Effects of Freeze–Thaw Cycles on Uptake Preferences of Plants for Nutrient: A Review
Abstract
1. Introduction
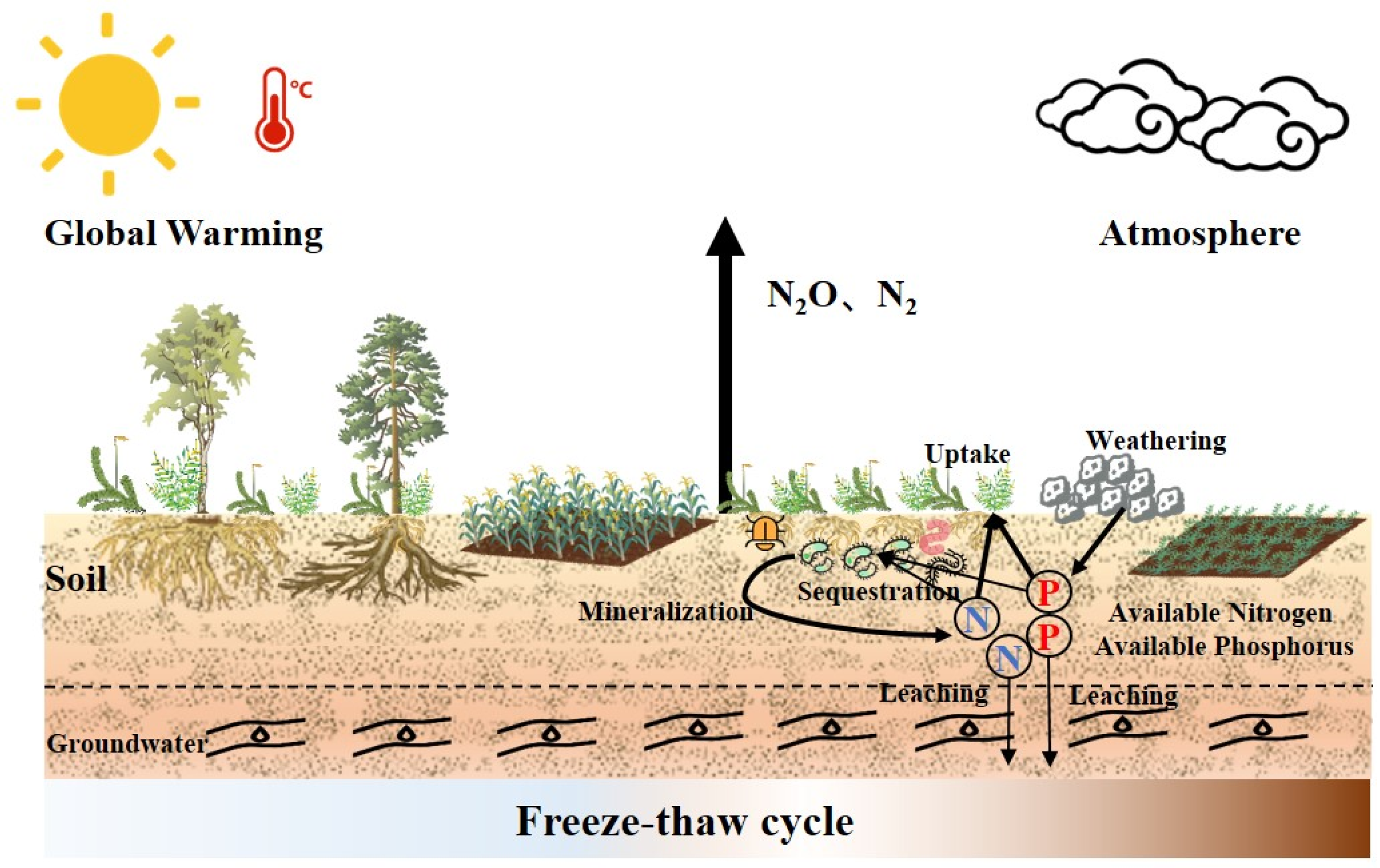
2. Soil N and P Cycling Under the Freeze–Thaw Cycle Conditions
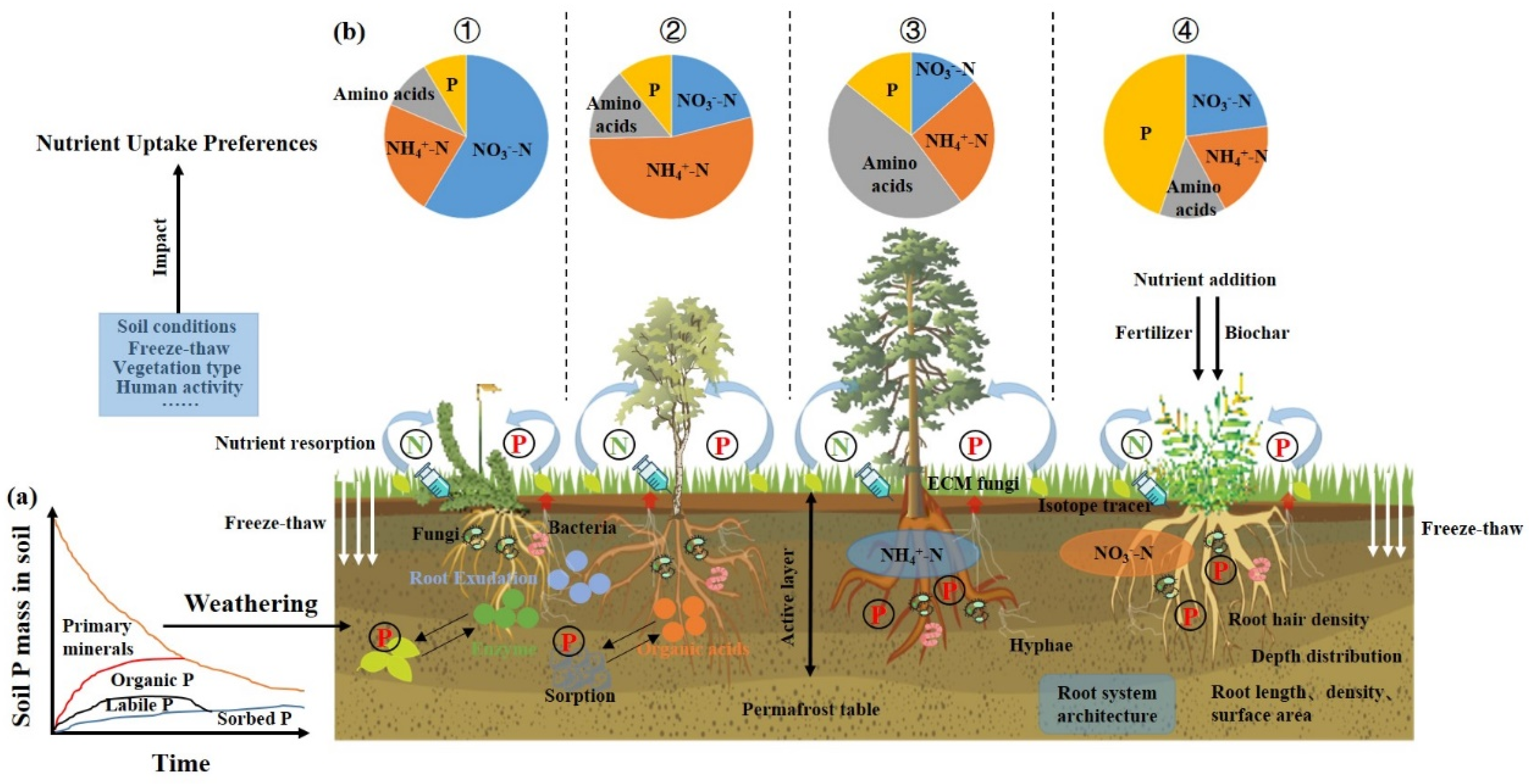
3. N Utilization Preferences of Plants Under the Freeze–Thaw Cycle Conditions
4. P Utilization of Plants Under the Freeze–Thaw Cycle Conditions
5. Envisioning the Future
5.1. Enhanced Research on the Subterranean Components of Plants Can Provide a Deeper Understanding of Their Nutrient Utilization Strategies
5.2. Incorporate Additional Nutrients into the Study and Examine Their Interrelationships
5.3. Revising Research Ideas and Extending the Research Timeline
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Zhang, L.; Wei, X.; Chen, Y.; Yang, W.; Tan, B.; Yue, K.; Ni, X.; Wu, F. Litter removal reduced soil nitrogen mineralization in repeated freeze-thaw cycles. Sci. Rep. 2019, 9, 2052. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.C.; Zhang, L.; Liu, J.; Peng, B.; Fan, Z.Z.; Dai, W.W.; Jiang, P.; Bai, E. Responses of terrestrial nitrogen pools and dynamics to different patterns of freeze-thaw cycle: A meta-analysis. Glob. Change Biol. 2018, 24, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Matzner, E.; Borken, W.J. Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. Eur. J. Soil. Sci. 2008, 59, 274–284. [Google Scholar]
- Yi, Y.; Kimball, J.; Rawlins, M.; Moghaddam, M.; Euskirchen, E. The role of snow cover and soil freeze/thaw cycles affecting boreal-arctic soil carbon dynamics. Biogeosci. Discuss. 2015, 12, 11113–11157. [Google Scholar]
- Lee, H.; Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K. IPCC, 2023: Climate Change 2023: Synthesis Report, Summary for Policymakers. In Con-Tribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar]
- Groffman, P.M.; Hardy, J.P.; Fashu-Kanu, S.; Driscoll, C.T.; Cleavitt, N.L.; Fahey, T.J.; Fisk, M.C. Snow depth, soil freezing and nitrogen cycling in a northern hardwood forest landscape. Biogeochemistry 2011, 102, 223–238. [Google Scholar] [CrossRef]
- Groffman, P.M.; Driscoll, C.T.; Fahey, T.J.; Hardy, J.P.; Fitzhugh, R.D.; Tierney, G.L. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 2001, 56, 135–150. [Google Scholar] [CrossRef]
- Wang, E.; Chen, J.; Liu, L.; Cui, L.; Xue, J.; Ren, J.; Du, Q. Effect of soil texture on water and salt transport in freeze—Thaw soil in the shallow groundwater area. Water 2023, 15, 2587. [Google Scholar] [CrossRef]
