The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of the EO
2.2. Enantioselective Analysis
2.3. Anti-Inflammatory Activity
3. Discussion
3.1. Chemical Composition and Main Components
3.2. Enantioselective GC-MS Analysis of Enantiomeric Distribution
3.3. Anti-Inflammatory Activity
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation and EO Distillation
4.3. Qualitative Chemical Analysis
4.4. GC-FID Quantitative Analyses
4.5. Enantioselective Analysis of the EO
4.6. Oxidative-Burst Assay (Antiinflammatory)
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biodiversity A–Z. Megadiverse Countries Definition. Available online: https://www.biodiversitya-z.org/content/megadiverse-countries (accessed on 17 July 2023).
- Armijos, C.; Ramírez, J.; Salinas, M.; Vidari, G.; Suárez, A.I. Pharmacology and Phytochemistry of Ecuadorian Medicinal Plants: An Update and Perspectives. Pharmaceuticals 2021, 14, 1145. [Google Scholar] [CrossRef] [PubMed]
- Calvopiña, K.; Malagón, O.; Capetti, F.; Sgorbini, B.; Verdugo, V.; Gilardoni, G. A New Sesquiterpene Essential Oil from the Native Andean Species Jungia Rugosa Less (Asteraceae): Chemical Analysis, Enantiomeric Evaluation, and Cholinergic Activity. Plants 2021, 10, 2102. [Google Scholar] [CrossRef] [PubMed]
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Finzi, P.V.; Vidari, G. Phytochemical and Ethnomedicinal Study of Huperzia Species Used in the Traditional Medicine of Saraguros in Southern Ecuador; AChE and MAO Inhibitory Activity. J. Ethnopharmacol. 2016, 193, 546–554. [Google Scholar] [CrossRef]
- Armijos, C.; Valarezo, E.; Cartuche, L.; Zaragoza, T.; Finzi, P.V.; Mellerio, G.G.; Vidari, G. Chemical Composition and Antimicrobial Activity of Myrcianthes Fragrans Essential Oil, a Natural Aromatizer of the Traditional Ecuadorian Beverage Colada Morada. J. Ethnopharmacol. 2018, 225, 319–326. [Google Scholar] [CrossRef]
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and Ethnopharmacology of the Ecuadorian Flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef]
- García, V.J.; Márquez, C.O.; Rodríguez, M.V.; Orozco, J.J.; Aguilar, C.D.; Ríos, A.C. Páramo Ecosystems in Ecuador’s Southern Region: Conservation State and Restoration. Agronomy 2020, 10, 1922. [Google Scholar] [CrossRef]
- Morocho, C.C.; Chuncho, G. Paramos of Ecuador, importance and effects: A review. Zero Latit. For. 2019, 9, 71–83. [Google Scholar]
- Calderón-Loor, M.; Cuesta, F.; Pinto, E.; Gosling, W.D. Carbon Sequestration Rates Indicate Ecosystem Recovery Following Human Disturbance in the Equatorial Andes. PLoS ONE 2020, 15, e0230612. [Google Scholar] [CrossRef]
- Poulenard, J.; Podwojewski, P.; Janeau, J.-L. Runoff and Soil Erosion under Rainfall Simulation of Andisols from the Ecuadorian Paramo: Effect of Tillage and Burning. Catena 2001, 45, 185–207. [Google Scholar] [CrossRef]
- Binder, S.; Isbell, F.; Polasky, S.; Catford, J.A.; Tilman, D. Grassland Biodiversity Can Pay. Proc. Natl. Acad. Sci. USA 2018, 115, 3876–3881. [Google Scholar] [CrossRef]
- Sklenář, P.; Jørgensen, P.M. Distribution Patterns of Páramo Plants in Ecuador. J. Biogeogr. 1999, 26, 681–691. [Google Scholar] [CrossRef]
- Joslin, A.J.; Jepson, W.E. Territory and Authority of Water Fund Payments for Ecosystem Services in Ecuador’s Andes. Geoforum 2018, 91, 10–20. [Google Scholar] [CrossRef]
- Brück, S.A.; Medina Torres, B.D.; Teixeira de Moraes Polizeli, M. de L. The Ecuadorian Paramo in Danger: What We Know and What Might Be Learned from Northern Wetlands. Glob. Ecol. Conserv. 2023, 47, e02639. [Google Scholar]
- Barta, B.; Mouillet, C.; Espinosa, R.; Andino, P.; Jacobsen, D.; Christoffersen, K.S. Glacial-Fed and Páramo Lake Ecosystems in the Tropical High Andes. Hydrobiologia 2018, 813, 19–32. [Google Scholar]
- García, V.J.; Márquez, C.O.; Isenhart, T.M.; Rodríguez, M.; Crespo, S.D.; Cifuentes, A.G. Evaluating the Conservation State of the Páramo Ecosystem: An Object-Based Image Analysis and CART Algorithm Approach for Central Ecuador. Heliyon 2019, 5, e02701. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG III. Bot. J. Linn. Soc. 2009, 161, 105–121. [Google Scholar]
- The Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.M.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Soltis, D.E.; Mabberley, D.J.; Sennikov, A.N.; Soltis, P.S.; et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Wang, H.-X.; Liu, H.; Moore, M.J.; Landrein, S.; Liu, B.; Zhu, Z.-X.; Wang, H.-F. Plastid Phylogenomic Insights into the Evolution of the Caprifoliaceae (Dipsacales). Mol. Phylogenetics Evol. 2020, 142, 2–51. [Google Scholar]
- Xu, Z.; Chang, L. Caprifoliaceae. Identif. Control. Common Weeds 2017, 3, 405–416. [Google Scholar]
- Tropicos. Home. Available online: https://www.tropicos.org/home (accessed on 14 August 2024).
- Larsen, B.B. A Taxonomic Revision of Phyllactis and Valeriana Sect. Bracteata (Valerianaceae). Nord. J. Bot. 1986, 6, 427–446. [Google Scholar]
- Jorgensen, P.; Leon-Yanez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; pp. 286–288. [Google Scholar]
- View of Medicinal Plants of the Andes and the Amazon—The Magic and Medicinal Flora of Northern Peru. Available online: https://ethnobotanyjournal.org/index.php/era/article/view/1283/759 (accessed on 22 January 2025).
