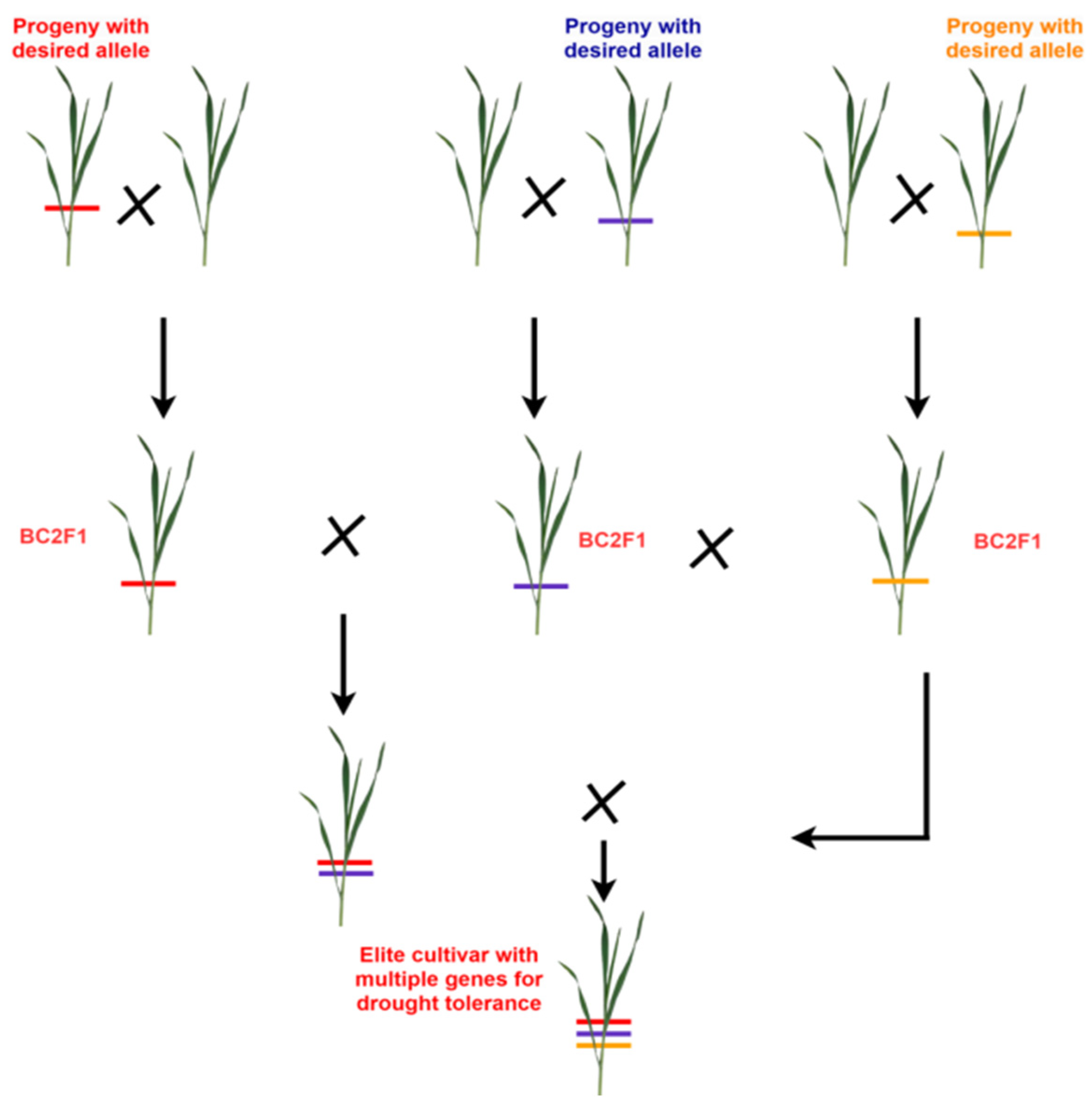
4.1.2. Backcross Breeding
4.1.3. Mutation Breeding and TILLING
4.1.4. Recurrent Selection
4.2. Molecular and Metabolic Approaches
4.2.1. Development of DNA Based Markers for Drought Tolerance in Wheat
4.2.2. Marker-Assisted Selection
4.2.3. Quantitative Trait Loci (QTLs), MQTLs, and e-QTLs
4.2.4. GWAS, Genetic Linkage Mapping, and Transcriptomics for Drought Tolerance
4.2.5. Phenomics and Metabolomics
| Annotated Metabolites | Sample | Analytical Platform | References |
|---|---|---|---|
| Targeted and non-target analysis: amino acids, organic acids, sugars, sugar alcohols, and organic antioxidants | Root and leaf tissue | GC-MS | [145] |
| Non-target analysis: amino acids, organic acids, sugars, polyols, glycolysis cycle, and GABA shunt metabolites | Shoot tissue | TOF | [146] |
| Non-target analysis: amino acids, organic acids, sugars | Leaf tissue | GC-MS | [147] |
| Non-target analysis: lipids, sugars, oxidative stress compounds, and phytohormones | Root tissue | RPLC-Q-TOF | [148] |
| Increase in total phenolics, flavonoids, anthocyanins | Leaf tissue | Colorimetric method | [149,150] |
| Induction in sugars, amino acids, organic acids | Leaf tissue | GC–MS | [146,151] |
| Tannins | seeds | RT-PCR | [152] |
| Flavonoid | Leaf tissue | Colorimetric method | [150] |
| AA (serine, asparagine, methionine, lysine) | Seeds | GC/MS | [147] |
| Organic compounds, phenols, flavonoid | Leaf tissue | GC/MS | [17] |
| Soluble sugars and proline, proteins, inorganic solutes | Seeds | Biochemical methods | [153] |
| Proline, protein content, total soluble sugars | Leaf tissue | Biochemical methods | [154] |
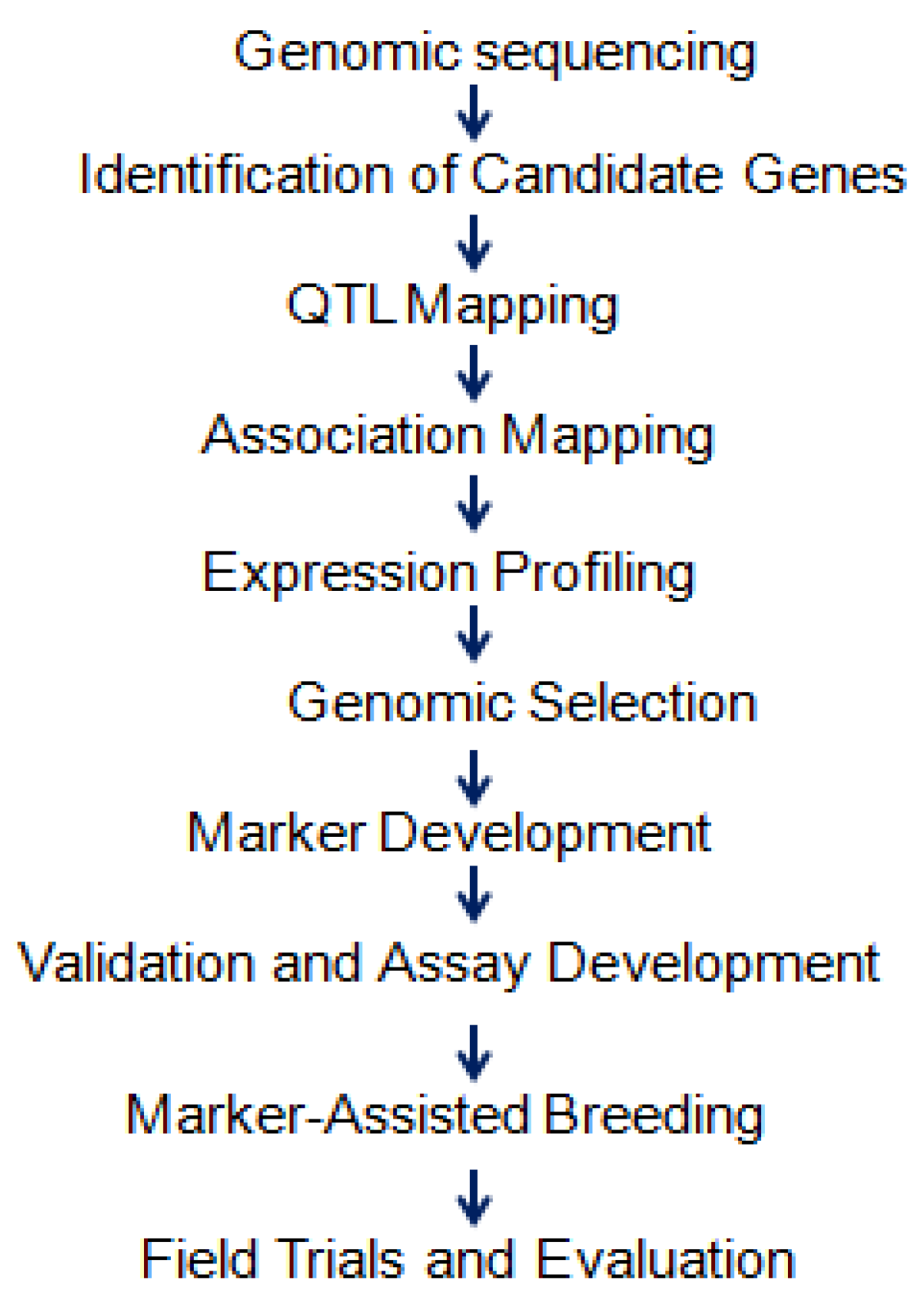
4.3. Genomics-Assisted Breeding Approaches
4.3.1. Genomic Breeding Approaches for Designing Drought Tolerance in Wheat
4.3.2. Genomic Selection
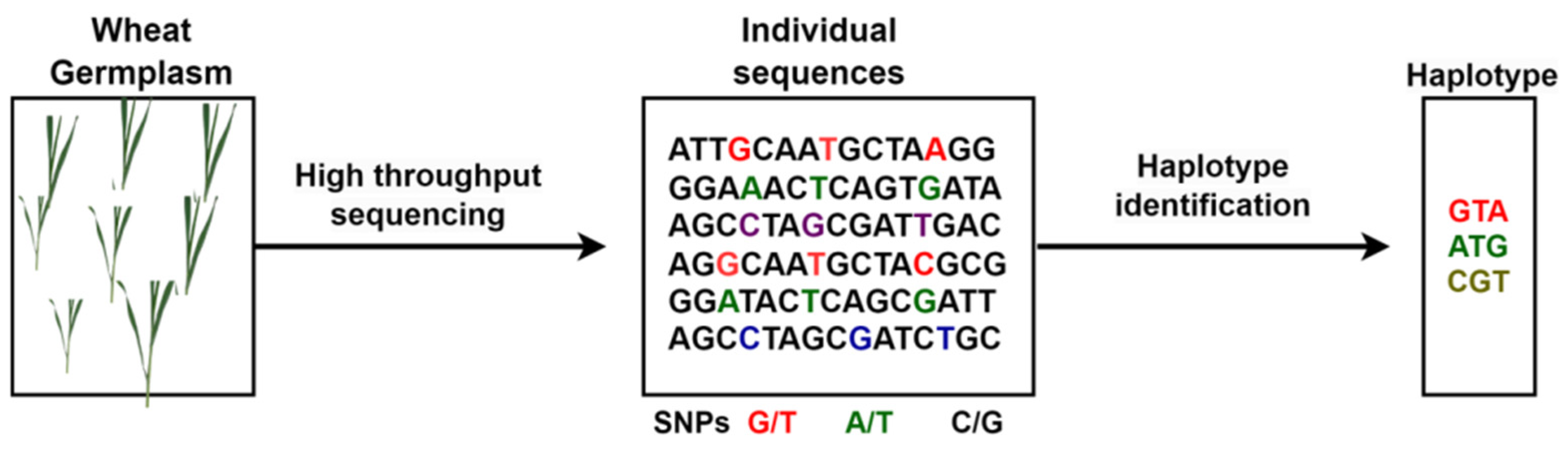
4.3.3. Haplotype
| Traits | Chromosomes | Study Approach | Population Type | Reference |
|---|---|---|---|---|
| Drought susceptibility index | - | RAPD | wheat genotypes | [163] |
| DH, PH, TKW | 12A | SSR and RFLP | Back cross | [164] |
| Normal and drought stress | - | SSR Marker | RILs | [165] |
| Normal and drought stress | - | SSR Marker | DH | [166] |
| Normal (ramandi 2014) | - | SSR and DArT | RILs | [167] |
| NDVI and grain yield | 2A, 2D, 3B, and 5A | SSR/MABC | HD2733 × C306 (donor) | [168] |
| Grain yield and other traits | 2A, 3B, 4B | MABC | BC1F1 | [105] |
| Grain quality and rust resistance | - | MABC | F2–F5 | [9] |
| Grain yield | - | MABC | F3 to F8 | [168] |
| Root length, root weight, root density, root diameter | 2A, 2B, 2D, 5B | Marker–trait association | Core collection | [169] |
| NDVI, GFD, TKG, grain yield | - | MARS | BC1/BC2 F2 | [116] |
| Grain yield and biological yield | 1D, 4A | MARS | Backcross populations | [170,171] |
| NUE and photosynthesis | 1B and 5A. | Haplotype breeding | Wheat cultivars | [172] |
| DH, PH, and TKW | 3D, 4A, 5B, 7A, and 7B | GWAS | Diverse wheat genotypes | [173] |
| Grain yield | - | Genomic Selection | Wheat lines from CIMMYT | [174] |
| Root growth angle | DRO1-like genes | Genome editing | NARC 2009 and Galaxy variety | [175] |
