Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis
Abstract
1. Introduction
2. Results
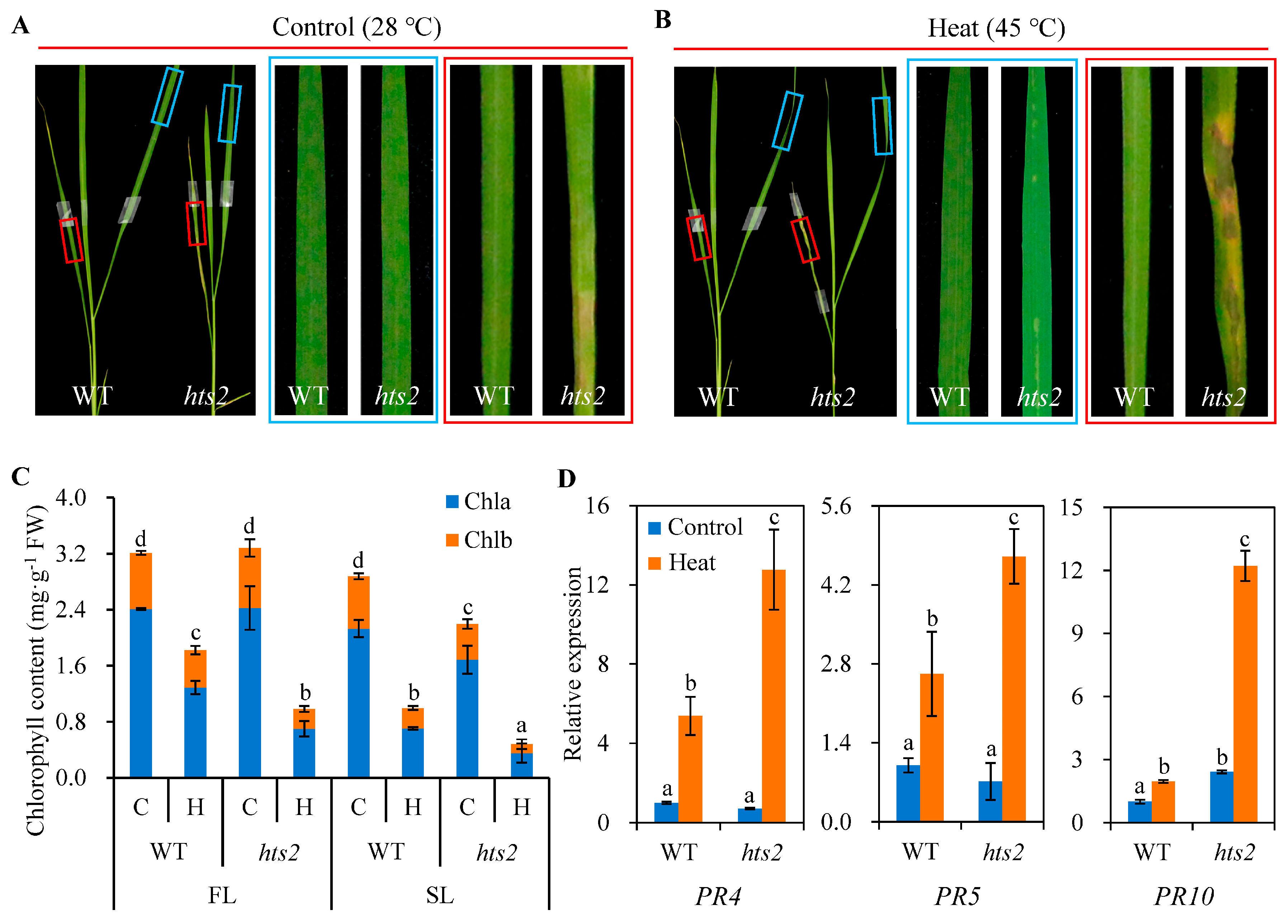
2.1. hts2 Mutant Is Hypersensitive to Heat Stress
2.2. Heat Treatment Accelerated Lesion-Mimic Symptoms Formation in hts2 Mutant
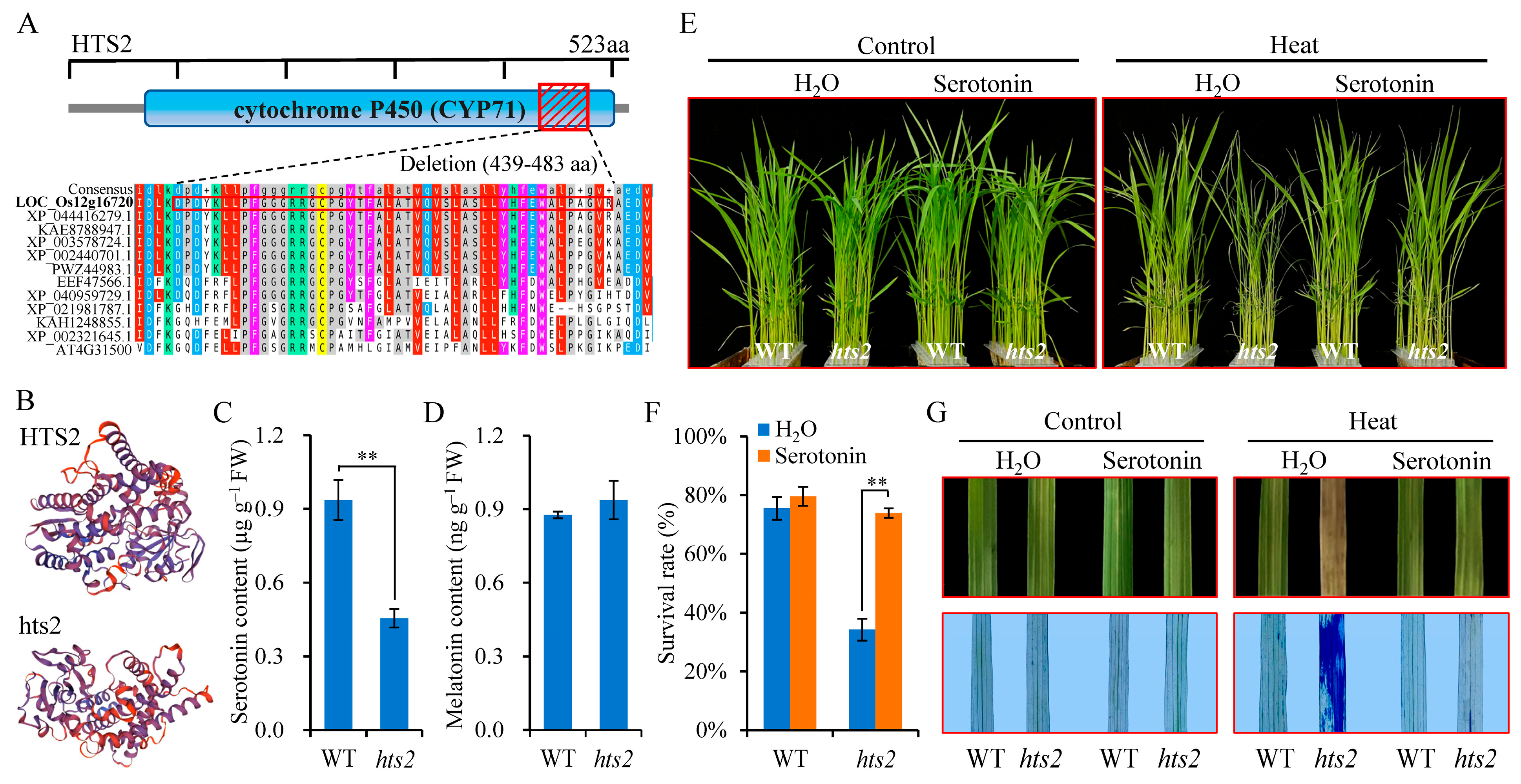
2.3. Map-Based Cloning of HTS2
2.4. The Heat-Sensitive Phenotype of hts2 Can Be Restored by Serotonin Complementation
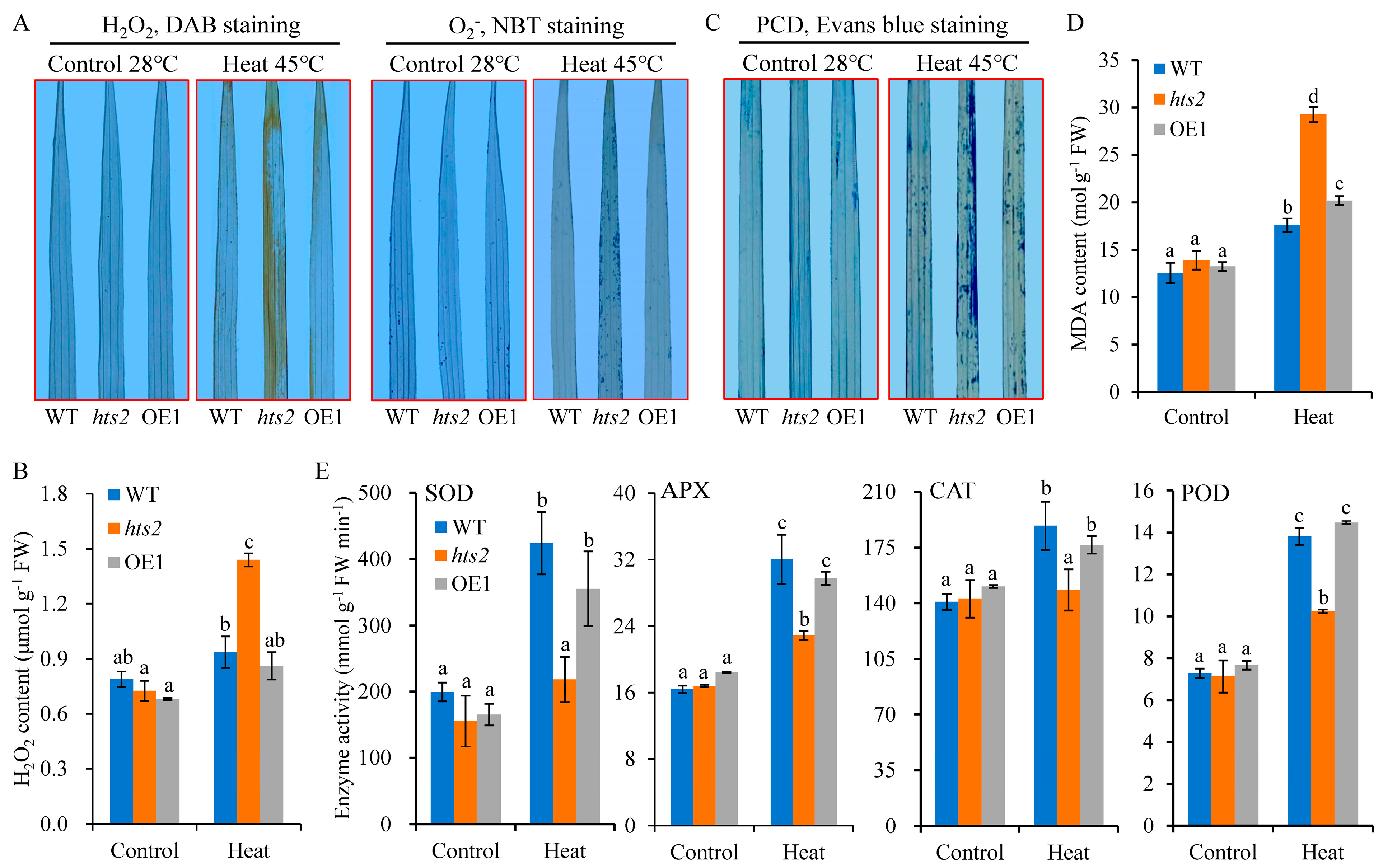
2.5. HTS2 Deficiency Accelerates Heat Stress-Induced ROS Accumulation and Cell Death
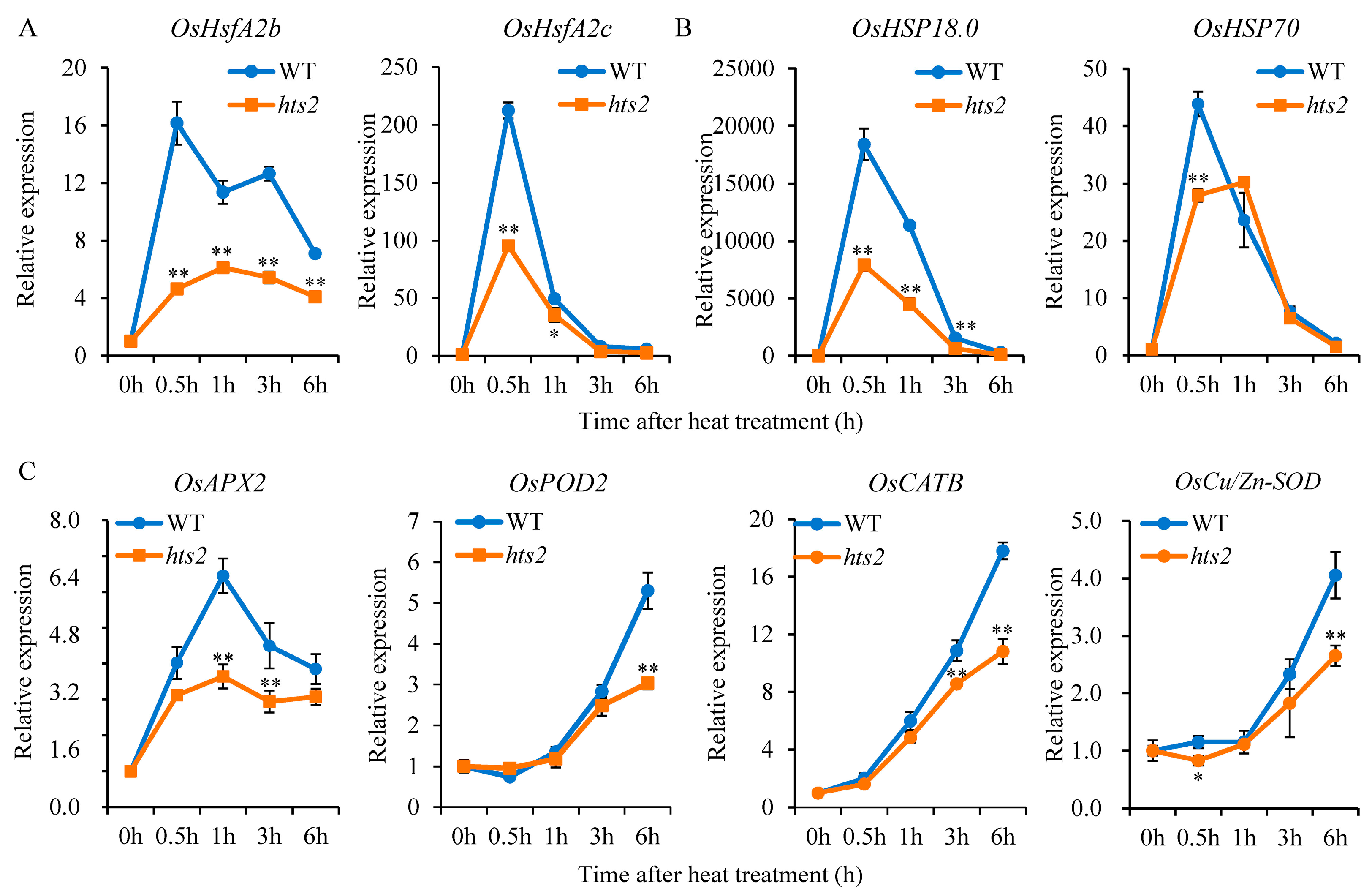
2.6. HTS2 Modulates the Expression of HsfA2 and Its Downstream Heat-Responsive Genes Under High Temperature Condition
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Map-Based Cloning of HTS2
4.3. Plasmid Construction and Plant Transformation
4.4. RNA Extraction and Quantitative Real-Time PCR
4.5. Cell Physiology Analysis
4.6. H2O2 and Methyl Viologen Treatment
4.7. Quantification of Serotonin and Melatonin Contents
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.Y.; Li, X.M.; Lin, H.X.; Chong, K. Crop improvement through temperature resilience. Annu. Rev. Plant Biol. 2019, 70, 753–780. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Ainsworth, E.A.; Bahuguna, R.N.; Broadley, M.R.; Busch, W.; Carpita, N.C.; Castrillo, G.; Chory, J.; DeHaan, L.R.; Duarte, C.M.; et al. Climate change challenges, plant science solutions. Plant Cell 2023, 35, 24–66. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- VanWallendael, A.; Soltani, A.; Emery, N.C.; Peixoto, M.M.; Olsen, J.; Lowry, D.B. A molecular view of plant local adaptation: Incorporating stress-response networks. Annu. Rev. Plant Biol. 2019, 70, 559–583. [Google Scholar] [CrossRef]