- Bussmann, R.W.; Sharon, D.; Garcia, M. From Chamomile to Aspirin? Medicinal Plant Use among Clients at Laboratorios Beal in Trujillo, Peru. Ethnobot. Res. Appl. 2009, 7, 399–407. [Google Scholar] [CrossRef]
- Choi, H.-S.; Hong, K.-B.; Han, S.H.; Suh, H.J. Valerian/Cascade Mixture Promotes Sleep by Increasing Non-Rapid Eye Movement (NREM) in Rodent Model. Biomed. Pharmacother. 2018, 99, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Tammadon, M.R.; Nobahar, M.; Hydarinia-Naieni, Z.; Ebrahimian, A.; Ghorbani, R.; Vafaei, A.A. The Effects of Valerian on Sleep Quality, Depression, and State Anxiety in Hemodialysis Patients: A Randomized, Double-Blind, Crossover Clinical Trial. Oman Med. J. 2021, 36, e255. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.A.; Bravo-Valverde, R.L.; Kaplowitz, B.R.; Cott, J.M. Valerian as a Hypnotic for Hispanic Patients. Cult. Divers. Ethn. Minor. Psychol. 2000, 6, 84–92. [Google Scholar]
- Das, G.; Shin, H.-S.; Tundis, R.; Gonçalves, S.; Tantengco, O.A.G.; Campos, M.G.; Acquaviva, R.; Malfa, G.A.; Romano, A.; Robles, J.A.H.; et al. Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System. Plants 2021, 10, 846. [Google Scholar] [CrossRef]
- Agnihotri, S.; Wakode, S.; Ali, M. Chemical Composition, Antimicrobial and Topical Anti-Inflammatory Activity of Valeriana Jatamansi Jones. Essential Oil. J. Essent. Oil Bear. Plants 2011, 14, 417–422. [Google Scholar] [CrossRef]
- Khuda, F.; Iqbal, Z.; Zakiullah; Khan, A.; Nasir, F. Antimicrobial and Anti-Inflammatory Activities of Leaf Extract of Valeriana Wallichii DC. Pak. J. Pharm. Sci. 2012, 25, 715–719. [Google Scholar]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in Essential Oils: Chemistry, Classification, and Potential Impact on Human Health and Industry. Phytomedicine Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Cho, I.H.; Namgung, H.-J.; Choi, H.-K.; Kim, Y.-S. Volatiles and Key Odorants in the Pileus and Stipe of Pine-Mushroom (Tricholoma Matsutake Sing.). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Grujic-Jovanovic, S.; Skaltsa, H.D.; Marin, P.; Sokovic, M. Composition and Antibacterial Activity of the Essential Oil of Six Stachys Species from Serbia. Flavour Fragr. J. 2004, 19, 139–144. [Google Scholar]
- Beck, J.J.; Higbee, B.S.; Merrill, G.B.; Roitman, J.N. Comparison of Volatile Emissions from Undamaged and Mechanically Damaged Almonds. J. Sci. Food Agric. 2008, 88, 1363–1368. [Google Scholar] [CrossRef]
- Escudero, A.; Campo, E.; Fariña, L.; Cacho, J.; Ferreira, V. Analytical Characterization of the Aroma of Five Premium Red Wines. Insights into the Role of Odor Families and the Concept of Fruitiness of Wines. J. Agric. Food Chem. 2007, 55, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, E.E.; Puertas, M.A.; Combariza, M.Y. Volatile Secondary Metabolites from Spilanthes Americana Obtained by Simultaneous Steam Distillation-Solvent Extraction and Supercritical Fluid Extraction. J. Chromatogr. A 1996, 752, 223–232. [Google Scholar] [CrossRef]
- Lin, J.; Rouseff, R.L.; Barros, S.; Naim, M. Aroma Composition Changes in Early Season Grapefruit Juice Produced from Thermal Concentration. J. Agric. Food Chem. 2002, 50, 813–819. [Google Scholar] [CrossRef]
- Chisholm, M.G.; Jell, J.A.; Cass, D.M. Characterization of the Major Odorants Found in the Peel Oil of Citrus Reticulata Blanco Cv. Clementine Using Gas Chromatography–Olfactometry. Flavour Fragr. J. 2003, 18, 275–281. [Google Scholar] [CrossRef]
- Cavalli, J.; Tomi, F.; Bernardini, A.; Casanova, J. Composition and Chemical Variability of the Bark Oil of Cedrelopsis Grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003, 18, 532–538. [Google Scholar] [CrossRef]
- Mondello, L.; Zappia, G.; Cotroneo, A.; Bonaccorsi, I.; Chowdhury, J.U.; Yusuf, M.; Dugo, G. Studies on the Essential Oil-Bearing Plants of Bangladesh. Part VIII. Composition of Some Ocimum Oils O. Basilicum L. Var. Purpurascens; O. Sanctum L. Green; O. Sanctum L. Purple; O. Americanum L., Citral Type; O. Americanum L., Camphor Type. Flavour Fragr. J. 2002, 17, 335–340. [Google Scholar] [CrossRef]
- Gancel, A.-L.; Ollitrault, P.; Froelicher, Y.; Tomi, F.; Jacquemond, C.; Luro, F.; Brillouet, J.-M. Leaf Volatile Compounds of Six Citrus Somatic Allotetraploid Hybrids Originating from Various Combinations of Lime, Lemon, Citron, Sweet Orange, and Grapefruit. J. Agric. Food Chem. 2005, 53, 2224–2230. [Google Scholar] [CrossRef]
