Comprehensive Genetic and Morphological Analysis of Algerian Carob (Ceratonia siliqua L.) Accessions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Morphological Characterization
2.3. Molecular Analysis
2.3.1. DNA Isolation
2.3.2. SSR Analysis
2.4. Statistical Analyses
3. Results
3.1. Morphological Analysis
3.2. Genetic Analysis of the Carob Collection
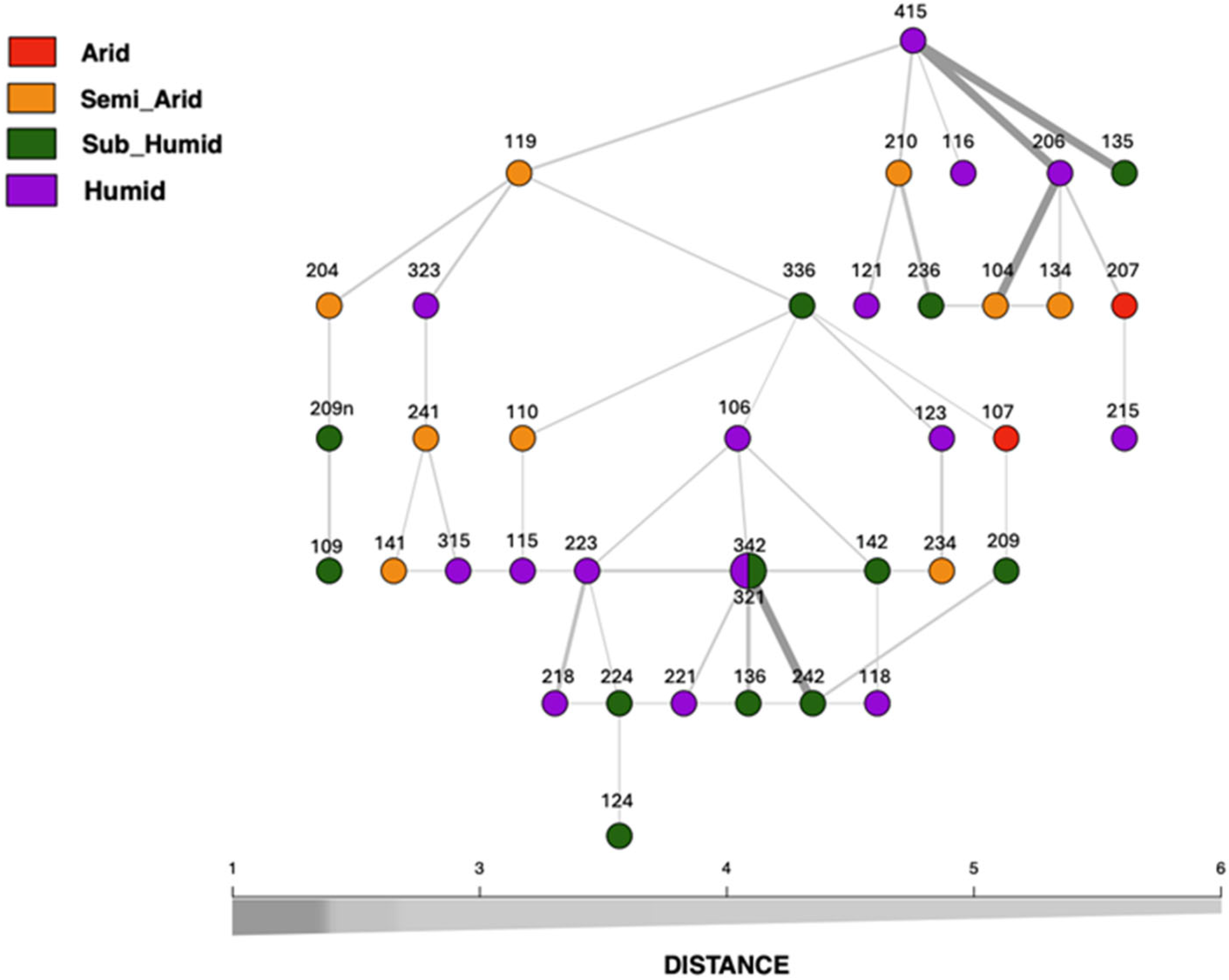
3.3. Clustering Patterns and Genetic Relationships Among Studied Carob Accessions
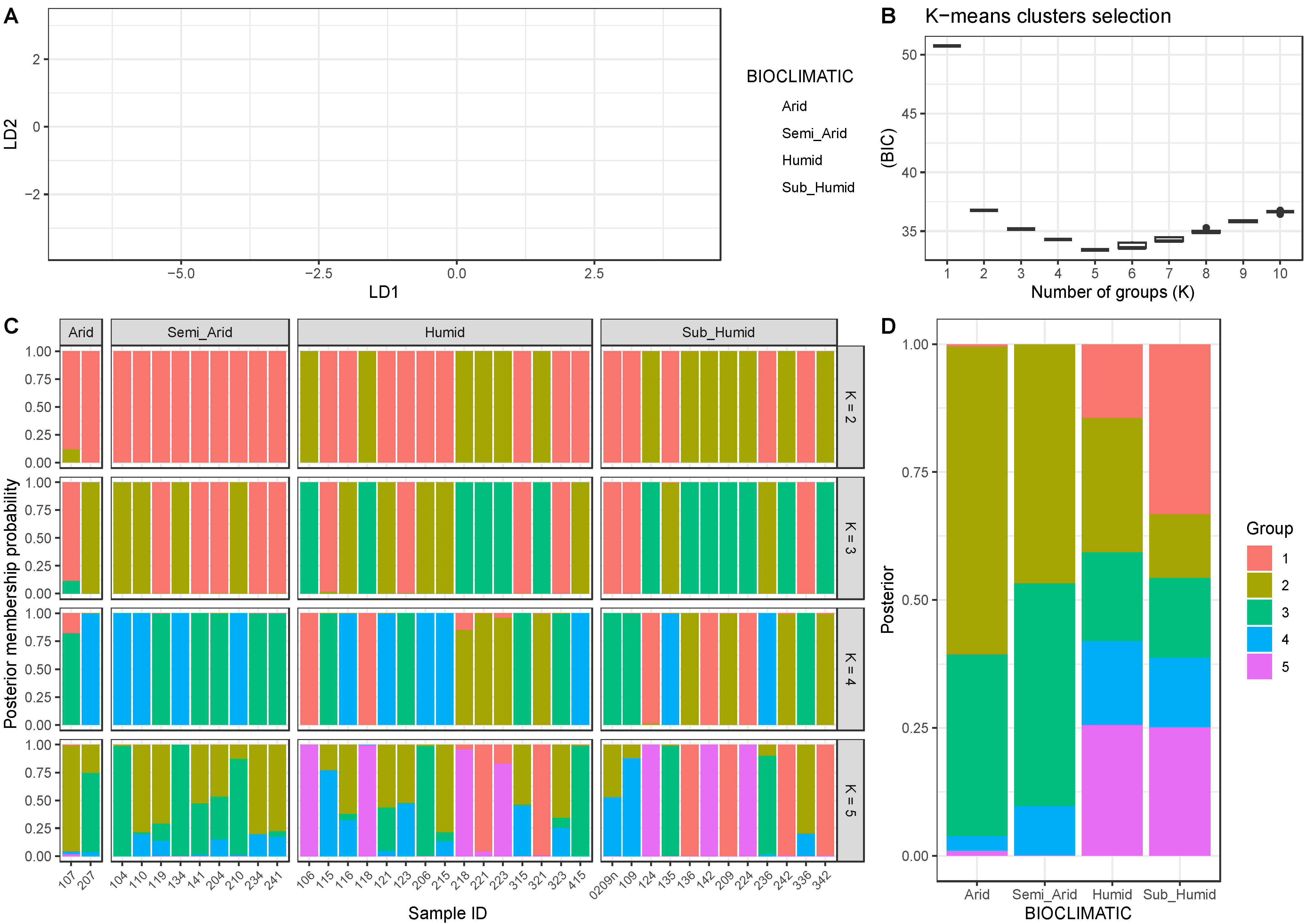
3.4. Genetic Structure Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cegledi, E.; Dobroslavić, E.; Zorić, Z.; Repajić, M.; Elez Garofulić, I. Antioxidant Activity of Carob Tree (Ceratonia siliqua L.) Leaf Extracts Obtained by Advanced Extraction Techniques. Processes 2024, 12, 658. [Google Scholar] [CrossRef]
- Viruel, J.; Le Galliot, N.; Pironon, S.; Nieto Feliner, G.; Suc, J.; Lakhal-Mirleau, F.; Juin, M.; Selva, M.; Bou Dagher Kharrat, M.; Ouahmane, L.; et al. A Strong East–West Mediterranean Divergence Supports a New Phylogeographic History of the Carob Tree (Ceratonia Siliqua, Leguminosae) and Multiple Domestications from Native Populations. J. Biogeogr. 2020, 47, 460–471. [Google Scholar] [CrossRef]
- Mahdad, Y.M.; Gaouar, S.B.S. Origin, Distribution and Domestication of the Carob Tree (Ceratonia siliqua L.). Turk. J. Bot. 2023, 47, 89–96. [Google Scholar] [CrossRef]
- Baumel, A.; Nieto Feliner, G.; Médail, F.; La Malfa, S.; Di Guardo, M.; Bou Dagher Kharrat, M.; Lakhal-Mirleau, F.; Frelon, V.; Ouahmane, L.; Diadema, K.; et al. Genome-wide Footprints in the Carob Tree (Ceratonia Siliqua) Unveil a New Domestication Pattern of a Fruit Tree in the Mediterranean. Mol. Ecol. 2022, 31, 4095–4111. [Google Scholar] [CrossRef]
- El Kahkahi, R.; Zouhair, R.; Ait Chitt, M.; Errakhi, R. Morocco Carob (Ceratonia siliqua L.) Populations: Morphological Variability of Pods and Kernel. Int. J. Pure Appl. Biosci. 2014, 2, 38–47. [Google Scholar]
- Smaili, O.; Chebouti-Meziou, N.; Scollo, F.; La Malfa, S.; Gentile, A.; Distefano, G.; Luca, L.P.; Catalano, C. Evaluation of the Morphological and Physicochemical Diversity of Carob (Ceratonia siliqua, Fabaceae) Germplasm from Algeria. Forests 2024, 15, 1423. [Google Scholar] [CrossRef]
- Thomas, P.A.; Garcia-Martí, X.; Mukassabi, T.A.; Tous, J. International Biological Flora: Ceratonia siliqua. J. Ecol. 2024, 112, 1885–1922. [Google Scholar] [CrossRef]