- Li, B.J.; Gao, K.; Ren, H.M.; Tang, W.Q. Molecular mechanisms governing plant responses to high temperatures. J. Integr. Plant Biol. 2018, 60, 757–779. [Google Scholar] [CrossRef]
- Bakery, A.; Vraggalas, S.; Shalha, B.; Chauchan, H.; Benhamed, M.; Fragkostefanakis, S. Heat stress transcription factors as the central molecular rheostat to optimize plant survival and recovery from heat stress. New Phytol. 2024, 244, 51–64. [Google Scholar] [CrossRef]
- Raturi, V.; Zinta, G. HSFA1 heat shock factors integrate warm temperature and heat signals in plants. Trends Plant Sci. 2024, 29, 1165–1167. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Molecular regulation of plant responses to environmental temperatures. Mol. Plant 2020, 13, 544–564. [Google Scholar] [CrossRef]
- He, N.Y.; Chen, L.S.; Sun, A.Z.; Zhao, Y.; Yin, S.N.; Guo, F.Q. A nitric oxide burst at the shoot apex triggers a heat-responsive pathway in Arabidopsis. Nat. Plants 2022, 8, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Mu, X.R.; Gao, J.; Lin, H.X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Xu, Y.F.; Chu, C.C.; Yao, S.G. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Xia, S.; Liu, H.; Cui, Y.; Yu, H.; Rao, Y.; Yan, Y.; Zeng, D.; Hu, J.; Zhang, G.; Gao, Z.; et al. UDP-N-acetylglucosamine pyrophosphorylase enhances rice survival at high temperature. New Phytol. 2022, 233, 344–359. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Liao, M.; Ma, Z.M.; Kang, Y.R.; Zhang, B.M.; Gao, X.L.; Yu, F.; Yang, P.F.; Ke, Y.G. ENHANCED DISEASE SUSCEPTIBILITY 1 promotes hydrogen peroxide scavenging to enhance rice thermotolerance. Plant Physiol. 2023, 192, 3106–3119. [Google Scholar] [CrossRef]