- Blanc, M.-C.; Bradesi, P.; Gonçalves, M.J.; Salgueiro, L.; Casanova, J. Essential Oil of Dittrichia Viscosa Ssp.Viscosa: Analysis by13C-NMR and Antimicrobial Activity. Flavour Fragr. J. 2006, 21, 324–332. [Google Scholar] [CrossRef]
- Mahajan, S.S.; Goddik, L.; Qian, M.C. Aroma Compounds in Sweet Whey Powder. J. Dairy Sci. 2004, 87, 4057–4063. [Google Scholar] [CrossRef]
- Gauvin, A.; Ravaomanarivo, H.; Smadja, J. Comparative Analysis by Gas Chromatography–Mass Spectrometry of the Essential Oils from Bark and Leaves of Cedrelopsis Grevei Baill, an Aromatic and Medicinal Plant from Madagascar. J. Chromatogr. A 2004, 1029, 279–282. [Google Scholar] [PubMed]
- Duquesnoy, E.; Dinh, N.H.; Castola, V.; Casanova, J. Composition of a Pyrolytic Oil from Cupressus Funebris Endl. of Vietnamese Origin. Flavour Fragr. J. 2006, 21, 453–457. [Google Scholar] [CrossRef]
- Boti, J.B.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Casanova, J. Chemical Variability ofJuniperus Oxycedrus Ssp.Oxycedrus Berry and Leaf Oils from Corsica, Analysed by Combination of GC, GC–MS and13C-NMR. Flavour Fragr. J. 2006, 21, 268–273. [Google Scholar]
- Pollak, F.C.; Berger, R.G. Geosmin and Related Volatiles in Bioreactor-Cultured Streptomyces Citreus CBS 109.60. Appl. Environ. Microbiol. 1996, 62, 1295. [Google Scholar] [CrossRef]
- Tamura, H.; Boonbumrung, S.; Yoshizawa, T.; Varanyanond, W. Volatile Components of the Essential Oils in the Pulp of Four Yellow Mangoes (Mangifera Indica L.) in Thailand. FSTR 2000, 6, 68–73. [Google Scholar]
- Lee, J.-G.; Lee, C.-G.; Kwag, J.-J.; Buglass, A.J.; Lee, G.-H. Determination of Optimum Conditions for the Analysis of Volatile Components in Pine Needles by Double-Shot Pyrolysis–Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2005, 1089, 227–234. [Google Scholar]
- Paolini, J.; Muselli, A.; Bernardini, A.; Bighelli, A.; Casanova, J.; Costa, J. Thymol Derivatives from Essential Oil of Doronicum Corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar]
- Couladis, M.; Chinou, I.B.; Tzakou, O.; Loukis, A. Composition and Antimicrobial Activity of the Essential Oil of Ballota Pseudodictamnus L. Bentham. Phytother. Res. 2002, 16, 723–726. [Google Scholar]
- Brat, P.; Rega, B.; Alter, P.; Reynes, M.; Brillouet, J.-M. Distribution of Volatile Compounds in the Pulp, Cloud, and Serum of Freshly Squeezed Orange Juice. J. Agric. Food Chem. 2003, 51, 3442–3447. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Nakahara, K.; Shoji, A.; Shibamoto, T. Volatile Chemicals Identified in Extracts from Leaves of Japanese Mugwort (Artemisia Princeps Pamp.). J. Agric. Food Chem. 2000, 48, 3463–3469. [Google Scholar]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the Essential Oil of Teucrium Ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007, 22, 101–104. [Google Scholar]
- Paolini, J.; Tomi, P.; Bernardini, A.-F.; Bradesi, P.; Casanova, J.; Kaloustian, J. Detailed Analysis of the Essential Oil from Cistus Albidus L. by Combination of GC/RI, GC/MS and C-NMR Spectroscopy. Nat. Prod. Res. 2008, 22, 1270–1278. [Google Scholar] [PubMed]
- Muselli, A.; Rossi, P.; Desjobert, J.; Bernardini, A.; Berti, L.; Costa, J. Chemical Composition and Antibacterial Activity of Otanthus Maritimus (L.) Hoffmanns. & Link Essential Oils from Corsica. Flavour Fragr. J. 2007, 22, 217–223. [Google Scholar]
- Ngassoum, M.B.; Yonkeu, S.; Jirovetz, L.; Buchbauer, G.; Schmaus, G.; Hammerschmidt, F.-J. Chemical Composition of Essential Oils ofLantana Camara Leaves and Flowers from Cameroon and Madagascar. Flavour Fragr. J. 1999, 14, 245–250. [Google Scholar]
- Aicha, N.; Skandrani, I.; Kilani, S. Chemical Composition, Mutagenic and Antimutagenic Activities of Essential Oils from (Tunisian) Artemisia Campestris and Artemisia Herba-Alba. J. Essent. Oil Res. 2008, 20, 37–43. [Google Scholar]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Essential Oil Constituents of Three Varieties of Kenyan Sweet Oranges Citrus Sinensis. Flavour Fragr. J. 2005, 20, 80–85. [Google Scholar]
- Ledauphin, J.; Guichard, H.; Saint-Clair, J.-F.; Picoche, B.; Barillier, D. Chemical and Sensorial Aroma Characterization of Freshly Distilled Calvados. 2. Identification of Volatile Compounds and Key Odorants. J. Agric. Food Chem. 2003, 51, 433–442. [Google Scholar]