- Idžojtić, M. WITHDRAWN: Description of Cones, Flowers, Fruits and Seeds of Woody Seed Plants. In Dendrology: Cones, Flowers, Fruits and Seeds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 31–721. [Google Scholar]
- Tous, J.; Romero, A.; Batlle, I. The Carob Tree: Botany, Horticulture, and Genetic Resources. In Horticultural Reviews, 1st ed.; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; Volume 41. [Google Scholar]
- Battle, I.; Tous, J. Promoting the Conservation and Use of Under-Utilised and Neglected Crops 17 Carob Tree Ceratonia Siliqua L.; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1997. [Google Scholar]
- Laaraj, S.; Salmaoui, S.; Addi, M.; El-rhouttais, C.; Tikent, A.; Elbouzidi, A.; Taibi, M.; Hano, C.; Noutfia, Y.; Elfazazi, K. Carob (Ceratonia siliqua L.) Seed Constituents: A Comprehensive Review of Composition, Chemical Profile, and Diverse Applications. J. Food Qual. 2023, 2023, 1–14. [Google Scholar] [CrossRef]
- Patmore, J.V.; Goff, H.D.; Fernandes, S. Cryo-Gelation of Galactomannans in Ice Cream Model Systems. Food Hydrocoll. 2003, 17, 161–169. [Google Scholar] [CrossRef]
- Martins-Loução, M.A.; Correia, P.J.; Romano, A. Carob: A Mediterranean Resource for the Future. Plants 2024, 13, 1188. [Google Scholar] [CrossRef]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-benna, J.; Sebai, H.; Marzouki, L. Chemical Constituents and Pharmacological Actions of Carob Pods and Leaves (Ceratonia siliqua L.) on the Gastrointestinal Tract: A Review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Azab, A. Carob (Ceratonia siliqua): Super Food and Medicine. Literature Update. Eur. Chem. Bull. 2020, 9, 306–312. [Google Scholar] [CrossRef]
- Caruso, M.; La Malfa, S.; Pavlíček, T.; Frutos Tomñs, D.; Gentile, A.; Tribulato, E. Characterisation and Assessment of Genetic Diversity in Cultivated and Wild Carob (Ceratonia siliqua L.) Genotypes Using AFLP Markers. J. Hortic. Sci. Biotechnol. 2008, 83, 177–182. [Google Scholar] [CrossRef]
- Barracosa, P.; Lima, M.B.; Cravador, A. Analysis of Genetic Diversity in Portuguese Ceratonia siliqua L. Cultivars Using RAPD and AFLP Markers. Sci. Hortic. 2008, 118, 189–199. [Google Scholar] [CrossRef]
- La Malfa, S.; Currò, S.; Bugeja Douglas, A.; Brugaletta, M.; Caruso, M.; Gentile, A. Genetic Diversity Revealed by EST-SSR Markers in Carob Tree (Ceratonia siliqua L.). Biochem. Syst. Ecol. 2014, 55, 205–211. [Google Scholar] [CrossRef]