- Wang, J.J.; Xu, J.; Wang, L.; Zhou, M.Y.; Nian, J.Q.; Chen, M.M.; Lu, X.L.; Liu, X.; Wang, Z.; Cen, J.S.; et al. SEMI-ROLLED LEAF 10 stabilizes catalase isozyme B to regulate leaf morphology and thermotolerance in rice (Oryza sativa L.). Plant Biotechnol. J. 2023, 21, 819–838. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Zhang, Q.; Liu, D.L.; Wang, H.Q.; Yin, J.Y.; Wang, R.; He, M.L.; Cui, M.; Shang, Z.L.; Wang, D.K.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, S.; Li, Z.; Cheng, J.; Hu, P.; Zhu, T.; Wang, X.; Jin, M.; Wang, X.; Li, L.; et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020, 183, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ji, P.; Liao, J.; Duan, X.; Luo, Z.; Yu, X.; Jiang, C.J.; Xu, C.; Yang, H.; Peng, B.; et al. CRISPR/Cas knockout of the NADPH oxidase gene OsRbohB reduces ROS overaccumulation and enhances heat stress tolerance in rice. Plant Biotechnol. J. 2025, 23, 226–351. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Sarkar, A.K. Serotonin: A frontline player in plant growth and stress responses. Physiol. Plant 2023, 175, e13968. [Google Scholar] [CrossRef]
- Kaur, H.; Mukherjee, S.; Baluska, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal Behav. 2015, 10, e1049788. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Dharmawardhana, P.; Ren, L.; Amarasinghe, V.; Monaco, M.; Thomason, J.; Ravenscroft, D.; McCouch, S.; Ware, D.; Jaiswal, P. A genome scale metabolic network for rice and accompanying analysis of tryptophan, auxin and serotonin biosynthesis regulation under biotic stress. Rice 2013, 6, 15. [Google Scholar] [CrossRef]