- Shimoda, M.; Yoshimura, Y.; Yoshimura, T.; Noda, K.; Osajima, Y. Volatile Flavor Compounds of Sweetened Condensed Milk. J. Food Sci. 2001, 66, 804–807. [Google Scholar]
- Adams, D.R.P. Identification of Essential Oil Components by Gas Chromatography; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Raal, A.; Arak, E.; Orav, A.; Kailas, T.; Müürisepp, M. Variation in the Composition of the Essential Oil of Commercial Valeriana Officinalis L. Roots from Different Countries. J. Essent. Oil Res. 2008, 20, 524–529. [Google Scholar]
- Feng, Y.-X.; Wang, Y.; Chen, Z.-Y.; Guo, S.-S.; You, C.-X.; Du, S.-S. Efficacy of Bornyl Acetate and Camphene from Valeriana Officinalis Essential Oil against Two Storage Insects. Environ. Sci. Pollut. Res. 2019, 26, 16157–16165. [Google Scholar]
- Letchamo, W.; Ward, W.; Heard, B.; Heard, D. Essential Oil of Valeriana Officinalis L. Cultivars and Their Antimicrobial Activity As Influenced by Harvesting Time under Commercial Organic Cultivation. J. Agric. Food Chem. 2004, 52, 3915–3919. [Google Scholar] [CrossRef] [PubMed]
- Castillo, L.N.; Calva, J.; Ramírez, J.; Vidari, G.; Armijos, C. Chemical Analysis of the Essential Oils from Three Populations of Lippia Dulcis Trevir. Grown at Different Locations in Southern Ecuador. Plants 2024, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Cumbicus, N.; Gilardoni, G. A Novel Chemical Profile of a Selective In Vitro Cholinergic Essential Oil from Clinopodium Taxifolium (Kunth) Govaerts (Lamiaceae), a Native Andean Species of Ecuador. Molecules 2021, 26, 45. [Google Scholar] [CrossRef]
- Andrade, J.M.; Lucero Mosquera, H.; Armijos, C. Ethnobotany of Indigenous Saraguros: Medicinal Plants Used by Community Healers “Hampiyachakkuna” in the San Lucas Parish, Southern Ecuador. BioMed. Res. Int. 2017, 2017, 9343724. [Google Scholar] [CrossRef]
- Ramírez, J.; Andrade, M.D.; Vidari, G.; Gilardoni, G. Essential Oil and Major Non-Volatile Secondary Metabolites from the Leaves of Amazonian Piper Subscutatum. Plants 2021, 10, 1168. [Google Scholar] [CrossRef]
- Eiasu, B.K.; Dyafta, V.; Araya, H.T. Effect of Leaf Age on Essential Oil Yield and Composition in Rose-Scented Geranium. HortScience 2022, 57, 1524–1528. [Google Scholar] [CrossRef]
- Muhammad, S.; Nasshorudin, D. Novel Closed System Extraction of Essential Oil: Impact on Yield and Physical Characterization. Int. Conf. Biotechnol. Environ. Manag. 2014, 75, 6. [Google Scholar]
- Bhatt, I.D.; Dauthal, P.; Rawat, S.; Gaira, K.S.; Jugran, A.; Rawal, R.S.; Dhar, U. Characterization of Essential Oil Composition, Phenolic Content, and Antioxidant Properties in Wild and Planted Individuals of Valeriana Jatamansi Jones. Sci. Hortic. 2012, 136, 61–68. [Google Scholar] [CrossRef]
- Seidler-Lozykowska, K.; Mielcarek, S.; Baraniak, M. Content of Essential Oil and Valerenic Acids in Valerian (Valeriana Offcinalis L.) Roots at the Selected Developmental Phases. J. Essent. Oil Res. 2009, 21, 413–416. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C. Chemical Differentiation of Rhizome and Root Essential Oils of Indian Valerian (Valeriana Jatamansi Jones). J. Essent. Oil Bear. Plants 2012, 16, 835–840. [Google Scholar] [CrossRef]
- Pokharel, B.R.; Pandey, S.; Manandhar, M.D.; Pant, B. Comparative Study of Essential Oil in Wild and in Vitro Cultures of Valeriana Jatamansi Jones in Nepal. Plant Biotechnol. Rep. 2023, 17, 379–387. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Goyal, R.; Gupta, V.; Dhar, K.L. Gabaergic Effect of Valeric Acid from Valeriana Wallichii in Amelioration of ICV STZ Induced Dementia in Rats. Rev. Bras. Farmacogn. 2016, 26, 484–489. [Google Scholar] [CrossRef]
- Minchán-Herrera, P.; Ybañez-Julca, R.O.; Quispe-Díaz, I.M.; Venegas-Casanova, E.A.; Jara-Aguilar, R.; Salas, F.; Zevallos-Escobar, L.; Yáñez, O.; Pino-Rios, R.; Calderon, P.B.; et al. Valeriana Pilosa Roots Essential Oil: Chemical Composition, Antioxidant Activities, and Molecular Docking Studies on Enzymes Involved in Redox Biological Processes. Phyton-Int. J. Exp. Bot. 2024, 93, 1337. [Google Scholar] [CrossRef]
- Shinjyo, N.; Waddell, G.; Green, J. Valerian Root in Treating Sleep Problems and Associated Disorders—A Systematic Review and Meta-Analysis. J. Evid. Based Integr. Med. 2020, 25, 2515690X20967323. [Google Scholar] [CrossRef]