- Di Guardo, M.; Scollo, F.; Ninot, A.; Rovira, M.; Hermoso, J.F.; Distefano, G.; La Malfa, S.; Batlle, I. Genetic Structure Analysis and Selection of a Core Collection for Carob Tree Germplasm Conservation and Management. Tree Genet. Genomes 2019, 15, 41. [Google Scholar] [CrossRef]
- Mahfoud, H.; Ameen, T.; Kazngi, F.; Nasser, S. Morphological and Genetic Variability of Natural Syrian Carob (Ceratonia silique L.). Int. J. Agric. Environ. Sci. 2018, 5, 70–76. [Google Scholar] [CrossRef]
- Afif, M.; Messaoud, C.; Boulila, A.; Chograni, H.; Bejaoui, A.; Rejeb, M.N.; Boussaid, M. Genetic Structure of Tunisian Natural Carob Tree (Ceratonia siliqua L.) Populations Inferred from RAPD Markers. Ann. For. Sci. 2008, 65, 710. [Google Scholar] [CrossRef]
- Gadoum, A.; Chahbar, M.; Adda, A.; Sahnoune, M.; Aid, F. Morphometrical Variation of Carob Tree (Ceratoniasiliqua L.) in Algeria. Genet. Biodivers. J. 2021, 5, 103–115. [Google Scholar] [CrossRef]
- Kocherane, R.; Krouchi, F.; Derridj, A. Genetic Resources of Carob Tree (Ceratonia siliqua L.) in Algeria: Insight from Pod and Seed Morphology. AgroBiologia 2019, 9, 1581–1600. [Google Scholar]
- Mahdad, Y.M.; Mediouni, R.M.; Selka, N.; Viruel, J.; Gaouar, S.B.S. Functional Diversity Based on Morphometric Analysis and Identification of the Algerian Carob Tree (Ceratonia siliqua L.) Cultivars. Genet. Biodivers. J. 2022, 6, 1–20. [Google Scholar] [CrossRef]
- Boublenza, I.; El haitoum, A.; Ghezlaoui, S.; Mahdad, M.; Vasaï, F.; Chemat, F. Algerian Carob (Ceratonia siliqua L.) Populations. Morphological and Chemical Variability of Their Fruits and Seeds. Sci. Hortic. 2019, 256, 108537. [Google Scholar] [CrossRef]
- Zemouri, Z.; Djabeur, A.; Frimehdi, N.; Khelil, O.; Kaid-Harche, M. The Seed Diversity of Carob (Ceratonia siliqua L.) and the Relationship between Seeds Color and Coat Dormancy. Sci. Hortic. 2020, 274, 109679. [Google Scholar] [CrossRef]
- Ayad, R.; Ayad, R.; Bourekoua, H.; Lefahal, M.; Makhloufi, E.H.; Akkal, S.; Medjroubi, K.; Nieto, G. Process Optimization of Phytoantioxidant and Photoprotective Compounds from Carob Pods (Ceratonia siliqua L.) Using Ultrasonic Assisted Extraction Method. Molecules 2022, 27, 8802. [Google Scholar] [CrossRef]
- Ayad, R.; Ayad, R.; Zineddine, L.; El Houda Berghida, N. Algerian Wild Green Carob (Ceratonia siliqua L.): Physicochemical Characteristics and Antioxidant Potency. Bull. Chem. Soc. Ethiop. 2023, 38, 187–198. [Google Scholar] [CrossRef]