- Fu, Z.; Wu, Q.; Zhang, W.; He, H.; Wang, L. Water migration and segregated ice formation in frozen ground: Current advances and future perspectives. Front. Earth Sci. 2022, 10, 826961. [Google Scholar]
- Han, Z.M.; Deng, M.W.; Yuan, A.Q.; Wang, J.H.; Li, H.; Ma, J.C. Vertical variation of a black soil’s properties in response to freeze-thaw cycles and its links to shift of microbial community structure. Sci. Total Environ. 2018, 625, 106–113. [Google Scholar] [CrossRef]
- Sorensen, P.O.; Finzi, A.C.; Giasson, M.A.; Reinmann, A.B.; Sanders-DeMott, R.; Templer, P.H. Winter soil freeze-thaw cycles lead to reductions in soil microbial biomass and activity not compensated for by soil warming. Soil. Biol. Biochem. 2018, 116, 39–47. [Google Scholar] [CrossRef]
- Su, M.; Kleineidam, K.; Schloter, M. Influence of litter quality on the abundance of genes involved in nitrification and denitrification after freezing and thawing of an arable soil. Biol. Fertil. Soils 2010, 46, 537–541. [Google Scholar] [CrossRef]
- Whitfield, C.J.; Casson, N.J.; North, R.L.; Venkiteswaran, J.J.; Ahmed, O.; Leathers, J.; Nugent, K.J.; Prentice, T.; Baulch, H.M. The effect of freeze-thaw cycles on phosphorus release from riparian macrophytes in cold regions. Can. Water Resour. J. Rev. Can. Des. Ressour. Hydr. 2019, 44, 160–173. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Li, P.; Xu, G.C.; Shi, P.; Zhang, Y. Effects of freeze-thaw cycles on aggregate-associated organic carbon and glomalin-related soil protein in natural-succession grassland and Chinese pine forest on the Loess Plateau. Geoderma 2019, 334, 1–8. [Google Scholar] [CrossRef]
- Charrier, G.; Charra-Vaskou, K.; Kasuga, J.; Cochard, H.; Mayr, S.; Ameglio, T. Freeze-Thaw Stress: Effects of Temperature on Hydraulic Conductivity and Ultrasonic Activity in Ten Woody Angiosperms. Plant Physiol. 2014, 164, 992–998. [Google Scholar] [CrossRef]
- Swift, J.; Adame, M.; Tranchina, D.; Henry, A.; Coruzzi, G.M. Water impacts nutrient dose responses genome-wide to affect crop production. Nat. Commun. 2019, 10, 1374. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Townsend, A.R.; Schmidt, S.K. Phosphorus Limitation of Microbial Processes in Moist Tropical Forests: Evidence from Short-term Laboratory Incubations and Field Studies. Ecosystems 2002, 5, 0680–0691. [Google Scholar] [CrossRef]
- Lipson, D.A.; Monson, R.K. Plant-microbe competition for soil amino acids in the alpine tundra: Effects of freeze-thaw and dry-rewet events. Oecologia 1998, 113, 406–414. [Google Scholar] [CrossRef]
- Connolly, B.M.; Orrock, J.L. Climatic variation and seed persistence: Freeze-thaw cycles lower survival via the joint action of abiotic stress and fungal pathogens. Oecologia 2015, 179, 609–616. [Google Scholar] [CrossRef]