| YLD, PH, TNPM, TKG, GNS, SL, HI | 1A, 1B, 1D, 2B, 3A, 3B, 6A, 6B and 7A | Genetic linkage mapping | RILs | [175,176] |
| NDVI, CT, PH, GWPS, TKG and YLD | 2A, 5D, 5A and 4B | Genetic linkage mapping | RILs | [177] |
| Root length and root weight | 1B, 2A, 2B, 2D, 3D, 4A, 4B, 5A, 5B, 6A, 6B, 6D, 7A | GWAS | Core collection | [178] |
| Root numbers, root weight, seed weight, seed length | 1B, 2A, 2B, 3B, 5A, 5B, 6A, 7A | GWAS | Landraces | [179] |
| Flavonoid biosynthesis | - | Transcriptomics | Wheat cultivars | [180] |
| Dehydrins and aquaporins | - | Transcriptomics | Landraces | [181] |
| PH, TNPM, DH, juvenile growth habit | 2B, 4D | Exome sequencing | RILs | [62] |
| Aquaporins, LEA proteins | 5B, 6D, 6B, 2B | RNA Seq | Landraces | [182] |
| RWC, TKW, awn length, coleoptiles length, shoot length | 2A | QTL analysis | Core collection | [183] |
| Grain yield | 3BL | QTL analysis | DH (RAC875 × Kukri) | [184] |
| YLD, CT, potential quantum efficiency, chlorophyll content | 1A, 1D, 2B, 3A, 3B, 4B, 4D, 5B, 6A | QTL analysis | RILs (C306 × HUW206) | [139] |
4.3.4. Genome Editing and Sequencing
4.3.5. Bioinformatics and Speed Breeding
5. Future Road Map
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shewry, P.R.; Popineau, Y.; Lafiandra, D.; Belton, P. Wheat glutenin subunits and dough elasticity: Findings of the EUROWHEAT project. Trends Food Sci. Technol. 2000, 11, 433–441. [Google Scholar] [CrossRef]
- Gill, B.S.; Appels, R.; Botha-Oberholster, A.M.; Buell, C.R.; Bennetzen, J.L.; Chalhoub, B.; Chumley, F.; Dvorák, J.; Iwanaga, M.; Keller, B.; et al. A workshop report on wheat genome sequencing: International Genome Research on Wheat Consortium. Genetics 2004, 168, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Shukla, R.S.; Singh, S.K.; Pandey, S. Holistic approach to ascertain genetic variability and responsive trait selection for different heat regimes of central India via field screening of wheat recombinant inbred lines. J. Agron. Crop Sci. 2024, 210, e12674. [Google Scholar] [CrossRef]
- Sehgal, D.; Dhakate, P.; Ambreen, H.; Shaik, K.H.B.; Rathan, N.D.; Anusha, N.M.; Deshmukh, R.; Vikram, P. Wheat omics: Advancements and opportunities. Plants 2023, 12, 426. [Google Scholar] [CrossRef]
- Khare, V.; Pandey, S.; Singh, S.; Shukla, R. Identification of drought tolerant recombinant inbred lines (RILs) based on selection indices in bread wheat. tritici. J. Cereal Res. 2022, 14, 44–56. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on maize and wheat production. PLoS ONE 2016, 11, e0156362. [Google Scholar] [CrossRef]
- Hazaymeh, K.; Hassan, Q.K. Remote sensing of agricultural drought monitoring: A state of art review. Aims Environ. Sci. 2016, 3, 604–630. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Gautam, T.; Dhillon, G.; Gautam, S.; Singh, R.; Singh, V.; Prasad, P.; Kaur, S.; Chhuneja, P.; Sharma, P.; Balyan, H.; et al. Marker-assisted pyramiding of genes/QTL for grain quality and rust resistance in wheat (Triticum aestivum L.). Mol. Breed. 2020, 40, 49. [Google Scholar] [CrossRef]
- CIMMYT (International Maize and Wheat Improvement Center), 2014.Wheat Improvement e The Mandate of CIMMYT’s Global Wheat Program (2014-11-12). Available online: https://www.cimmyt.org/es/nuestro-trabajo/trigo/ (accessed on 15 April 2023).