- Huynh, L.; Pacher, T.; Tran, H.; Novak, J. Comparative Analysis of the Essential Oils of Valeriana Hardwickii Wall. from Vietnam and Valeriana Officinalis L. from Austria. J. Essent. Oil Res. 2013, 25, 409–414. [Google Scholar]
- Bardakci, H.; Demirci, B.; Yesilada, E.; Kirmizibekmez, H.; Baser, K.H.C. Chemical Composition of the Essential Oil of the Subterranean Parts of Valeriana Alliariifolia. Acad. Chem. Globe Publ. 2012, 6, 89–92. [Google Scholar]
- Wang, J.; Zhao, J.; Liu, H.; Zhou, L.; Liu, Z.; Wang, J.; Han, J.; Yu, Z.; Yang, F. Chemical Analysis and Biological Activity of the Essential Oils of Two Valerianaceous Species from China: Nardostachys Chinensis and Valeriana Officinalis. Molecules 2010, 15, 6411–6422. [Google Scholar] [CrossRef]
- Raina, A.P.; Negi, K.S. Essential Oil Composition of Valeriana Jatamansi Jones from Himalayan Regions of India. Indian J. Pharm. Sci. 2015, 77, 218–222. [Google Scholar] [CrossRef]
- Paudel, P.N.; Satyal, P.; Satyal, R.; Setzer, W.N.; Gyawali, R. Chemical Composition, Enantiomeric Distribution, Antimicrobial and Antioxidant Activities of Origanum Majorana L. Essential Oil from Nepal. Molecules 2022, 27, 6136. [Google Scholar] [CrossRef]
- Matailo, A.; Bec, N.; Calva, J.; Ramírez, J.; Andrade, J.M.; Larroque, C.; Vidari, G.; Armijos, C. Selective BuChE Inhibitory Activity, Chemical Composition, and Enantiomer Content of the Volatile Oil from the Ecuadorian Plant Clinopodium Brownei. Rev. Bras. Farmacogn. 2019, 29, 749–754. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of α-Pinene and β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [PubMed]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, Antimicrobial Evaluation, and Structure–Activity Relationship of α-Pinene Derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [PubMed]
- Gehan, I.; Entsar, I. In Vitro Antimicrobial and Antioxidant Activities of Monoterpenes against Some Food-Borne Pathogens. J. Plant Prot. Path. Mansoura Univ. 2019, 10, 87–94. [Google Scholar]
- Orhan, İ.E.; Özçelik, B.; Kartal, M.; Kan, Y. Antimicrobial and Antiviral Effects of Essential Oils from Selected Umbelliferae and Labiatae Plants and Individual Essential Oil Components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar]
- Tiwari, M.; Kakkar, P. Plant Derived Antioxidants—Geraniol and Camphene Protect Rat Alveolar Macrophages against t-BHP Induced Oxidative Stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J.; Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus Officinalis L. Essential Oils from Spain: Composition, Antioxidant Capacity, Lipoxygenase and Acetylcholinesterase Inhibitory Capacities, and Antimicrobial Activities. Plant Biosyst. 2018, 152, 1282–1292. [Google Scholar]
- De Alfonso, I.; Vacas, S.; Primo, J. Role of α-Copaene in the Susceptibility of Olive Fruits to Bactrocera Oleae (Rossi). J. Agric. Food Chem. 2014, 62, 11976–11979. [Google Scholar]
- Mozuraitis, R.; Stranden, M.; Ramirez, M.I.; Borg-Karlson, A.-K.; Mustaparta, H. Chemical Constituents of Supercritical Extracts from Prunus Yedoensis, Saururus Chinensis, Zanthoxylum Piperitum and Their Anti-Inflammatory Activities. Chem. Senses 2002, 27, 505–509. [Google Scholar]
- Mustaparta, H.; Stranden, M. Chapter Ten—Olfaction and Learning in Moths and Weevils Living on Angiosperm and Gymnosperm Hosts. In Recent Advances in Phytochemistry; Romeo, J.T., Ed.; Chemical Ecology and Phytochemistry of Forest Ecosystems; Elsevier: Amsterdam, The Netherlands, 2005; Volume 39, pp. 269–292. [Google Scholar]
- Bae, G.-S.; Heo, K.-H.; Choi, S.B.; Jo, I.-J.; Kim, D.-G.; Shin, J.-Y.; Seo, S.-H.; Park, K.-C.; Lee, D.-S.; Oh, H.; et al. Beneficial Effects of Fractions of Nardostachys Jatamansi on Lipopolysaccharide-Induced Inflammatory Response. Evid.-Based Complement. Altern. Med. 2014, 2014, 837835. [Google Scholar]
- Wu, J.; Guo, J.; Du, X.; Mcgeer, P.L. Anti-Inflammatory and Neuroprotective Effects of Kissoone B and Extracts of Valeriana Amurensis. Rev. Bras. Farmacogn. 2020, 30, 474–481. [Google Scholar]
- Yoon, C.-S.; Kim, K.-W.; Lee, S.-C.; Kim, Y.-C.; Oh, H. Anti-Neuroinflammatory Effects of Sesquiterpenoids Isolated from Nardostachys Jatamansi. Bioorganic Med. Chem. Lett. 2018, 28, 140–144. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.M.; Danielli, L.J.; Apel, M.A.; Cassel, E.; Vargas, R.M.F.; Von Poser, G.L.; Müller, L.G.; Rates, S.M.K. A Valepotriate-Enriched Fraction from Valeriana Glechomifolia Meyer Inhibits Leukocytes Migration and Nociception in Formalin Test in Rodents. Rev. Bras. Farmacogn. 2019, 29, 477–482. [Google Scholar] [CrossRef]