- Skinner, D.Z.; Bellinger, B.S. Freezing tolerance of winter wheat as influenced by extended growth at low temperatures and exposure to freeze-thaw cycles. Can. J. Plant Sci. 2017, 97, 250–256. [Google Scholar] [CrossRef]
- Song, Y.; Zou, Y.C.; Wang, G.P.; Yu, X.F. Altered soil carbon and nitrogen cycles due to the freeze-thaw effect: A meta-analysis. Soil. Biol. Biochem. 2017, 109, 35–49. [Google Scholar] [CrossRef]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Zemunik, G.; Turner, B.L.; Lambers, H.; Laliberté, E. Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat. Plants 2015, 1, 15050. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Ciais, P.; Jou, D.; Piao, S.; Obersteiner, M.; Vicca, S.; Janssens, I.A.; Sardans, J. The bioelements, the elementome, and the biogeochemical niche. Ecology 2019, 100, e02652. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Mitchell, M.J.; Groffman, P.M.; Christenson, L.M.; Hardy, J.P. Winter in northeastern North America: A critical period for ecological processes. Front. Ecol. Environ. 2005, 3, 314–322. [Google Scholar] [CrossRef]
- Kreyling, J.; Beierkuhnlein, C.; Jentsch, A. Effects of soil freeze–thaw cycles differ between experimental plant communities. Basic Appl. Ecol. 2010, 11, 65–75. [Google Scholar] [CrossRef]
- Kreyling, J.; Thiel, D.; Simmnacher, K.; Willner, E.; Jentsch, A.; Beierkuhnlein, C. Geographic origin and past climatic experience influence the response to late spring frost in four common grass species in central Europe. Ecography 2012, 35, 268–275. [Google Scholar] [CrossRef]
- Li, H.; Liu, B.; McCormack, M.L.; Ma, Z.; Guo, D. Diverse belowground resource strategies underlie plant species coexistence and spatial distribution in three grasslands along a precipitation gradient. New Phytol. 2017, 216, 1140–1150. [Google Scholar] [CrossRef]
- Lucci, G.M. Pastures and drought: A review of processes and implications for nitrogen and phosphorus cycling in grassland systems. Soil Res. 2019, 57, 101–112. [Google Scholar] [CrossRef]
- Sonesson, M.; Callaghan, T.V. Strategies of Survival in Plants of the Fennoscandian Tundra. Arctic 1991, 44, 95–105. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; Sinauer: Sunderland, MA, USA, 1853. [Google Scholar]
- Fitzhugh, R.D.; Driscoll, C.T.; Groffman, P.M.; Tierney, G.L.; Fahey, T.J.; Hardy, J.P. Effects of soil freezing disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 2001, 56, 215–238. [Google Scholar]