- Mukherjee, S.; David, A.; Yadav, S.; Baluska, F.; Bhatla, S.C. Salt stress-induced seedling growth inhibition coincides with differential distribution of serotonin and melatonin in sunflower seedling roots and cotyledons. Physiol. Plant 2014, 152, 714–728. [Google Scholar] [CrossRef]
- Erland, L.A.; Turi, C.E.; Saxena, P.K. Serotonin: An ancient molecule and an important regulator of plant processes. Biotechnol. Adv. 2016, 34, 1347–1361. [Google Scholar] [CrossRef]
- Akcay, U.C.; Okudan, N. Exogenous serotonin improves drought and salt tolerance in tomato seedlings. Plant Growth Regul. 2023, 101, 239–249. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, P.; Wang, R.; Sun, L.; Ju, Q.; Xu, J. Comparative physiological responses and transcriptome analysis reveal the roles of melatonin and serotonin in regulating growth and metabolism in Arabidopsis. BMC Plant Biol. 2018, 18, 362. [Google Scholar] [CrossRef]
- Li, J.J.; Zhang, H.Y.; Yue, D.F.; Chen, S.Y.; Yin, Y.X.; Zheng, C.F.; Chen, Y. Endogenous serotonin induced by cold acclimation increases cold tolerance by reshaping the MEL/ROS/RNS redox network in Kandelia obovata. J. For. Res. 2024, 35, 112. [Google Scholar] [CrossRef]
- Hayashi, K.; Fujita, Y.; Ashizawa, T.; Suzuki, F.; Nagamura, Y.; Hayano-Saito, Y. Serotonin attenuates biotic stress and leads to lesion browning caused by a hypersensitive response to Magnaporthe oryzae penetration in rice. Plant J. 2016, 85, 46–56. [Google Scholar] [CrossRef]