- Mylonas, I.; Stavrakoudis, D.; Katsantonis, D.; Korpetis, E. Better farming practices to combat climate change. In Climate Change and Food Security with Emphasis on Wheat; Academic Press: Cambridge, MA, USA, 2020; pp. 1–29. [Google Scholar] [CrossRef]
- Merchuk-Ovnat, L.; Barak, V.; Fahima, T.; Ordon, F.; Lidzbarsky, G.A.; Krugman, T.; Saranga, Y. Ancestral QTL alleles from wild emmer wheat improve drought resistance and productivity in modern wheat cultivars. Front. Plant Sci 2016, 7, 452. [Google Scholar] [CrossRef]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Sankar, B.; Murali, P.V.; Gomathinayagam, M.; Lakshmanan, G.M.A.; Panneerselvam, R. Water deficit stress effects on reactive oxygen mobilization in Catharanthus roseus; impacts on ajmalicine accumulation. Colloids Surf. B Biointerfaces 2008, 62, 105–111. [Google Scholar] [PubMed]
- Poggi, G.M.; Corneti, S.; Aloisi, I.; Ventura, F. Environment-oriented selection criteria to overcome controversies in breeding for drought resistance in wheat. J. Plant Physiol. 2023, 280, 153895. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Gopi, R.; Somasundaram, R.; Panneerselvam, R. Water Deficit Stress Mitigation by Calcium Chloride in Catharanthus roseus: Effects on Oxidative Stress, Proline Metabolism and Indole Alkaloid Accumulation. Colloids Surf. B Biointerfaces 2007, 60, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Pratap, A.; Douglas, C.; Prajapati, U.; Kumari, G.; War, A.R.; Tomar, R.; Pandey, A.K.; Dubey, S. Breeding Progress and Future Challenges: Biotic Stresses. In The Mungbean Genome; Springer: Cham, Switzerland, 2020; pp. 55–80. [Google Scholar]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide Application Improves the Drought Tolerance in Maize Through Modulation of Enzymatic Antioxidants and Leaf Gas Exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Shi, J.F.; Mao, X.G.; Jing, R.L.; Pang, X.B.; Wang, Y.G. Chang, X.P. Gene Expression Profiles of Response toWater Stress at the Jointing Stage in Wheat. Agric. Sci. China 2010, 9, 325–330. [Google Scholar] [CrossRef]
- Acevedo, E.; Silva, P.; Silva, H. Wheat Growth and Physiology. Bread Wheat Improvement and Production. Food and Agriculture Organization of The United Nations, Rome. 2002. Available online: https://www.fao.org/3/y4011e/y4011e06.htm#bm06 (accessed on 20 March 2022).
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological responses of wheat to drought stress and its mitigation approaches. Acta Physiol. Plant. 2018, 40, 80. [Google Scholar] [CrossRef]
- Almaghrabi, O.A. Impact of drought stress on germination and seedling growth parameters of some wheat cultivars. Life Sci. J. 2012, 1, 590–598. [Google Scholar]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Sarto, J.R.W.; Rampim, L.; Rosset, J.S.; Bassegio, D.; da Costa, P.F.; Inagaki, A.M. Wheat phenology and yield under drought: A review. Aust. J. Crop. Sci. 2017, 11, 941–946. [Google Scholar] [CrossRef]
- Elhani, S.; Martos, V.; Rharrabti, Y.; Royo, C.; del Moral, L.G. Contribution of main stem and tillers to durum wheat (Triticum turgidum L. var. durum) grain yield and its components grown in Mediterranean environments. Field Crops Res 2007, 103, 25–35. [Google Scholar] [CrossRef]
- Ribot, G.G.; Silva, P.; Acevedo, E. Morphological and physiological traits of assistance in the selection of high yielding varieties of durum wheat (Triticum turgidum L. spp. Durum) for the rainfed Mediterranean environments of central Chile. Am. J. Plant Sci. 2012, 3, 1809–1819. Available online: http://www.scirp.org/journal/PaperInformaton.aspx?PaperID=25993 (accessed on 5 February 2024). [CrossRef]
- van Ginkel, M.; Calhoun, D.S.; Gebeyehu, G.; Miranda, A.; Tian-you, C.; Pargas Lara, R.; Trethowan, R.M.; Sayre, K.; Crossa, J.; Rajaram, S. Plant traits related to yield of wheat in early, late, or continuous drought conditions. Euphytica 1998, 100, 109–121. [Google Scholar]
- Cattivelli, L.; Rizza, F.; Badeck, F.-W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crops Res. 2008, 105, 1–14. [Google Scholar]
- Jatayev, S.; Sukhikh, I.; Vavilova, V.; Smolenskaya, S.E.; Goncharov, N.P.; Kurishbayev, A.; Zotova, L.; Absattarova, A.; Serikbay, D.; Hu, Y.G.; et al. Green revolution ‘stumbles’ in a dry environment: Dwarf wheat with Rht genes fails to produce higher grain yield than taller plants under drought. Plant Cell Environ. 2020, 43, 2355–2364. [Google Scholar] [CrossRef]
- Mohammadi, R.; Etminan, A.; Shoshtari, L. Agro-physiological characterization of durum wheat genotypes under drought conditions. Exp. Agric. 2019, 55, 484–499. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of drought stress on some agronomic and morpho-physiological traits in durum wheat genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Villegas, D.; Garcia del Moral, L.F.; Rharrabti, Y.; Martos, V.; Royo, C. Morphological traits above the flag leaf node as indicators of drought susceptibility index in durum wheat. J. Agron. Crop Sci. 2007, 193, 103–116. [Google Scholar] [CrossRef]
- Vosoghi Rad, M.; Jami Moeini, M.; Taherian, M.; Armin, M. Accumulation and remobilization of assimilates in different genotypes of durum wheat under terminal drought stress. J. Crop. Sci. Biotechnol. 2022, 25, 199–214. [Google Scholar] [CrossRef]
- Clarke, J.M. Effect of leaf rolling on leaf water loss in Triticum spp. Can. J. Plant Sci. 1986, 66, 885–891. [Google Scholar]
- Yang, R.C.; Jana, S.; Clarke, J.M. Phenotypic diversity and associations of some potentially drought-responsive characters in durum wheat. Crop Sci. 1991, 31, 1484–1491. [Google Scholar] [CrossRef]
- Huang, B.; DaCosta, M.; Jiang, Y. Research advances in mechanisms of turfgrass tolerance to abiotic stresses: From physiology to molecular biology. Crit. Rev. Plant Sci. 2014, 33, 141–189. [Google Scholar] [CrossRef]
- Impa, S.M.; Sunoj, V.S.J.; Krassovskaya, I.; Bheemanahalli, R.; Obata, T.; Jagadish, S.V.K. Carbon Balance and Source-Sink Ihsan, M.Z.; El-Nakhlawy, F.S.; Ismail, S.M.; Fahad, S.; Daur, I. Wheat phenological development and growth studies as affected by drought and late season high temperature stress under arid environment. Front. Plant Sci. 2016, 7, 795. [Google Scholar] [CrossRef]
- Kamrani, M. Relationship among agro-morphological traits in bread wheat (Triticum aestivum L.) genotypes under irrigated and rain-fed conditions. J. Agron. 2015, 14, 254–263. [Google Scholar] [CrossRef][Green Version]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J 2013, 1–12. [Google Scholar] [CrossRef]
- Yang, J.; Zang, J. Grain filling of cereals under soil drying. New Phytol. 2006, 169, 223–236. [Google Scholar] [CrossRef]
- Monneveux, P.; Reynolds, M.P.; Gonzaalez-Santoyo, H.; Pena, R.J.; Mayr, L.; Zapata, F. Relationships between grain yield, flag leaf morphology, carbon isotope discrimination and ash content in irrigated wheat. J. Agron. Crop Sci. 2004, 190, 395–401. [Google Scholar] [CrossRef]
- Ali, Z.; Merrium, S.; Habib-ur-Rahman, M.; Hakeem, S.; Saddique, M.A.B.; Sher, M.A. Wetting mechanism and morphological adaptation; leaf rolling enhancing atmospheric water acquisition in wheat crop—A review. Environ. Sci. Pollut. Res. 2022, 29, 30967–30985. [Google Scholar] [CrossRef]
- Kadioglu, A.; Terzi, R. A dehydration avoidance mechanism: Leaf rolling. Bot. Rev. 2007, 73, 290–302. [Google Scholar] [CrossRef]
- O’toole, J.C.; Cruz, R.T.; Singh, T.N. Leaf rolling and transpiration. Plant Sci. Lett. 1979, 16, 111–114. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, X.; Morita, S.; Sekiya, N.; Araki, H.; Gu, H.; Liu, X. Are crop deep roots always beneficial for combating drought: A review of root structure and function, regulation and phenotyping. Agric. Water Manag. 2022, 271, 107781. [Google Scholar] [CrossRef]