- Hirai, K.; Noguchi, K.; Mizoguchi, T.; Kaneko, S.; Takahashi, M. Contribution of subsoil and season for nitrogen mineralization under field condition in forest soil. Jpn. J. For. Environ. 2007, 49, 51–59. [Google Scholar]
- Alm, J.; Saarnio, S.; Nykänen, H.; Silvola, J.; Martikainen, P. Winter CO2, CH4 and N2O fluxes on some natural and drained boreal peatlands. Biogeochemistry 1999, 44, 163–186. [Google Scholar]
- Joseph, G.; Henry, H.A. Soil nitrogen leaching losses in response to freeze–thaw cycles and pulsed warming in a temperate old field. Soil. Biol. Biochem. 2008, 40, 1947–1953. [Google Scholar]
- Maljanen, M.; Hytönen, J.; Martikainen, P.J. Cold-season nitrous oxide dynamics in a drained boreal peatland differ depending on land-use practice. Can. J. For. Res. 2010, 40, 565–572. [Google Scholar]
- Risk, N.; Snider, D.; Wagner-Riddle, C. Mechanisms leading to enhanced soil nitrous oxide fluxes induced by freeze–thaw cycles. Can. J. Soil Sci. 2013, 93, 401–414. [Google Scholar]
- Wipf, S.; Sommerkorn, M.; Stutter, M.I.; Wubs, E.R.J.; van der Wal, R. Snow cover, freeze-thaw, and the retention of nutrients in an oceanic mountain ecosystem. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Landscape Function and Disturbance in Arctic Tundra; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013.
- Gruhn, P.; Goletti, F.; Yudelman, M. Integrated Nutrient Management, Soil Fertility, and Sustainable Agriculture: Current Issues and Future Challenges; Intl Food Policy Res Inst: London, UK, 2000. [Google Scholar] [CrossRef]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar]
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Ghosh, U.; Chatterjee, A.; Bremer, E. Determining the Moisture and Plant Effect on Nutrient Release, and Plant Nutrient Uptake Using Ion Exchange Resin Membrane. Commun. Soil Sci. Plan. 2018, 49, 782–790. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yang, F. Critical review on soil phosphorus migration and transformation under freezing-thawing cycles and typical regulatory measurements. Sci. Total Environ. 2021, 751, 141614. [Google Scholar]
- Yang, G.; Peng, Y.; Abbott, B.W.; Biasi, C.; Wei, B.; Zhang, D.; Wang, J.; Yu, J.; Li, F.; Wang, G. Phosphorus rather than nitrogen regulates ecosystem carbon dynamics after permafrost thaw. Glob. Change Biol. 2021, 27, 5818–5830. [Google Scholar]
- Street, L.E.; Mielke, N.; Woodin, S.J. Phosphorus Availability Determines the Response of Tundra Ecosystem Carbon Stocks to Nitrogen Enrichment. Ecosystems 2018, 21, 1155–1167. [Google Scholar] [CrossRef]