- Kumar, G.; Saad, K.R.; Arya, M.; Puthusseri, B.; Mahadevappa, P.; Shetty, N.P.; Giridhar, P. The synergistic role of serotonin and melatonin during temperature stress in promoting cell division, ethylene and isoflavones biosynthesis in Glycine max. Curr. Plant Biol. 2021, 26, 100206. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef]
- Qiu, T.; Zhao, X.; Feng, H.; Qi, L.; Yang, J.; Peng, Y.L.; Zhao, W. OsNBL3, a mitochondrion-localized pentatricopeptide repeat protein, is involved in splicing nad5 intron 4 and its disruption causes lesion mimic phenotype with enhanced resistance to biotic and abiotic stresses. Plant Biotechnol. J. 2021, 19, 2277–2290. [Google Scholar] [CrossRef]
- Fujiwara, T.; Maisonneuve, S.; Isshiki, M.; Mizutani, M.; Chen, L.; Wong, H.L.; Kawasaki, T.; Shimamoto, K. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenase that catalyzes conversion of tryptamine to serotonin in rice. J. Biol. Chem. 2010, 285, 11308–11313. [Google Scholar] [CrossRef]
- Singh, V.P.; Jaiswal, S.; Wang, Y.; Feng, S.; Tripathi, D.K.; Singh, S.; Gupta, R.; Xue, D.; Xu, S.; Chen, Z.H. Evolution of reactive oxygen species cellular targets for plant development. Trends Plant Sci. 2024, 29, 865–877. [Google Scholar] [CrossRef]
- Xing, Y.H.; Lu, H.Y.; Zhu, X.F.; Deng, Y.F.; Xie, Y.J.; Luo, Q.H.; Yu, J.S. How rice responds to temperature changes and defeats heat stress. Rice 2024, 17, 73. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A beta-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Peng, Y.; Zhang, Q.; Xia, S.; Ruan, B.; Xu, Q.; Yu, X.; Zhou, T.; Liu, H.; Zeng, D.; et al. Disruption of EARLY LESION LEAF 1, encoding a cytochrome P450 monooxygenase, induces ROS accumulation and cell death in rice. Plant J. 2021, 105, 942–956. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xu, J.; Wang, F.; Tang, Y.; Wei, Z.; Ji, Z.; Wang, C.; Zhao, K. Mutation types of CYP71P1 cause different phenotypes of mosaic spot lesion and premature leaf senescence in rice. Front. Plant Sci. 2021, 12, 641300. [Google Scholar] [CrossRef]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Cote, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and membrane binding properties of serotonin protect lipids from oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef]
- Azmitia, E.C. Serotonin and brain: Evolution, neuroplasticity, and homeostasis. Int. Rev. Neurobiol. 2007, 77, 31–56. [Google Scholar] [CrossRef]
- Kang, K.; Kim, Y.S.; Park, S.; Back, K. Senescence-induced serotonin biosynthesis and its role in delaying senescence in rice leaves. Plant Physiol. 2009, 150, 1380–1393. [Google Scholar] [CrossRef]
- Mukherjee, S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol. Biochem. 2018, 132, 33–45. [Google Scholar] [CrossRef]
- Pelagio-Flores, R.; Ruiz-Herrera, L.F.; Lopez-Bucio, J. Serotonin modulates Arabidopsis root growth via changes in reactive oxygen species and jasmonic acid-ethylene signaling. Physiol. Plant 2016, 158, 92–105. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef]
- Park, S.; Byeon, Y.; Back, K. Transcriptional suppression of tryptamine 5-hydroxylase, a terminal serotonin biosynthetic gene, induces melatonin biosynthesis in rice (Oryza sativa L.). J. Pineal Res. 2013, 55, 131–137. [Google Scholar] [CrossRef]