- Turkez, H.; Togar, B.; Di Stefano, A.; Taspınar, N.; Sozio, P. Protective Effects of Cyclosativene on H2O2-Induced Injury in Cultured Rat Primary Cerebral Cortex Cells. Cytotechnology 2015, 67, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Ma, L.; Dong, L.; Zhang, X.; Chen, J.; Fu, X. Anti-Inflammatory, Antinociceptive Activity of an Essential Oil Recipe Consisting of the Supercritical Fluid CO2 Extract of White Pepper, Long Pepper, Cinnamon, Saffron and Myrrh in Vivo. J. Oleo Sci. 2014, 63, 1251–1260. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical Composition and Anticancer, Antiinflammatory, Antioxidant and Antimalarial Activities of Leaves Essential Oil of Cedrelopsis Grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef]
- Martins, F.T.; Doriguetto, A.C.; de Souza, T.C.; de Souza, K.R.D.; Dos Santos, M.H.; Moreira, M.E.C.; Barbosa, L.C.A. Composition, and Anti-Inflammatory and Antioxidant Activities of the Volatile Oil from the Fruit Peel of Garcinia Brasiliensis. Chem. Biodivers. 2008, 5, 251–258. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.J.; Hyun, C.-G.; Lee, N.H. Chemical Constituents of Supercritical Extracts from Prunus Yedoensis, Saururus Chinensis, Zanthoxylum Piperitum and Their Anti-Inflammatory Activities. Int. J. Pharmacol. 2013, 9, 258–264. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Sahoo, A.; Kamila, P.K.; Das, P.K.; Mohanty, S.; Nayak, S.; Panda, P.C. Chemical Composition, Antioxidant, Anti-Inflammatory and Anticancer Activities of Bark Essential Oil of Cryptocarya Amygdalina from India. J. Essent. Oil Bear. Plants 2021, 24, 617–631. [Google Scholar] [CrossRef]
- Costaa, E.V.; Brasil da Silva, T.; Pereira Soares, M.B. Chemical Composition of the Essential Oil from the Fresh Fruits of Xylopia Laevigata and Its Cytotoxic Evaluation. Nat. Prod. Commun. 2016, 11, 417–418. [Google Scholar]
- Alitonou, G.; Avlessi, F.; Sohounhloue, D.C.K.; Bessière, J.M.; Menut, C. Chemical and Biological Investigation on Volatile Constituents of Pentadesma Butyracea Sabine (Clusiaceae) From Benin. J. Essent. Oil Res. 2010, 22, 138–140. [Google Scholar] [CrossRef]
- Núñez Sellés, A.J.; Agüero, J.A.; Paz, L.N. GC-MS Analysis of Mango Stem Bark Extracts (Mangifera Indica L.), Haden Variety. Possible Contribution of Volatile Compounds to Its Health Effects. Open Chem. 2021, 19, 27–38. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.; Fu, Y.; Wang, W. Composition and Biological Activities of the Essential Oil from Schisandra Chinensis Obtained by Solvent-Free Microwave Extraction. LWT Food Sci. Technol. 2011, 44, 2047–2052. [Google Scholar]
- Lai, P.; Su, X.-D.; Gao, Y.; Xiang, Y.; Lai, P.; Xing, X. Chemical Composition and Biological Activities of the Essential Oil from Aristolochia Fordiana Hemsl. Rec. Nat. Prod. 2019, 13, 346–354. [Google Scholar] [CrossRef]
- Dong, T.; Thuan Lee, A.; Van Tran, D. Essential Oil of the Leaves of Psychotria Asiatica L.: Chemical Composition, Antioxidant, Anti-Inflammatory, and Cytotoxic Properties. Nat. Prod. Res. 2024, 2, 1–6. [Google Scholar]
- Calva, J.; Silva, M.; Morocho, V. Composition and Anti-Acetylcholinesterase Properties of the Essential Oil of the Ecuadorian Endemic Species Eugenia Valvata McVaugh. Molecules 2023, 28, 8112. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar]
- Stojanović, N.M.; Ranđelović, P.J.; Simonović, M.; Radić, M.; Todorović, S.; Corrigan, M.; Harkin, A.; Boylan, F. Essential Oil Constituents as Anti-Inflammatory and Neuroprotective Agents: An Insight through Microglia Modulation. Int. J. Mol. Sci. 2024, 25, 5168. [Google Scholar] [CrossRef]
- Bichão, H.; Borg-Karlson, A.K.; Wibe, A.; Araújo, J.; Mustaparta, H. Molecular Receptive Ranges of Olfactory Receptor Neurones Responding Selectively to Terpenoids, Aliphatic Green Leaf Volatiles and Aromatic Compounds, in the Strawberry Blossom Weevil Anthonomus Rubi. Chemoecology 2005, 15, 211–226. [Google Scholar] [CrossRef]
- Ramírez, J.; Gilardoni, G.; Jácome, M.; Montesinos, J.; Rodolfi, M.; Guglielminetti, M.L.; Cagliero, C.; Bicchi, C.; Vidari, G. Chemical Composition, Enantiomeric Analysis, AEDA Sensorial Evaluation and Antifungal Activity of the Essential Oil from the Ecuadorian Plant Lepechinia Mutica Benth (Lamiaceae). Chem. Biodivers. 2017, 14, e1700292. [Google Scholar] [CrossRef]