- Singh, A. Soil Salinization Management for Sustainable Development: A Review. J. Environ. Manag. 2021, 277, 111383. [Google Scholar] [CrossRef]
- Muhammad, A.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Shad Ali, G.; Yang, L.; Du, L.; Reddy, A.S.; Poovaiah, B.W. Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal. Behav. 2010, 5, 991–994. [Google Scholar] [CrossRef]
- Nio, S.; Cawthray, G.; Wade, L.; Colmer, T. Pattern of solutes accumulated during leaf osmotic adjustment as related to duration of water deficit for wheat at the reproductive stage. Plant Physiol. Biochem. 2011, 49, 1126–1137. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought tolerance in plants: Physiological and molecular responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Lata, C.; Muthamilarasan, M.; Prasad, M. Drought Stress Responses and Signal Transduction in Plants. In Elucidation of Abiotic Stress Signaling in Plants: Functional Genomics Perspectives; Pandey, G., Ed.; Springer: New York, NY, USA, 2015; pp. 195–225. ISBN 978-1-4939-2540-7. [Google Scholar]
- Kim, T.H. Mechanism of ABA Signal Transduction: Agricultural Highlights for Improving Drought Tolerance. J. Plant Biol. 2014, 57, 1–8. [Google Scholar]
- Kaur, G.; Asthir, B. Molecular Responses to Drought Stress in Plants. Biol. Plant 2017, 61, 201–209. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K.; Shinozaki, K. The Transcriptional Regulatory Network in the Drought Response and Its Crosstalk in Abiotic Stress Responses Including Drought, Cold, and Heat. Front. Plant Sci. 2014, 5, 170. [Google Scholar]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar]
- Xue, G.P.; McIntyre, C.L.; Chapman, S.; Bower, N.I.; Way, H.; Reverter, A.; Clarke, B.; Shorter, R. Differential gene expression of wheat progeny with contrasting levels of transpiration efficiency. Plant Mol. Biol. 2006, 61, 863–881. [Google Scholar]
- Ribaut, J.M.; Vicente, M.C.; Delannay, X. Molecular breeding in developing countries: Challenges and perspectives. Curr. Opin. Plant Biol. 2010, 13, 213–218. [Google Scholar] [PubMed]
- Esposito, S.; Agostino, N.; Taranto, F.; Sonnante, G.; Sestili, F.; Lafiandra, D.; De Vita, P. Whole-exome sequencing of selected bread wheat recombinant inbred lines as a useful resource for allele mining and bulked segregant analysis. Front. Genet. 2022, 13, 1058471. [Google Scholar]
- Nehe, A.; King, J.; King, I.P.; Murchie, E.H.; Foulkes, M.J. Identifying variation for N-use efficiency and associated traits in amphidiploids derived from hybrids of bread wheat and the genera Aegilops, Secale, Thinopyrum and Triticum. PLoS ONE 2022, 17, e0266924. [Google Scholar] [CrossRef]
- Fernandez-Gallego, J.A.; Kefauver, S.C.; Vatter, T.; Gutiérrez, N.A.; Nieto-Taladriz, M.T.; Araus, J.L. Low-cost assessment of grain yield in durum wheat using RGB images. Eur. J. Agron. 2019, 105, 146–156. [Google Scholar]
- Nehe, A.S.; Foulkes, M.J.; Ozturk, I.; Rasheed, A.; York, L.; Kefauver, S.C.; Ozdemir, F.; Morgounov, A. Root and canopy traits and adaptability genes explain drought tolerance responses in winter wheat. PLoS ONE 2021, 16, e0242472. [Google Scholar] [CrossRef]
- Kefauver, S.C.; El-Haddad, G.; Vergara, O.; Araus, J.L. RGB picture vegetation indexes for High-Throughput Phenotyping Platforms (HTPPs). In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XVII, Toulouse, France, 21–24 September 2015. [Google Scholar]
- Rebetzke, G.J.; Ellis, M.H.; Bonnett, D.G.; Richards, R.A. Molecular mapping of genes for Coleoptile growth in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 1173–1183. [Google Scholar]
- Bai, C.; Ge, Y.; Ashton, R.W.; Evans, J.; Milne, A.; Hawkesford, M.J.; Whalley, W.R.; Parry, M.A.J.; Melichar, J.; Feuerhelm, D.; et al. The relationships between seedling root screens, root growth in the field and grain yield for wheat. Plant Soil 2019, 440, 311–326. [Google Scholar] [CrossRef]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 2007, 58, 177–186. [Google Scholar] [PubMed]
- Kenobi, K.; Atkinson, J.A.; Wells, D.M.; Gaju, O.; Silva De, J.G.; Foulkes, M.J.; Dryden, I.L.; Wood, A.T.A.; Bennett, M.J. Linear discriminant analysis reveals differences in root architecture in wheat seedlings related to nitrogen uptake efficiency. J. Exp. Bot. 2017, 68, 4969–4981. [Google Scholar] [PubMed]
- Babar, M.A.; Reynolds, M.P.; Van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance indices as a potential indirect selection criterion for wheat yield under irrigation This research was partially funded by the Oklahoma Wheat Research Foundation (OWRF), Oklahoma Wheat Commission and CIMMYT, Mexico. Crop Sci. 2006, 46, 578–588. [Google Scholar]
- Zhou, L.; Ming, B.; Wang, K.; Shen, D.; Fang, L.; Yang, H.; Xue, J.; Xie, R.; Hou, P.; Ye, J.; et al. Establishment of critical nitrogen-concentration dilution curves based on leaf area index and aboveground biomass for drip-irrigated spring maize in Northeast China. Crop J. 2025, 13, 1–9. [Google Scholar] [CrossRef]
- Rivero, R.M.; Kojima, M.; Gepstein, A.; Sakakibara, H.; Mittler, R.; Gepstein, S.; Blumwald, E. Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Proc. Natl. Acad. Sci. USA 2007, 104, 19631–19636. [Google Scholar]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 2009, 150, 1530–1540. [Google Scholar]
- Wani, S.H.; Khan, H.; Riaz, A.; Joshi, D.C.; Hussain, W.; Rana, M.; Kumar, A.; Athiyannan, N.; Singh, D.; Ali, N.; et al. Genetic diversity for developing climate-resilient wheats to achieve food security goals. Adv. Agron. 2022, 171, 255–303. [Google Scholar]
- Ceoloni, C.; Kuzmanovic, L.; Ruggeri, R.; Rossini, F.; Fort, P.; Cuccurullo, A.; Bitti, A. Harnessing genetic diversity of wild gene pools to enhance wheat crop production and sustainability: Challenges and opportunities. Diversity 2017, 9, 55. [Google Scholar] [CrossRef]
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef]
- Khan, A.; Sovero, V.; Gemenet, D. Genome-assisted breeding for drought resistance. Curr. Genom. 2016, 17, 330–342. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Stalker, H.T.; Blair, M.W.; Bertioli, D.J.; Upadhyaya, H.; Nielen, S.; Ortiz, R. Enhancing crop gene pools with beneficial traits using wild relatives. Plant Breed. Rev. 2008, 30, 179–230. [Google Scholar]
- Maxted, N.; Ford-Lloyd, B.V.; Jury, S.; Kell, S.; Scholten, M. Towards a definition of a crop wild relative. Biodivers. Conserv. 2006, 15, 2673–2685. [Google Scholar] [CrossRef]
- Mondal, S.; Rutkoski, J.E.; Velu, G.; Singh, P.K.; Crespo-Herrera, L.A.; Guzman, C.; Bhavani, S.; Lan, C.; He, X.; Singh, R.P. Harnessing diversity in wheat to enhance grain yield, climate resilience, disease and insect pest resistance and nutrition through conventional and modern breeding approaches. Front. Plant Sci. 2016, 7, 991. [Google Scholar] [CrossRef] [PubMed]
- Sigmon, B.; Vollbrecht, E. Amazing grass: Developmental genetics of maize domestication. Biochem. Soc. Trans. 2005, 33, 1502. [Google Scholar] [CrossRef]
- Baboev, S.K.; Buranov, A.K.; Bozorov, T.A.; Adylov, B.S.; Morgunov, A.I.; Muminzhonov, H. Biological and agronomical assessment of wheat landraces cultivated in mountain areas of Uzbekistan. Sel’skokhozyaistvennaya Biol. (Agric. Biol.) 2017, 52, 553–560. [Google Scholar] [CrossRef]
- Trethowan, R.M.; Mujeeb-Kazi, A. Novel germplasm resources for improving environmental stress tolerances of hexaploid wheat. Crop Sci. 2008, 48, 1255–1265. [Google Scholar] [CrossRef]
- Placido, D.F.; Campbell, M.T.; Folsom, J.J.; Cui, X.; Kruger, G.R.; Baenziger, P.S.; Walia, H. Introgression of novel traits from a wild wheat relative improves drought adaptation in wheat. Plant Physiol. 2013, 161, 1806. [Google Scholar] [CrossRef]