- Chen, H.; Dai, Z.; Jager, H.I.; Wullschleger, S.D.; Xu, J.; Schadt, C.W. Influences of nitrogen fertilization and climate regime on the above-ground biomass yields of miscanthus and switchgrass: A meta-analysis. Renew. Sustain. Energy Rev. 2019, 108, 303–311. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat. Clim. Change 2015, 5, 465–469. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Zouber, A.; Lasanta, T.; Pueyo, Y. Dryness is accelerating degradation of vulnerable shrublands in semiarid Mediterranean environments. Ecol. Monogr. 2012, 82, 407–428. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, G. Decoupling of nutrient element cycles in soil and plants across an altitude gradient. Sci. Rep. 2016, 6, 34875. [Google Scholar] [CrossRef]
- Michelsen, A.; Graglia, E.; Schmidt, I.K.; Jonasson, S.; Sleep, D.; Quarmby, C. Differential responses of grass and a dwarf shrub to long-term changes in soil microbial biomass C, N and P following factorial addition of NPK fertilizer, fungicide and labile carbon to a heath. New Phytol. 1999, 143, 523–538. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Vimercati, L. Growth of cyanobacterial soil crusts during diurnal freeze-thaw cycles. J. Microbiol. 2019, 57, 243–251. [Google Scholar] [CrossRef]
- Zhou, Y.; Berruti, F.; Greenhalf, C.; Henry, H.A. Combined effects of biochar amendment, leguminous cover crop addition and snow removal on nitrogen leaching losses and nitrogen retention over winter and subsequent yield of a test crop (Eruca sativa L.). Soil Biol. Biochem. 2017, 114, 220–228. [Google Scholar] [CrossRef]
- Zhou, Y.; Berruti, F.; Greenhalf, C.; Tian, X.; Henry, H.A.L. Increased retention of soil nitrogen over winter by biochar application: Implications of biochar pyrolysis temperature for plant nitrogen availability. Agric. Ecosyst. Environ. 2017, 236, 61–68. [Google Scholar] [CrossRef]
- Liu, X.Y.; Koba, K.; Koyama, L.A.; Hobbie, S.E.; Weiss, M.S.; Inagaki, Y.; Shaver, G.R.; Giblin, A.E.; Hobara, S.; Nadelhoffer, K.J.; et al. Nitrate is an important nitrogen source for Arctic tundra plants. Proc. Natl. Acad. Sci. USA 2018, 115, 3398–3403. [Google Scholar] [CrossRef] [PubMed]
- Norby, R.J.; Sloan, V.L.; Iversen, C.M.; Childs, J. Controls on Fine-Scale Spatial and Temporal Variability of Plant-Available Inorganic Nitrogen in a Polygonal Tundra Landscape. Ecosystems 2019, 22, 528–543. [Google Scholar]
- Schimel, J.P.; Chapin, F.S., III. Tundra Plant Uptake of Amino Acid and NH4+ Nitrogen in Situ: Plants Complete Well for Amino Acid N. Ecology 1996, 77, 2142–2147. [Google Scholar] [CrossRef]