- Montalván, M.; Peñafiel, M.A.; Ramírez, J.; Cumbicus, N.; Bec, N.; Larroque, C.; Bicchi, C.; Gilardoni, G. Chemical Composition, Enantiomeric Distribution, and Sensory Evaluation of the Essential Oils Distilled from the Ecuadorian Species Myrcianthes Myrsinoides (Kunth) Grifo and Myrcia Mollis (Kunth) DC. (Myrtaceae). Plants 2019, 8, 511. [Google Scholar] [CrossRef]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C.; Gilardoni, G. Chemical, Enantioselective, and Sensory Analysis of a Cholinesterase Inhibitor Essential Oil from Coreopsis Triloba S.F. Blake (Asteraceae). Plants 2019, 8, 448. [Google Scholar] [CrossRef]
- Gilardoni, G.; Montalván, M.; Ortiz, M.; Vinueza, D.; Montesinos, J.V. The Flower Essential Oil of Dalea Mutisii Kunth (Fabaceae) from Ecuador: Chemical, Enantioselective, and Olfactometric Analyses. Plants 2020, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- de Saint Laumer, J.-Y.; Cicchetti, E.; Merle, P. Quantification in Gas Chromatography: Prediction of Flame Ionization Detector Response Factors from Combustion Enthalpies and Molecular Structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID Quantification Technique without Authentic Samples Using Predicted Response Factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Maldonado, Y.E.; Malagón, O.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Native Ecuadorian Species Steiractinia Sodiroi (Hieron.) S.F. Blake (Asteraceae): Chemical and Enantioselective Analyses. Sci. Rep. 2023, 13, 17180. [Google Scholar] [CrossRef]
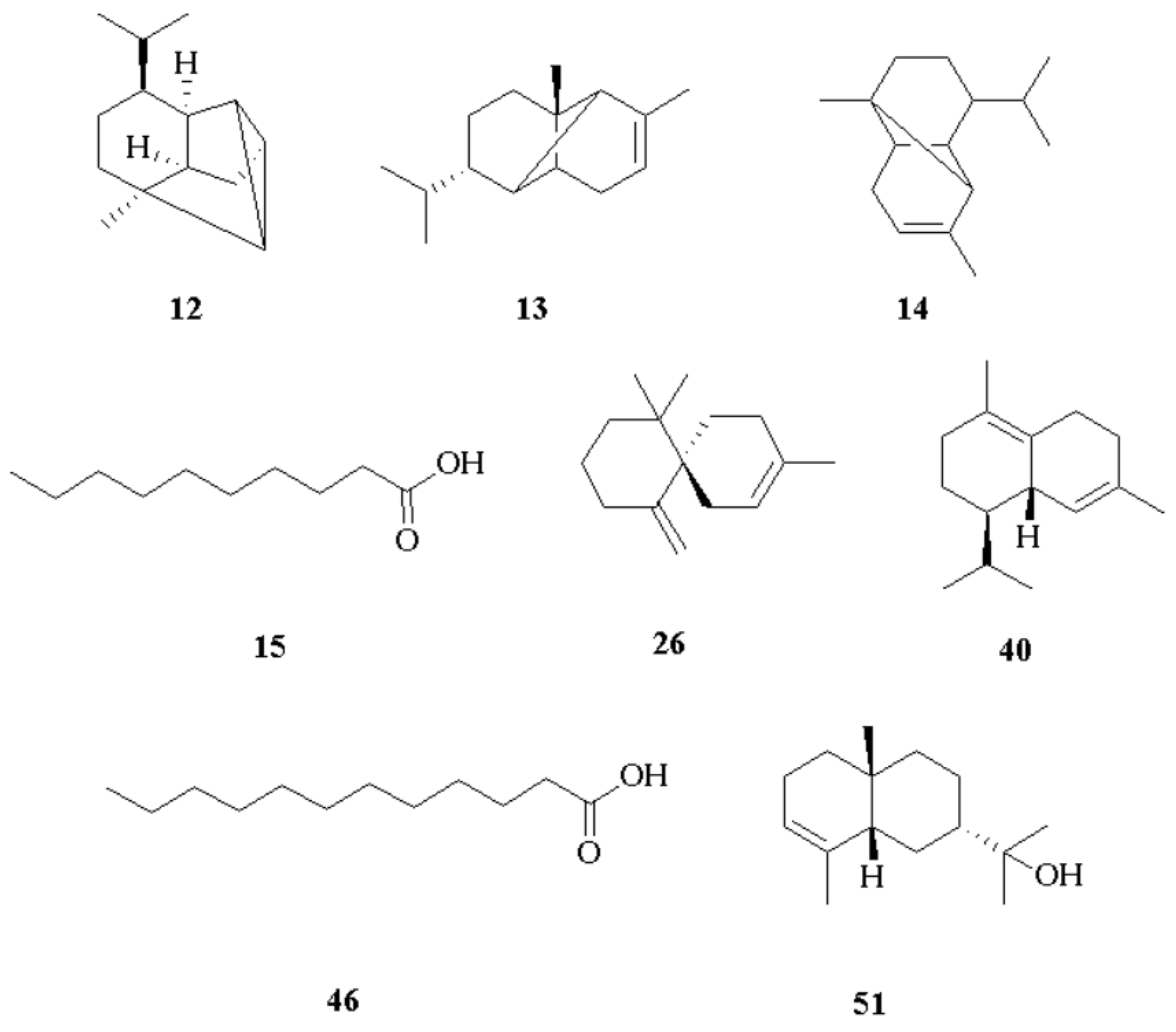


| Compounds | 5% Phenyl Methyl Polysiloxane | Polyethylene Glycol | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LRI a | LRI b | % | σ | LRI a | LRI c | % | σ | |||
| 1 | α-pinene | 929 | 932 | 0.1 | 0.03 | 1019 | 1026 | Trace | - | [33] |
| 2 | camphene | 943 | 946 | 0.1 | 0.07 | 1060 | 1060 | 0.2 | 0.05 | [34] |
| 3 | valeric acid | 946 | 939 | 0.1 | 0.06 | 1693 | - | 0.2 | 0.08 | § |
| 4 | β-pinene | 970 | 974 | Trace | - | 1106 | 1105 | 0.0 | 0.01 | [35] |
| 5 | 2-methoxy-3-(1-methylpropyl)-pyrazine | 1168 | 1168 | 0.1 | 0.05 | 1505 | 1509 | 0.1 | 0.01 | [36] |
| 6 | isobornyl acetate | 1280 | 1283 | 0.3 | 0.12 | 1582 | 1577 | 0.5 | 0.10 | [37] |
| 7 | (2E,4Z)-decadienal | 1287 | 1292 | 0.1 | 0.01 | 1776 | 1779 | 0.2 | 0.02 | [38] |
| 8 | (E,E)-2,4-decadienal | 1309 | 1315 | 0.2 | 0.14 | 1810 | 1814 | 0.2 | 0.01 | [39] |
| 9 | unidentified (MW = 196) | 1315 | - | 0.2 | 0.09 | 1587 | - | 0.5 | 0.12 | § |
| 10 | 8,9-didehydrocycloisolongifolene | 1321 | 1317 | 0.1 | 0.07 | 1436 | - | 0.1 | 0.02 | § |
| 11 | α-cubebene | 1345 | 1348 | 0.5 | 0.09 | 1450 | 1450 | 0.3 | 0.03 | [40] |
| 12 | cyclosativene | 1360 | 1369 | 4.5 | 0.80 | 1466 | 1465 | 4.4 | 0.70 | [41] |
| 13 | α-ylangene | 1362 | 1373 | 1468 | 1470 | [42] | ||||
| 14 | α-copaene | 1373 | 1374 | 9.0 | 2.44 | 1481 | 1483 | 8.8 | 2.20 | [43] |
| 15 | decanoic acid | 1378 | 1382 | 16.0 | 0.02 | 2297 | 2294 | 15.6 | 3.57 | [44] |
| 16 | β-cubebene | 1386 | 1387 | 2.9 | 0.19 | 1530 | 1527 | 2.8 | 0.16 | [42] |
| 17 | 7-epi-sesquithujene | 1390 | 1390 | 0.4 | 0.09 | 1570 | - | 0.3 | 0.06 | § |
| 18 | cyperene | 1394 | 1398 | 1.1 | 0.42 | 1510 | 1514 | 1.1 | 0.25 | [45] |
| 19 | β-longipinene | 1404 | 1400 | 0.4 | 0.13 | 1516 | - | 0.2 | 0.05 | § |
| 20 | β-funebrene | 1410 | 1413 | 0.2 | 0.07 | 1532 | 1618 | Trace | - | [46] |
| 21 | (E)-β-caryophyllene | 1413 | 1420 | 1.1 | 0.52 | 1583 | 1585 | 1.3 | 0.29 | [47] |
| 22 | β-gurjunene | 1423 | 1431 | 0.1 | 0.03 | 1578 | 1580 | 0.1 | 0.01 | [48] |
| 23 | α-guayen | 1444 | 1437 | 0.3 | 0.12 | 1604 | 1604 | 0.3 | 0.06 | [49] |
| 24 | α-humulene | 1447 | 1452 | 0.4 | 0.06 | 1656 | 1656 | 0.4 | 0.09 | [50] |
| 25 | alloaromadendrene | 1455 | 1458 | 1.1 | 0.25 | 1631 | 1631 | 0.9 | 0.20 | [51] |
| 26 | β-chamigrene | 1469 | 1476 | 3.2 | 1.01 | 1650 | - | 3.1 | 0.97 | § |
| 27 | γ-muurolene | 1472 | 1478 | 0.1 | 0.06 | 1699 | 1702 | 0.5 | 0.13 | [52] |
| 28 | germacrene D | 1475 | 1480 | 0.8 | 0.30 | 1679 | 1678 | 0.9 | 0.16 | [53] |
| 29 | widdra-2,4(14)-diene | 1478 | 1481 | 0.2 | 0.05 | 1568 | - | 0.3 | 0.07 | § |
| 30 | unidentified (MW = 204) | 1480 | - | 0.2 | 0.07 | 1705 | - | 0.1 | 0.07 | - |