- Zaharieva, M.; Ayana, N.G.; Hakimi AAl Misra, S.C.; Monneveux, P. Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: A review. Genet. Resour. Crop Evol. 2010, 57, 937–962. [Google Scholar] [CrossRef]
- Peleg, Z.; Fahima, T.; Krugman, T.; Abbo, S.; Yakir, D.; Korol, A.B.; Saranga, Y. Genomic dissection of drought resistance in durum wheat’ wild emmer wheat recombinant inbred line population. Plant Cell Environ. 2009, 32, 758–779. [Google Scholar] [CrossRef]
- Nevo, E.; Chen, G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010, 33, 670–685. [Google Scholar] [PubMed]
- Kumar, A.; Sharma, A.; Sharma, R.; Choudhary, A.; Srivastava, P.; Kaur, H.; Padhy, A.K. Morpho-physiological evaluation of Elymus semicostatus (Nees ex Steud.) Melderis as potential donor for drought tolerance in Wheat (Triticum aestivum L.). Genet. Resour. Crop. Evol. 2021, 69, 411–430. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Sharma, R.; Srivastva, P.; Choudhary, A. Exploration of wheat wild relative diversity from Lahaul valley: A cold arid desert of Indian Himalayas. Cereal Res. Commun. 2021, 50, 305–320. [Google Scholar] [CrossRef]
- Suneja, Y.; Gupta, A.K.; Bains, N.S. Stress adaptive plasticity: Aegilops tauschii and Triticum dicoccoides as Potential donors of drought associated morpho-physiological traits in wheat. Front. Plant Sci. 2019, 10, 211. [Google Scholar]
- Allard, R.W. Principles of Plant Breeding; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Jiang, G.L. Molecular markers and marker assisted breeding in plants. In Plant Breeding from Laboratories to Fields; Anderson, S.B., Ed.; InTech: Rijeka, Croatia; London, UK, 2013; pp. 45–83. [Google Scholar]
- Babu, R.; Nair, S.K.; Prasanna, B.M.; Gupta, H.S. Integrating marker-assisted selection in crop breeding–Prospects and challenges. Curr. Sci. 2004, 87, 601–619. [Google Scholar]
- Holland, J.B. Implementation of molecular markers for quantitative traits in breeding programs-challenges and opportunities. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Mishra, V.K.; Srivastava, K.; Kumar, M.; Singh, P. Marker-assisted foreground pyramiding of genes/QTL’s for grain quality and rust resistance genes in most popular wheat cv., HUW234 of Eastern Gangetic plains of India. J. Plant Biochem. Biotech. 2025, 17, 1–7. [Google Scholar]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2007, 363, 557–572. [Google Scholar]
- Salina, E.; Dobrovolskaya, O.; Efremova, T.; Leonova, I.; Roder, M.S. Microsatellite monitoring of recombination around the Vrn-B1 locus of wheat during early backcross breeding. Plant Breed. 2003, 122, 116–119. [Google Scholar]
- Hospital, F. Selection in backcross programmes. Philos. Trans. R. Soc. B 2005, 360, 1503–1511. [Google Scholar]
- Frisch, M.; Bohn, M.; Melchinger, A.E. Comparison of selection strategies for marker-assisted backcrossing of a gene. Crop Sci. 1999, 39, 1295–1301. [Google Scholar]
- Visscher, P.M.; Haley, C.S.; Thompson, R. Marker assisted introgression in backcross breeding programs. Genetics 1996, 144, 1923–1932. [Google Scholar] [PubMed]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2019, 42, 33–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandey, K.; Dangi, R.; Prajapati, U.; Kumar, S.; Maurya, N.K.; Singh, A.V.; Pandey, A.K.; Singh, J.; Rajan, R. Advance breeding and biotechnological approaches for crop improvement: A review. Int. J. Chem. Stud. 2019, 7, 837–841. [Google Scholar]
- Mwadzingeni, L.; Figlan, S.; Shimelis, H.; Mondal, S.; Tsilo, T. Genetic resources and breeding methodologies for improving drought tolerance in wheat. J. Crop Improv. 2016, 31, 648–672. [Google Scholar] [CrossRef]
- Rai, N.; Bellundagi, A.; Kumar, P.K.; Kalasapura Thimmappa, R.; Rani, S.; Sinha, N.; Krishna, H.; Jain, N.; Singh, G.P.; Singh, P.K.; et al. Marker-assisted backcross breeding for improvement of drought tolerance in bread wheat (Triticum aestivum L. em Thell). Plant Breed. 2018, 137, 514–526. [Google Scholar]
- Todkar, L.; Singh, G.P.; Jain, N.; Singh, P.K.; Prabhu, K.V. Introgression of drought tolerance QTLs through marker assisted backcross breeding in wheat (Triticum aestivum L.). Indian J. Genet. Plant Breed. 2020, 80, 209–212. [Google Scholar]
- Sharma, A.; Srivastava, P.; Mavi, G.S.; Kaur, S.; Kaur, J.; Bala, R.; Singh, T.P.; Sohu, V.S.; Chhuneja, P.; Bains, N.S.; et al. Resurrection of wheat cultivar PBW343 using marker-assisted gene pyramiding for rust resistance. Front. Plant Sci. 2021, 12, 570408. [Google Scholar] [CrossRef]
- Bado, S.; Forster, B.P.; Nielen, S.; Ali, A.M.; Lagoda, P.J.; Till, B.J.; Laimer, M. Plant mutation breeding: Current progress and future assessment. Plant Breed. Rev. 2015, 39, 23–88. [Google Scholar]
- Mohapatra, S.R.; Majhi, P.K.; Mondal, K.; Samantara, K. TILLING and Eco-TILLING: Concept, Progress, and Their Role in Crop Improvement. In Advanced Crop Improvement, Volume 1: Theory and Practice; Springer International Publishing: Cham, Switzerland, 2023; pp. 349–377. [Google Scholar]
- Kharkwal, M.C. Role of mutation breeding in crop improvement with special reference to Indian Subcontinent. In Mutation Breeding for Sustainable Food Production and Climate Resilience; Singapore: Singapore, 2023; pp. 355–428. [Google Scholar]
- Njau, P.N.; Kinyua, M.G.; Kimurto, P.K.; Okwaro, H.K.; Maluszynski, M. Drought tolerant wheat varieties developed through mutation breeding technique. J. Agric. Sci. Technol. 2005, 7, 18–29. [Google Scholar]
- Dormatey, R.; Sun, C.; Ali, K.; Coulter, J.A.; Bi, Z.; Bai, J. Gene Pyramiding for Sustainable Crop Improvement against Biotic and Abiotic Stresses. Agronomy 2020, 10, 1255. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, H.; Bi, H.; Fromm, M.; Yang, B.; Weeks, D.P. Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res. 2013, 41, e188. [Google Scholar] [PubMed]
- Eathington, S.R.; Crosbie, T.M.; Edwards, M.D.; Reiter, R.S.; Bull, J.K. Molecular markers in commercial breeding. Crop Sci. 2007, 47, 154163. [Google Scholar]
- Cai, J.; Wang, S.; Su, Z.; Li, T.; Zhang, X.; Bai, G. Meta-analysis of QTL for Fusarium head blight resistance in Chinese wheat landraces. Crop J. 2019, 7, 784–798. [Google Scholar]
- Jain, N.; Singh, G.P.; Singh, P.K.; Ramya, P.; Krishna, H.; Ramya, K.T.; Todkar, L.; Amasiddha, B.; Kumar, K.P.; Vijay, P.; et al. Molecular approaches for wheat improvement under drought and heat stress. Indian J. Genet. 2014, 74, 578–583. [Google Scholar]
- Bernardo, R.; Charcosset, A. Usefulness of gene information in marker-assisted recurrent selection: A simulation appraisal. Crop Sci. 2006, 46, 614–621. [Google Scholar]
- Ali, M.; Zhang, L.; DeLacy, I.; Arief, V.; Dieters, M.; Pfeiffer, W.H.; Wang, J.; Li, H. Modeling and simulation of recurrent phenotypic and genomic selections in plant breeding under the presence of epistasis. Crop J. 2020, 8, 866–877. [Google Scholar] [CrossRef]
- Borrell, A.K.; Mullet, J.E.; George-Jaeggli, B.; van Oosterom, E.J.; Hammer, G.L.; Klein, P.E.; Jordan, D.R. Drought adaptation of stay-green sorghum is associated with canopy development, leaf anatomy, root growth, and water uptake. J. Exp. Bot. 2014, 65, 6251–6263. [Google Scholar]
- Gokidi, Y.; Bhanu, A.N.; Singh, M.N. Marker assisted recurrent selection: An overview. Adv. Life Sci. 2016, 5, 6493–6499. [Google Scholar]
- Boopathi, N.M.; Shobhana, V.G. Genomics Assisted Breeding for Improving Disease and Pest Resistance in Crop Plants. In Plant Molecular Breeding in Genomics Era: Applications; Springer: Cham, Switzerland, 2024; pp. 41–77. [Google Scholar]
- Krishnappa, G.; Tyagi, B.S.; Gupta, V.; Gupta, A.; Venkatesh, K.; Kamble, U.R.; Singh, G.; Singh, G.P. Wheat breeding. In Fundamentals of Field Crop Breeding; Springer: Singapore, 2022; pp. 39–111. [Google Scholar]
- Azizi, M.M.F.; Lau, H.Y.; Abu-Bakar, N. Integration of advanced technologies for plant variety and cultivar identification. J. Biosci. 2021, 46, 91. [Google Scholar] [CrossRef]