- Lipson, D.A.; Schmidt, S.K.; Monson, R.K. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol. Biochem. 2000, 32, 441–448. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Duan, F.; An, X.; Liu, X.; Hao, D.; Gu, R.; Wang, Z.; Chen, F.; Yuan, L. Overexpression of the maize ZmAMT1;1a gene enhances root ammonium uptake efficiency under low ammonium nutrition. Plant Biotechnol. Rep. 2018, 12, 47–56. [Google Scholar] [CrossRef]
- Kielland, K. Amino Acid Absorption by Arctic Plants: Implications for Plant Nutrition and Nitrogen Cycling. Ecology 1994, 75, 2373–2383. [Google Scholar] [CrossRef]
- Andresen, L.C.; Jonasson, S.; Strom, L.; Michelsen, A. Uptake of pulse injected nitrogen by soil microbes and mycorrhizal and non-mycorrhizal plants in a species-diverse subarctic heath ecosystem. Plant Soil 2008, 313, 283–295. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Liao, J.; Xu, X.; Tian, D.; Zhang, R.; Peng, J.; Niu, S. Plant nitrogen uptake preference and drivers in natural ecosystems at the global scale. New Phytol. 2025. [Google Scholar] [CrossRef]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil Biol. Biochem. 2005, 37, 413–423. [Google Scholar]
- Näsholm, T.; Kielland, K.; Ganeteg, U. Uptake of organic nitrogen by plants. New Phytol. 2009, 182, 31–48. [Google Scholar] [PubMed]
- Warren, C.R. Organic N molecules in the soil solution: What is known, what is unknown and the path forwards. Plant Soil 2014, 375, 1–19. [Google Scholar]
- Sohlenkamp, C.; Shelden, M.; Howitt, S.; Udvardi, M. Characterization of Arabidopsis AtAMT2, a novel ammonium transporter in plants. FEBS Lett. 2000, 467, 273–278. [Google Scholar] [PubMed]
- Raab, T.K.; Lipson, D.A.; Monson, R.K. Non-mycorrhizal uptake of amino acids by roots of the alpine sedge Kobresia myosuroides: Implications for the alpine nitrogen cycle. Oecologia 1996, 108, 488–494. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Moilanen, L.; Kielland, K. Preferential use of organic nitrogen for growth by a non-mycorrhizal arctic sedge. Nature 1993, 361, 150–153. [Google Scholar]
- Henry, H.A.L.; Jefferies, R.L. Free amino acid, ammonium and nitrate concentrations in soil solutions of a grazed coastal marsh in relation to plant growth. Plant Cell Environ. 2002, 25, 665–675. [Google Scholar] [CrossRef]
- Andresen, L.C.; Michelsen, A. Off-season uptake of nitrogen in temperate heath vegetation. Oecologia 2005, 144, 585–597. [Google Scholar] [CrossRef]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Starr, E.; Karaoz, U.; Brodie, E.L.; Zhou, J.; Tringe, S.G.; Malmstrom, R.R.; Woyke, T.; Banfield, J.F.; Firestone, M.K. Niche differentiation is spatially and temporally regulated in the rhizosphere. ISME J. 2020, 14, 999–1014. [Google Scholar] [CrossRef]