- Serradj, A.M.K.; Rais, S.; Adnane, Y.; Mahdad, M.Y.; Mediouni, R.; Gaouar, S.B.S. Morpho-Geometric Identification of Leaflets from Male, Female and Hermaphrodite Populations of the Carob Tree (Ceratonia siliqua L.) in Algeria. Genet. Biodivers. J. 2024, 8, 57–75. [Google Scholar]
- Toumi, S.; Acem, K.; Abdelhamid, D.; Khedim, K.; Lagraa, I.; Loumani, Z. Morphobiometric Characterisation of Carob Tree Pods Cultivated in Algeria and Evaluation of Physicochemical, Nutritional, and Sensory Properties of Their Powders. Proc. Latv. Acad. Sci. Sect. B. Nat. Exact Appl. Sci. 2024, 78, 153–163. [Google Scholar] [CrossRef]
- Wilkie, A.D.P.P.; Forrest, L.L. The Collection and Storage of Plant Material for DNA Extraction: The Teabag Method. Gard. Bull. Singap. 2013, 65, 231–234. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- The R Project for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Vaughan, D. Dplyr: A Grammar of Data Manipulation; R Package, Version 1.1.4; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 18. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R Package for Genetic Analysis of Populations with Clonal, Partially Clonal, and/or Sexual Reproduction. PeerJ 2014, 2014, e281. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program cervus Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. Ape 5.0: An Environment for Modern Phylogenetics and Evolutionary Analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R Package for the Multivariate Analysis of Genetic Markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 10 January 2025).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Viruel, J.; Haguenauer, A.; Juin, M.; Mirleau, F.; Bouteiller, D.; Boudagher-Kharrat, M.; Ouahmane, L.; La Malfa, S.; Médail, F.; Sanguin, H.; et al. Advances in Genotyping Microsatellite Markers through Sequencing and Consequences of Scoring Methods for Ceratonia siliqua (Leguminosae). Appl. Plant Sci. 2018, 6, e01201. [Google Scholar] [CrossRef]
- Särkinen, T.; George, M. Predicting Plastid Marker Variation: Can Complete Plastid Genomes from Closely Related Species Help? PLoS ONE 2013, 8, e82266. [Google Scholar] [CrossRef]
- Li, B.; Lin, F.; Huang, P.; Guo, W.; Zheng, Y. Development of Nuclear SSR and Chloroplast Genome Markers in Diverse Liriodendron Chinense Germplasm Based on Low-Coverage Whole Genome Sequencing. Biol. Res. 2020, 53, 21. [Google Scholar] [CrossRef]