- Kage, U.; Kumar, A.; Dhokane, D.; Karre, S.; Kushalappa, A.C. Functional molecular markers for crop improvement. Crit. Rev. Biotechnol. 2016, 36, 917–930. [Google Scholar]
- Ateş Sonmezoglu, B.; Terzi, B. Characterization of some bread wheat genotypes using molecular markers for drought tolerance Physiol. Mol. Biol. Plants 2018, 24, 159–166. [Google Scholar] [CrossRef]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; Munhoz, C.D.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- Belete, Y.; Shimelis, H.; Laing, M.; Mathew, I. Genetic diversity and population structure of bread wheat genotypes determined via phenotypic and SSR marker analyses under drought-stress conditions. J. Crop Improv. 2021, 35, 303–325. [Google Scholar]
- Semahegn, Y.; Shimelis, H.; Laing, M.; Mathew, I. Evaluation of bread wheat (Triticum aestivum L.) genotypes for yield and related traits under drought stress conditions, Acta Agric. Scand.-B Soil Plant Sci. 2020, 70, 474–484. [Google Scholar]
- Phuke, R.M.; He, X.; Juliana, P.; Kabir, M.R.; Roy, K.K.; Marza, F.; Roy, C.; Singh, G.P.; Chawade, A.; Joshi, A.K.; et al. Identification of genomic regions and sources for wheat blast resistance through GWAS in Indian wheat genotypes. Genes 2022, 13, 596. [Google Scholar] [CrossRef]
- Vinh, N.T.; Paterson, A.H. Genome mapping and its implication for stress resistance in plants. In Abiotic Stresses: Plant Resistance through Breeding and Molecular Approaches; Ashraf, M., Harris, P.J.C., Eds.; Haworth Press: New York, NY, USA, 2005. [Google Scholar]
- Salvi, S.; Tuberosa, R. To clone or not to clone plant QTLs: Present and future challenges. Trends Plant Sci. 2005, 10, 297–304. [Google Scholar] [CrossRef]
- Quarrie, S.A.; Gulli, M.; Calestani, C.; Steed, A.; Marmiroli, N. Location of a gene regulating drought-induced abscisic acid production on the long arm of chromosome 5A of wheat. Theor. Appl. Genet. 1994, 89, 794–800. [Google Scholar]
- Quarrie, S.A.; Steed, A.; Calestani, C.; Semikhodskii, A.; Lebreton, C.; Chinoy, C.; Steele, N.; Pljevljakusić, D.; Waterman, E.; Weyen, J.; et al. A high-density genetic map of hexaploid wheat (Triticum aestivum L.) from the cross-Chinese Spring x SQ1 and its use to compare QTLs for grain yield across a range of environments. Theor. Appl. Genet. 2005, 110, 865–880. [Google Scholar]
- Tuberosa, R.; Salvi, S.; Sanguineti, M.C.; Landi, P.; Maccaferri, M. Conti, S. Mapping QTLS regulating morpho-physiological traits and yield: Case studies, shortcomings and perspectives in drought-stressed maize. Ann. Bot. 2002, 89, 941–963. [Google Scholar]
- Singh, R.K.; Prasad, A.; Muthamilarasan, M.; Parida, S.K.; Prasad, M. Breeding and biotechnological interventions for trait improvement: Status and prospects. Planta 2020, 252, 54. [Google Scholar]
- Khahani, B.; Tavakol, E.; Shariati, V. Genome-wide meta-analysis on yield and yield-related QTLs in barley (Hordeum vulgare L.). Mol. Breed. 2019, 39, 56. [Google Scholar]
- Darzi-Ramandi, H.; Shariati, V.; Tavakol, E.; Najafi-Zarini, H.; Bilgrami, S.S.; Razavi, K. Detection of consensus genomic regions associated with root architecture of bread wheat on groups 2 and 3 chromosomes using QTL meta-analysis. Aust. J Crop Sci 2017, 11, 777. [Google Scholar]
- Acuna-Galindo, M.A.; Mason, R.E.; Subramanian, N.K.; Hays, D.B. Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci. 2015, 55, 477–492. [Google Scholar]
- Kumar, S.; Sehgal, S.K.; Kumar, U.; Prasad, P.V.V.; Joshi, A.K.; Gill, B.S. Genomic characterization of drought tolerance-related traits in spring wheat. Euphytica 2012, 186, 265–276. [Google Scholar]
- Fàbregas, N.; Yoshida, T.; Fernie, A.R. Role of Raf-like Kinases in SnRK2 Activation and Osmotic Stress Response in Plants. Nat. Commun. 2020, 11, 6184. [Google Scholar]
- Salem, K.F.M.; Roder, M.S.; Borner, A. Identification and mapping quantitative trait loci for stem reserve mobilisation in wheat (Triticum aestivum L.). Cereal Res. Commun. 2007, 35, 1367–1374. [Google Scholar]
- Shukla, S.; Singh, K.; Patil, R.V.; Kadam, S.; Bharti, S.; Prasad, P.; Singh, N.K.; Khanna-Chopra, R. Genomic regions associated with grain yield under drought stress in wheat (Triticum aestivum L.). Euphytica 2015, 203, 449–467. [Google Scholar]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic approach to understand plant adaptations to water and salt stress. In Plant Metabolites and Regulation Under Environmental Stress; Academic Press: Cambridge, MA, USA, 2018; pp. 133–144. [Google Scholar]
- Ullah, N.; Yüce, M.; Gökçe, Z.N.Ö.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants 2018, 10, ply016. [Google Scholar]
- Yadav, A.K.; Carroll, A.J.; Estavillo, G.M.; Rebetzke, G.J.; Pogson, B.J. Wheat drought tolerance in the field is predicted by amino acid responses to glasshouse-imposed drought. J. Exp. Bot. 2019, 70, 4931–4948. [Google Scholar]
- Bernardo, L.; Carletti, P.; Badeck, F.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef]
- Kubota, N. The effects of drought and flooding on the phenolic compounds in peach fruits. Sci. Rep. Fac. Agric. Okayama Univ. 1988, 171, 17–21. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol Biochem 2014, 80, 60–66. [Google Scholar]
- Bowne, J.B.; Erwin, T.A.; Juttner, J.; Schnurbusch, T.; Langridge, P.; Bacic, A.; Roessner, U. Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol. Plant 2012, 5, 418–429. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol-gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef]
- Kumari, V.V.; Roy, A.; Vijayan, R.; Banerjee, P.; Verma, V.C.; Nalia, A.; Pramanik, M.; Mukherjee, B.; Ghosh, A.; Reja, M.H.; et al. Drought and Heat Stress in Cool-Season Food Legumes in Sub-Tropical Regions: Consequences, Adaptation, and Mitigation Strategies. Plants 2021, 10, 1038. [Google Scholar] [CrossRef]
- Chorfi, A.; Taïbi, K. Biochemical Screening for Osmotic Adjustment of Wheat genotypes under Drought Stress. Tropicult 2011, 29, 82–87. [Google Scholar]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laido, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- Meuwissen, T. Genomic selection: Marker assisted selection on a genome wide scale. J. Anim. Breed. Genet. 2007, 124, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, A.; Isobe, S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. 2012, 110, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [PubMed]
- Goddard, M.E.; Hayes, B.J. Genomic selection. J. Anim. Breed. Genet. 2007, 124, 323–330. [Google Scholar]
- Garg, S. Computational methods for chromosome-scale haplotype reconstruction. Genome Biol. 2021, 22, 101. [Google Scholar]
- Ammar, R.; Paton, T.A.; Torti, D.; Shlien, A.; Bader, G.D. Long read nanopore sequencing for detection of HLA and CYP2D6 variants and haplotypes. F1000Research 2015, 4, 17. [Google Scholar] [CrossRef]
- Bhat, J.A.; Yu, D.; Bohra, A.; Ganie, S.A.; Varshney, R.K. Features and applications of haplotypes in crop breeding. Commun Biol 2021, 4, 1266. [Google Scholar] [CrossRef]
- El Ameen, T.M. Molecular markers for drought tolerance in bread wheat. Afr. J. Biotechnol. 2013, 12, 3148–3152. [Google Scholar] [CrossRef]
- Moncada, P.; Martínez, C.P.; Borrero, J.; Chatel, M.; Gauch, H., Jr.; Guimaraes, E.; Tohme, J.; McCouch, S.R. Quantitative trait loci for yield and yield components in an Oryza sativa× Oryza rufipogon BC2F2 population evaluated in an upland environment. Theor. Appl. Genet. 2001, 102, 41–52. [Google Scholar]
- Zhang, Y.; Paschold, A.; Marcon, C.; Liu, S.; Tai, H.; Nestler, J.; Yeh, C.T.; Opitz, N.; Lanz, C.; Schnable, P.S.; et al. The Aux/IAA gene rum1 involved in seminal and lateral root formation controls vascular patterning in maize (Zea mays L.) primary roots. J. Exp. Bot 2014, 65, 4919–4930. [Google Scholar] [CrossRef]