- Larsen, K.S.; Michelsen, A.; Jonasson, S.; Beier, C.; Grogan, P. Nitrogen Uptake During Fall, Winter and Spring Differs Among Plant Functional Groups in a Subarctic Heath Ecosystem. Ecosystems 2012, 15, 927–939. [Google Scholar] [CrossRef]
- Larsen, K.S.; Ibrom, A.; Jonasson, S.; Michelsen, A.; Beier, C. Significance of cold-season respiration and photosynthesis in a subarctic heath ecosystem in Northern Sweden. Glob. Change Biol. 2007, 13, 1498–1508. [Google Scholar] [CrossRef]
- Starr, G.; Oberbauer, S.F. Photosynthesis of arctic evergreens under snow: Implications for tundra ecosystem carbon balance. Ecology 2003, 84, 1415–1420. [Google Scholar] [CrossRef]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of Soil and Fertilizer Phosphorus Use: Reconciling Changing Concepts of Soil Phosphorus Behaviour with Agronomic Information. Exp. Agric. 2009, 45, 128. [Google Scholar]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Wege, S.; Khan, G.A.; Jung, J.Y.; Vogiatzaki, E.; Pradervand, S.; Aller, I.; Meyer, A.J.; Poirier, Y. The EXS Domain of PHO1 Participates in the Response of Shoots to Phosphate Deficiency via a Root-to-Shoot Signal. Plant Physiol. 2016, 170, 385–400. [Google Scholar] [CrossRef]
- Abrahão, A.; Costa, P.d.B.; Lambers, H.; Andrade, S.A.L.; Sawaya, A.C.H.F.; Ryan, M.H.; Oliveira, R.S.; Dalling, J. Soil types select for plants with matching nutrient-acquisition and-use traits in hyperdiverse and severely nutrient-impoverished campos rupestres and cerrado in Central Brazil. J. Ecol. 2019, 107, 1302–1316. [Google Scholar] [CrossRef]
- He, H.; Eldridge, D.J.; Lambers, H. Mineral Nutrition of Plants in Australia’s Arid Zone. Ecol. Aust. Arid. Zone 2018, 77–102. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Römheld, V.; Marschner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- Lambers, H.; Bishop, J.G.; Hopper, S.D.; Laliberté, E.; Zúñiga-Feest, A. Phosphorus-mobilization ecosystem engineering: The roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Ann. Bot. 2012, 110, 329–348. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, H.Q.; Song, J.; Feng, S.J.; Yang, Z.M. miRNA778 and SUVH6 are involved in phosphate homeostasis in Arabidopsis. Plant Sci. 2015, 238, 273–285. [Google Scholar] [PubMed]
- Dorioz, J.M.; Wang, D.; Poulenard, J.; Trévisan, D. The effect of grass buffer strips on phosphorus dynamics—A critical review and synthesis as a basis for application in agricultural landscapes in France. Agric. Ecosyst. Environ. 2006, 117, 4–21. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Vestberg, M.; Repo, T.; Lehto, T. Arbuscular and ectomycorrhizal root colonisation and plant nutrition in soils exposed to freezing temperatures. Soil Biol. Biochem. 2016, 99, 85–93. [Google Scholar] [CrossRef]