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Dong, G.J.; Ma, X.H.; Wang, F.; Zhang, Y.L.; Xiong, E.H.; Wu, J.H.; Wang, H.Z.; Qian, Q.; Wu, L.M.; et al. UMP kinase activity is involved in proper chloroplast development in rice. Photosynth. Res. 2018, 137, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Hara, N.; Ono, K.; Onodera, H.; Tagiri, A.; Oka, S.; Tanaka, H. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2006, 47, 969–976. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.R.; Lou, Y.G.; et al. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef]
- Wang, L.; Lu, H.; Zhang, X.; He, Y.; Zhang, J.; Guo, X.; Fu, H.; Ye, G.; Shu, Q. Disruption of serotonin biosynthesis increases resistance to striped stem borer without changing innate defense response in rice. J. Pineal Res. 2023, 75, e12895. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, X.; Zhao, X.; Wang, F.; Wang, H.; Zhang, Y.; Ruan, B.; Dong, G.; Yu, Y.; Wu, L.; Chen, F. Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis. Plants 2025, 14, 1072. https://doi.org/10.3390/plants14071072
Lv X, Zhao X, Wang F, Wang H, Zhang Y, Ruan B, Dong G, Yu Y, Wu L, Chen F. Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis. Plants. 2025; 14(7):1072. https://doi.org/10.3390/plants14071072
Chicago/Turabian StyleLv, Xuantong, Xunan Zhao, Fang Wang, Haili Wang, Yanli Zhang, Banpu Ruan, Guojun Dong, Yanchun Yu, Limin Wu, and Fei Chen. 2025. "Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis" Plants 14, no. 7: 1072. https://doi.org/10.3390/plants14071072
APA StyleLv, X., Zhao, X., Wang, F., Wang, H., Zhang, Y., Ruan, B., Dong, G., Yu, Y., Wu, L., & Chen, F. (2025). Rice Cytochrome P450 Protein CYP71P1 Is Required for Heat Stress Tolerance by Regulating Serotonin Biosynthesis and ROS Homeostasis. Plants, 14(7), 1072. https://doi.org/10.3390/plants14071072