- Czyczyło-Mysza, I.; Tyrka, M.; Marcińska, I.; Skrzypek, E.; Karbarz, M.; Dziurka, M.; Hura, T.; Dziurka, K.; Quarrie, S.A. Quantitative trait loci for leaf chlorophyll fluorescence parameters, chlorophyll and carotenoid contents in relation to biomass and yield in bread wheat and their chromosome deletion bin assignments. Mol. Breed. 2013, 32, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Petrarulo, M.; Marone, D.; Ferragonio, P.; Cattivelli, L.; Rubiales, D.; De Vita, P.; Mastrangelo, A.M. Genetic analysis of root morphological traits in wheat. Mol. Genet. Genom. 2015, 290, 785–806. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.K.; Bellundagi, A.; Krishna, H.; Mallikarjuna, M.G.; Thimmappa, R.K.; Rai, N.; Shashikumara, P.; Sinha, N.; Jain, N.; Singh, P.K.; et al. Development of bread wheat (Triticum aestivum L.) variety HD3411 following marker-assisted backcross breeding for drought tolerance. Front. Genet. 2023, 14, 1046624. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ali, N.; Ahmad, H. Association Mapping of Root Traits for Drought Tolerance in Bread Wheat; IntechOpen: London, UK, 2017. [Google Scholar]
- Kumar, A.; Sandhu, N.; Dixit, S.; Yadav, S.; Swamy, B.P.M.; Shamsudin, N.A.A. Marker-assisted selection strategy to pyramid two or more QTLs for quantitative trait-grain yield under drought. Rice 2018, 11, 35. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P.; Mathews, K.L.; McIntyre, C.L.; Olivares-Villegas, J.J.; Chapman, S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef]
- Koua, A.P.; Siddiqui, M.N.; Heß, K.; Klag, N.; Kambona, C.M.; Duarte-Delgado, D.; Oyiga, B.C.; Léon, J.; Ballvora, A. Genome-wide dissection and haplotype analysis identified candidate loci for nitrogen use efficiency under drought conditions in winter wheat. Plant Genome 2023, 17, e20394. [Google Scholar] [CrossRef]
- Rabbi, S.M.H.A.; Kumar, A.; Mohajeri Naraghi, S.; Simsek, S.; Sapkota, S.; Solanki, S.; Alamri, M.S.; Elias, E.M.; Kianian, S.; Missaoui, A.; et al. Genome-Wide Association Mapping for Yield and Related Traits Under Drought Stressed and Non-stressed Environments in Wheat. Front. Genet. 2021, 12, 649988. [Google Scholar] [CrossRef]
- Crossa, J.; Camposde los, G.; Pérez, P.; Gianola, D.; Burgueño, J.; Araus, J.L.; Makumbi, D.; Singh, R.P.; Dreisigacker, D.; Yan, J.; et al. Prediction of Genetic Values of Quantitative Traits in Plant Breeding Using Pedigree and Molecular Markers. Genetics 2010, 186, 713–724. [Google Scholar] [CrossRef]
- Ashraf, A.; Rehman, O.U.; Muzammil, S.; Leon, J.; Naz, A.A.; Rasool, F.; Ali, G.M.; Zafar, Y.; Khan, M.R. Evolution of Deeper Rooting 1-like homoeologs in wheat entails the C-terminus mutations as well as gain and loss of auxin response elements. PLoS ONE 2019, 14, e0214145. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liu, S.; Mai, S.; Qin, Y.; Wang, S.; Zhou, Z.; Yang, K.; Huang, X.; Deng, Y.; et al. Identification and validation of quantitative trait loci for seven quality-related traits in common wheat (Triticum aestivum L.). Theor. Appl. Genet. 2025, 138, 57–68. [Google Scholar] [CrossRef]
- Rajaram, S.; Van Ginkle, M. Mexico, 50 years of international wheat breeding. In The World Wheat Book: A History of Wheat Breeding; Bonjean, A.P., Angus, W.J., Eds.; Lavoisier Publishing: Paris, France, 2001; pp. 579–604. [Google Scholar]
- Li, L.; Peng, Z.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genome-wide association study reveals genomic regions controlling root and shoot traits at late growth stages in wheat. J. Exp. Bot. 2019, 124, 993–1006. [Google Scholar]
- Rufo, R.; Salvi, S.; Royo, C.; Soriano, J.M. Exploring the genetic architecture of root-related traits in mediterranean bread wheat landraces by genome-wide association analysis. Agronomy 2020, 10, 613. [Google Scholar] [CrossRef]
- Niu, Y.; Li, J.; Sun, F.; Song, T.; Han, B.; Liu, Z.; Su, P. Comparative transcriptome analysis reveals the key genes and pathways involved in drought stress response of two wheat (Triticum aestivum L.) varieties. Genomics 2023, 115, 110688. [Google Scholar] [CrossRef] [PubMed]
- Barratt, L.J.; Reynolds, I.J.; Ortega, S.F.; Harper, A.L. Transcriptomic and co-expression network analyses on diverse wheat landraces identifies candidate master regulators of the response to early drought. Front. Plant Sci. 2023, 14, 1212559. [Google Scholar]
- Chu, C.; Wang, S.; Paetzold, L.; Wang, Z.; Hui, K.; Rudd, J.C.; Xue, Q.; Ibrahim, A.M.H.; Metz, R.; Johnson, C.D.; et al. RNA-seq analysis reveals different drought tolerance mechanisms in two broadly adapted wheat cultivars ‘TAM 111’ and ‘TAM 112’. Sci. Rep. 2021, 11, 4301. [Google Scholar] [CrossRef]
- Ahmad, M.Q.; Khan, S.H.; Khan, A.S.; Kazi, A.M.; Basra, S. Identification of QTLs for drought tolerance traits on wheat chromosome 2A using association mapping. Int. J. Agric. Biol. 2014, 16, 862–870. [Google Scholar]
- Bennett, D.; Reynolds, M.; Mullan, D.; Izanloo, A.; Kuchel, H.; Langridge, P.; Schnurbusch, T. Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theor. Appl. Genet. 2012, 125, 1473–1485. [Google Scholar] [CrossRef]
- Martignago, D.; Rico-Medina, A.; Blasco-Escámez, D.; Fontanet-Manzaneque, J.B.; Cano-Delgado, A.I. Drought resistance by engineering plant tissue-specific responses. Front. Plant Sci. 2020, 10, 1–19. [Google Scholar]
- Wei, B.; Jing, R.; Wang, C.; Chen, J.; Mao, X.; Chang, X.; Jia, J. Dreb1 genes in wheat (Triticum aestivum L.): Development of functional markers and gene mapping based on SNPs. Mol. Breed. 2009, 23, 13–22. [Google Scholar]
- Ma, L.; Li, T.; Hao, C.; Wang, Y.; Chen, X.; Zhang, X. Ta GS 5-3A, a grain size gene selected during wheat improvement for larger kernel and yield. Plant. Biotechnol. J. 2016, 14, 1269–1280. [Google Scholar] [CrossRef]
- Zhuang, J.; Chen, J.M.; Yao, Q.H.; Xiong, F.; Sun, C.C.; Zhou, X.R.; Zhang, J.; Xiong, A.S. Discovery and expression profile analysis of AP2/ERFfamily genes from Triticum aestivum. Mol. Biol. Rep. 2011, 38, 745–753. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, W.; Lv, J.; Jia, Y.; Wang, M.; Xia, G. Ectopic expression of a wheat MYB transcription factor gene, TaMYB73, improves salinity stress tolerance in Arabidopsis thaliana. J. Exp. Bot. 2011, 63, 1511–1522. [Google Scholar] [CrossRef]
- Tripathi, P.; Rabara, R.C.; Rushton, P.J. A systems biology perspective on the role of WRKY transcription factors in drought responses in plants. Planta 2014, 239, 255–266. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, J.; Li, Y.; Rong, X.; Sun, J.; Sun, T.; Li, M.; Wang, L.; Feng, Y.; Chai, R.; et al. Expression of TaWRKY44, a wheat WRKY gene, in transgenic tobacco confers multiple abiotic stress tolerances. Front. Plant Sci. 2015, 6, 615. [Google Scholar] [CrossRef]
- Cheuk, A.; Houde, M. Genome wide identification of C1-2i zinc finger proteins and their response to abiotic stress in hexaploid wheat. Mol. Genet. Genom. 2016, 291, 873–890. [Google Scholar] [CrossRef]
- Peremarti, A.; Mare, C.; Aprile, A.; Roncaglia, E.; Cattivelli, L.; Villegas, D.; Royo, C. Transcriptomic and proteomic analyses of a pale-green durum wheat mutant shows variations in photosystem components and metabolic deficiencies under drought stress. BMC Genom. 2014, 15, 125. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X. From markers to genome-based breeding in wheat. Theor. Appl. Genet. 2019, 132, 767–784. [Google Scholar]
- Wang, Y.; Abrouk, M.; Gourdoupis, S.; Koo, D.H.; Karafiatova, M.; Molnar, I.; Holu sova, K.; Dole zel, J.; Athiyannan, N.; Cavalet-Giorsa, E.; et al. An unusual tandem kinase fusion protein confers leaf rust resistance in wheat. Nat. Genet. 2023, 55, 914–920. [Google Scholar]