- Weintraub, M.N.; Schimel, J.P. The seasonal dynamics of amino acids and other nutrients in Alaskan Arctic tundra soils. Biogeochemistry 2005, 73, 359–380. [Google Scholar] [CrossRef]
- Maltais-Landry, G.; Frossard, E. Similar phosphorus transfer from cover crop residues and water-soluble mineral fertilizer to soils and a subsequent crop. Plant Soil 2015, 393, 193–205. [Google Scholar] [CrossRef]
- Fan, J.W.; Du, Y.L.; Wang, B.R.; Turner, N.C.; Wang, T.; Abbott, L.K.; Stefanova, K.; Siddique, K.H.M.; Li, F.M. Forage yield, soil water depletion, shoot nitrogen and phosphorus uptake and concentration, of young and old stands of alfalfa in response to nitrogen and phosphorus fertilisation in a semiarid environment. Field Crop Res. 2016, 198, 247–257. [Google Scholar] [CrossRef]
- Gu, Y.-J.; Han, C.-L.; Kong, M.; Shi, X.-Y.; Zdruli, P.; Li, F.-M. Plastic film mulch promotes high alfalfa production with phosphorus-saving and low risk of soil nitrogen loss. Field Crop Res. 2018, 229, 44–54. [Google Scholar] [CrossRef]
- Suen, D.F.; Tsai, Y.H.; Cheng, Y.T.; Radjacommare, R.; Ahirwar, R.N.; Fu, H.; Schmidt, W. The deubiquitinase OTU5 regulates root responses to phosphate starvation. Plant Physiol. 2018, 176, 2441–2455. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar]
| Ecosystem | Climatic Regions | Number of Studies 1 |
|---|---|---|
| Forest | (Sub)Tropical | 6 |
| Forest | Temperate | 14 |
| Forest | Boreal | 2 |
| Grassland | Alpine | 13 |
| Grassland | Temperate | 12 |
| Tundra | Alpine | 2 |
| Tundra | Arctic | 4 |
| Other | Other | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, W.; Li, S. Effects of Freeze–Thaw Cycles on Uptake Preferences of Plants for Nutrient: A Review. Plants 2025, 14, 1122. https://doi.org/10.3390/plants14071122
Liu F, Zhang W, Li S. Effects of Freeze–Thaw Cycles on Uptake Preferences of Plants for Nutrient: A Review. Plants. 2025; 14(7):1122. https://doi.org/10.3390/plants14071122
Chicago/Turabian StyleLiu, Fang, Wei Zhang, and Siqi Li. 2025. "Effects of Freeze–Thaw Cycles on Uptake Preferences of Plants for Nutrient: A Review" Plants 14, no. 7: 1122. https://doi.org/10.3390/plants14071122
APA StyleLiu, F., Zhang, W., & Li, S. (2025). Effects of Freeze–Thaw Cycles on Uptake Preferences of Plants for Nutrient: A Review. Plants, 14(7), 1122. https://doi.org/10.3390/plants14071122