| 31 | unidentified (MW = 204) | 1485 | - | 0.2 | 0.14 | - | - | Trace | - | - |
| 32 | cis-β-guaiene | 1487 | 1492 | 0.3 | 0.03 | 1678 | 1671 | 0.3 | 0.05 | [54] |
| 33 | epicubebol | 1489 | 1493 | 0.4 | 0.05 | 1938 | 1928 | 0.5 | 0.09 | [55] |
| 34 | eciphyllene | 1494 | 1501 | 2.1 | 0.80 | 1705 | - | 1.1 | 0.31 | § |
| 35 | trans-β-guaiene | 1495 | 1639 | 1474 | - | 0.8 | 0.36 | § | ||
| 36 | premnaspirodiene | 1499 | 1505 | 0.4 | 0.12 | 1664 | - | 0.3 | 0.07 | § |
| 37 | unidentified (MW = 204) | 1502 | - | 0.1 | 0.04 | 1794 | - | 0.2 | 0.02 | - |
| 38 | cubebol | 1510 | 1514 | 0.3 | 0.14 | 1939 | 1930 | 0.8 | 0.13 | [56] |
| 39 | unidentified (MW = 220) | 1512 | - | 1.7 | 0.35 | 2000 | - | 1.3 | 0.11 | - |
| 40 | δ-cadinene | 1520 | 1522 | 9.7 | 0.70 | 1750 | 1752 | 9.5 | 1.25 | [57] |
| 41 | (E)-iso-γ-bisabolene | 1522 | 1529 | 0.7 | 0.20 | 1760 | 1762 | 0.4 | 0.08 | [58] |
| 42 | γ-cuprenene | 1527 | 1532 | 0.2 | 0.02 | 2060 | - | 0.2 | 0.02 | § |
| 43 | α-copaen-11-ol | 1535 | 1539 | 0.9 | 0.12 | 2053 | - | 0.7 | 0.11 | § |
| 44 | silphiperfol-5-en-3-one B | 1541 | 1550 | 0.2 | 0.03 | 1944 | - | 0.3 | 0.07 | § |
| 45 | β-calacorene | 1557 | 1564 | 0.1 | 0.04 | 1907 | 1912 | 0.1 | 0.04 | [59] |
| 46 | dodecanoic acid | 1569 | 1567 | 13.4 | 0.95 | 2485 | 2487 | 12.3 | 1.33 | [60] |
| 47 | viridiflorol | 1597 | 1592 | 0.4 | 0.03 | 2183 | - | 0.2 | 0.02 | § |
| 48 | silphiperfol-6-en-5-one | 1622 | 1624 | 0.7 | 0.13 | 2080 | - | 0.5 | 0.03 | § |
| 49 | alloaromadendrene oxide | 1636 | 1639 | 0.8 | 0.20 | 2065 | - | 0.3 | 0.02 | § |
| 50 | unidentified (MW = 202) | 1666 | - | 0.2 | 0.22 | 2008 | - | 0.4 | 0.04 | - |
| 51 | 7-epi-α-eudesmol | 1669 | 1662 | 5.0 | 0.87 | 1740 | - | 4.9 | 0.77 | § |
| 52 | pentadecanal | 1710 | 1716 | 0.5 | 0.07 | 2029 | 2024 | 0.2 | 0.04 | [61] |
| 53 | unidentified (MW = 200) | 1723 | - | 0.4 | 0.28 | 2201 | - | 0.4 | 0.04 | - |
| 54 | unidentified (MW = 204) | 1746 | - | 0.1 | 0.00 | 2141 | - | 0.4 | 0.02 | - |
| 55 | hexadecanoic acid | 1959 | 1959 | 0.5 | 0.18 | 2923 | 2928 | 0.3 | 0.12 | [62] |
| 56 | 3-(Z)-cembrene A | 1966 | 1965 | 0.9 | 0.11 | 2242 | - | 0.7 | 0.11 | § |
| monoterpene hydrocarbons | 0.2 | 0.2 | ||||||||
| oxygenated monoterpenes | 0.3 | 0.5 | ||||||||
| sesquiterpene hydrocarbons | 41.5 | 40.5 | ||||||||
| oxygenated sesquiterpenes | 8.3 | 7.7 | ||||||||
| diterpene sesquiterpenes | 0.9 | 0.7 | ||||||||
| others | 32.8 | 30.9 | ||||||||
| total | 84.0 | 80.5 | ||||||||
| Chiral Selector | Enantiomer | LRI | Enantiomeric Distribution % | e.e. % |
|---|---|---|---|---|
| DAC | (1S,5S)-(−)-α-pinene | 926 | - | 100 |
| DAC | (1S,5S)-(+)-α-pinene | 929 | 100 | |
| DET | (1R,4S)-(+)-camphene | 932 | - | 100 |
| DET | (1R,4S)-(–)-camphene | 922 | 100 | |
| DET | (1S,5S)-(−)-β-pinene | 961 | 100 | 100 |
| DET | (1S,5S)-(+)-β-pinene | 944 | - | |
| DET | (1R,2S,6S,7S,8S)-(–)-α-copaene | 1323 | 100 | 100 |
| DET | (1R,2S,6S,7S,8S)-(+)-α-copaene | 1319 | - | |
| DET | (R)-(+)-germacrene D | 1461 | 22.58 | 54.83 |
| DET | (S)-(−)-germacrene D | 1467 | 77.42 |
| Concentration (µg/mL) | V. rigida Essential Oil | Aspirin |
|---|---|---|
| 3.1 | 2.32 ± 0.28 | 15.12 ± 2.07 ** |
| 6.2 | 6.26 ± 0.97 | 23.81 ± 3.21 ** |
| 12.5 | 24.46 ± 1.91 | 31.16 ± 2.97 * |
| 25.0 | 46.39 ± 1.40 | 44.75 ± 4.74 |
| 50.0 | 51.42 ± 1.07 | 55.28 ± 2.30 |
| 100.0 | 61.42 ± 2.13 | 68.52 ± 5.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, L.M.; Vinueza, D.R.; Gilardoni, G.; Mota, A.J.; Malagón, O. The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity. Plants 2025, 14, 1062. https://doi.org/10.3390/plants14071062
Flores LM, Vinueza DR, Gilardoni G, Mota AJ, Malagón O. The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity. Plants. 2025; 14(7):1062. https://doi.org/10.3390/plants14071062
Chicago/Turabian StyleFlores, Linda M., Diego R. Vinueza, Gianluca Gilardoni, Antonio J. Mota, and Omar Malagón. 2025. "The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity" Plants 14, no. 7: 1062. https://doi.org/10.3390/plants14071062
APA StyleFlores, L. M., Vinueza, D. R., Gilardoni, G., Mota, A. J., & Malagón, O. (2025). The Essential Oil from the Roots of Valeriana rigida Ruiz & Pav. Growing in the Paramos of Chimborazo (Ecuador): Chemical Analysis, Enantioselective Profile, and Preliminary Biological Activity. Plants, 14(7), 1062. https://doi.org/10.3390/plants14071062