- Rahmanzadeh, A.; Khahani, B.; Taghavi, S.M.; Khojasteh, M.; Osdaghi, E. Genome-wide meta-QTL analyses provide novel insight into disease resistance repertoires in common bean. BMC Genom. 2022, 23, 680. [Google Scholar]
- Awan, M.J.A.; Pervaiz, K.; Rasheed, A.; Amin, I.; Saeed, N.A.; Dhugga, K.S.; Mansoor, S. Genome edited wheat-current advances for the second green revolution. Biotechnol. Adv. 2022, 60, 108006. [Google Scholar]
- Naik, A.; Jogi, M.; Shreenivas, B.V. Assessing the impact of climate change on global crop yields and farming practices. Arch. Curr. Res. Int. 2024, 24, 696–712. [Google Scholar] [CrossRef]
- Bakala, H.S.; Devi, J.; Singh, G.; Singh, I. Drought and heat stress: Insights into tolerance mechanisms and breeding strategies for pigeonpea improvement. Planta 2024, 259, 123. [Google Scholar] [CrossRef]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.H.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 2000, 155, 1–9. [Google Scholar] [CrossRef]
- Vendruscolo, E.C.G.; Schuster, I.; Pileggi, M.; Scapim, C.A.; Molinari, H.B.C.; Marur, C.J.; Vieira, L.G.E. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat. J. Plant Physiol. 2007, 164, 1367–1376. [Google Scholar] [CrossRef]
- Şimşek, Ö.; Isak, M.A.; Dönmez, D.; Dalda Şekerci, A.; İzgü, T.; Kaçar, Y.A. Advanced Biotechnological Interventions in Mitigating Drought Stress in Plants. Plants 2024, 13, 717. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Saripalli, G.; Sharma, P.K.; Poland, J. Wheat genomics: Genomes, pangenomes, and beyond. Trends Genet. 2024, 40, 982–992. [Google Scholar] [CrossRef]


| Trait | Early-Season Drought (Pre-Anthesis) | Terminal Drought (Post-Anthesis) |
|---|---|---|
| Early vigor | ˄ | ˅ |
| Peduncle length | ˄ | ˅ |
| Relative water content | ˄ | ˄ |
| Leaf area index | ˄ | ˅ |
| More number of tillers | ˄ | ˅ |
| Low number of tillers | ˅ | ˄ |
| Tall size | ˄ | ˅ |
| Semi-dwarf | ˅ | ˄ |
| Early flowering and maturity | ˅ | ˄ |
| Prolonged—or short but high rate—grain filling | ˅ | ˄ |
| Flag leaf area | ˅ | ˄ |
| Member | Trait | Technique Used | Reference |
|---|---|---|---|
| Wheat landraces | Drought tolerance | Crossing and selection | [83] |
| Emmer wheat | Drought tolerance | Synthetic, backcross | [84] |
| Agropyron elongatum | Root development | In situ hybridization and backcrossed | [85] |
| Exotic germplasm (wheat landraces) | Root mass to deeper soil profiles | Interspecific hybridization | [86] |
| Aegilops geniculata | - | Interspecific hybridization | [86] |
| Wild emmer | Morphophysiological traits | Crossed with wild emmer (G18-16) and durum (Langdon) | [87] |
| Triticum dicoccoides | Drought tolerance | QTL analysis and positional cloning of QTLs | [88] |
| Elymus semicostatus (Nees ex Steud.) | Leaf sheath compactness, number of florets, spike curvature, spike density | Screening for morpho-physiological traits for drought tolerance | [89] |
| Ae. Tauschii (DD genome) | Cellular thermotolerance | Diploid Xhexaploid cross approach | [90] |
| Aegilops tauschii, Triticum dicoccoides | Root and shoot growth, membrane injury | Crossing and selection | [91] |
| Traits | MQTL | Chr (cM) | Position (cM) | Reference |
|---|---|---|---|---|
| Plant height | MQTL-PH1 | 1A | 54.37 | [136] |
| Root number | MQTL6 | 3A | 45.8 | [137] |
| Root volume, root surface area, root length | MQTL7 | 3A | 75.5 | [137] |
| CID, Col, KN, SD, | MQTL2 | 1A | 60 | [138] |
| SG, WSC, WS, Yld | ||||
| Photo, WSC | MQTL3 | 1A | 89 | [138] |
| Chlorophyll content | Qchl.ksu-3B | 3B | 67.2 | [139] |
| Days to maturity | QDm-7D | 7D | 2.7 | [140] |
| Days to heading | MQTL-HD3 | 5B | 92.66 | [136] |
| Stem reserve mobilization | QSrm.ipk-5D | 5D | 19 | [141] |
| Stem reserve mobilization | QSrm.ipk-2D | 2D | 142 | [141] |
| Grain yield | qGYWD.3B.2 | 3B | 97.6 | [142] |
| Grain yield | Qyld.csdh.7AL | 7A | 155.9 | [142] |
| Thousand grain weight | QTgw-7D-b | 7D | 12.5 | [140] |
| Drought tolerance | MQTL-DT2 | 4B | 41.52 | [136] |
| Database | Database Salient Feature | URL |
|---|---|---|
| CerealsDB | Genotyping information for over 6000 wheat accessions and describe new webtools for exploring and visualizing the data and also describe a new database of quantitative trait loci that links phenotypic traits to CerealsDB SNP markers and allelic scores for each of those markers | https://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/indexNEW.php (accessed on 22 March 2024) |
| WheatGmap | Wheat gene mapping | https://www.wheatgmap.org (accessed on 20 March 2024) |
| PmiRExAt | A new online database resource that caters plant miRNA expression atlas. | http://pmirexat.nabi.res.in (accessed on 15 May 2024) |
| Triti-Map | Wheat gene and regulatory elements mapping | http://bioinfo.cemps.ac.cn/tritimap/ (accessed on 22 March 2024) |
| expVIP | Wheat transcriptome resources for expression analysis | http://www.wheat-expression.com/ (accessed on 20 March 2024) |
| WheatExp | Homologue-specific database of gene expression profiles for polyploid wheat. | https://wheat.pw.usda.gov/WheatExp/ (accessed on 20 March 2024) |
| Wheat Panache | Wheat genome-wide copy number variations (CNVs) visualization | http://www.appliedbioinformatics.com.au/wheat_panache (accessed on 20 March 2024) |
| WheatGenome | Genome viewer with BLAST search portal, wheat auto SNPdb, links to wheat genetic maps and a wheat genome Wiki to allow interaction between diverse wheat genome sequencing activities | http://wheatgenome.info (accessed on 20 March 2024) |
| wDBTF | Collates 3820 wheat sTFs sequences | http://wwwappli.nantes.inrae (accessed on 20 March 2024) |
| MASWheat | Marker-assisted selection database for wheat | https://maswheat.ucdavis.edu/ (accessed on 20 March 2024) |
| WISP | The Wheat Improvement Strategic Program | http://www.wheatisp.org/ (accessed on 20 March 2024) |
| OpenWildWheat | Sequencing resources of Ae. tauschii accessions | https://openwildwheat.org/ (accessed on 20 March 2024) |
| Wheat Omics | Multi-omics data analysis | http://wheatomics.sdau.edu.cn/ (accessed on 20 March 2024) |
| Wheat Atlas | Atlas of wheat germplasm and production statistics | http://wheatatlas.org (accessed on 20 March 2024) |
| Wheat IS | An International Wheat Information System, supporting the wheat research community | http://www.wheatis.org/ (accessed on 20 March 2024) |
| Grain genes | Datasets useful to researchers working on wheat, barley, rye, and oat | https://wheat.pw.usda.gov (accessed on 22 March 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, C.; Yadav, S.; Khare, V.; Gupta, V.; Patial, M.; Kumar, S.; Mishra, C.N.; Tyagi, B.S.; Gupta, A.; Sharma, A.K.; et al. Wheat Drought Tolerance: Unveiling a Synergistic Future with Conventional and Molecular Breeding Strategies. Plants 2025, 14, 1053. https://doi.org/10.3390/plants14071053
Singh C, Yadav S, Khare V, Gupta V, Patial M, Kumar S, Mishra CN, Tyagi BS, Gupta A, Sharma AK, et al. Wheat Drought Tolerance: Unveiling a Synergistic Future with Conventional and Molecular Breeding Strategies. Plants. 2025; 14(7):1053. https://doi.org/10.3390/plants14071053
Chicago/Turabian StyleSingh, Charan, Sapna Yadav, Vikrant Khare, Vikas Gupta, Madhu Patial, Satish Kumar, Chandra Nath Mishra, Bhudeva Singh Tyagi, Arun Gupta, Amit Kumar Sharma, and et al. 2025. "Wheat Drought Tolerance: Unveiling a Synergistic Future with Conventional and Molecular Breeding Strategies" Plants 14, no. 7: 1053. https://doi.org/10.3390/plants14071053
APA StyleSingh, C., Yadav, S., Khare, V., Gupta, V., Patial, M., Kumar, S., Mishra, C. N., Tyagi, B. S., Gupta, A., Sharma, A. K., Ahlawat, O. P., Singh, G., & Tiwari, R. (2025). Wheat Drought Tolerance: Unveiling a Synergistic Future with Conventional and Molecular Breeding Strategies. Plants, 14(7), 1053. https://doi.org/10.3390/plants14071053