- Jayaswall, K.; Sharma, H.; Jayaswal, D.; Sagar, R.; Bhandawat, A.; Kumar, A.; Sharma, I.; Chaturvedi, P.; Manjunathagowda, D.C.; Kumar, D.; et al. Development of Chloroplast Derived SSR Markers for Genus Allium and Their Characterization in the Allies for Genetic Improvement of Alliums. S. Afr. J. Bot. 2023, 162, 304–313. [Google Scholar] [CrossRef]
- Sexton, J.P.; Hangartner, S.B.; Hoffmann, A.A. Genetic isolation by environment or distance: Which pattern of gene flow is most common? Evolution 2014, 68, 1–15. [Google Scholar] [CrossRef]
- Hamasha, H.R.; Schmidt-Lebuhn, A.N.; Durka, W.; Schleuning, M.; Hensen, I. Bioclimatic Regions Influence Genetic Structure of Four Jordanian Stipa Species. Plant Biol. 2013, 15, 882–891. [Google Scholar] [CrossRef]
- Zuriaga, E.; Blanca, J.M.; Cordero, L.; Sifres, A.; Blas-Cerdán, W.G.; Morales, R.; Nuez, F. Genetic and Bioclimatic Variation in Solanum Pimpinellifolium. Genet. Resour. Crop Evol. 2009, 56, 39–51. [Google Scholar] [CrossRef]
- Torello Marinoni, D.; Akkak, A.; Beltramo, C.; Guaraldo, P.; Boccacci, P.; Bounous, G.; Ferrara, A.M.; Ebone, A.; Viotto, E.; Botta, R. Genetic and Morphological Characterization of Chestnut (Castanea sativa Mill.) Germplasm in Piedmont (North-Western Italy). Tree Genet. Genomes 2013, 9, 1017–1030. [Google Scholar] [CrossRef]
- Sorkheh, K.; Khaleghi, E. Molecular Characterization of Genetic Variability and Structure of Olive(Olea europaea L.) Germplasm Collection Analyzed by Agromorphologicaltraits and Microsatellite Markers. Turk. J. Agric. For. 2016, 40, 583–596. [Google Scholar] [CrossRef]
- Marra, F.P.; Caruso, T.; Costa, F.; Di Vaio, C.; Mafrica, R.; Marchese, A. Genetic Relationships, Structure and Parentage Simulation among the Olive Tree (Olea europaea L. Subsp. europaea) Cultivated in Southern Italy Revealed by SSR Markers. Tree Genet. Genomes 2013, 9, 961–973. [Google Scholar] [CrossRef]
- Rajpurohit, D.; Jhang, T. In Situ and Ex Situ Conservation of Plant Genetic Resources and Traditional Knowledge. In Plant Genetic Resources and Traditional Knowledge for Food Security; Springer: Singapore, 2015; pp. 137–162. [Google Scholar]


| Sample ID | Regions | Latitude | Longitude | Altitude (m) | Bioclimate | Type |
|---|---|---|---|---|---|---|
| 116 | Algiers | 36°43′17” N | 3°09′01” E | 45 | Humid | Cultivated |
| 123 | Annaba | 36°55′18′′ N | 7°44′00′′ E | 241 | Humid | Wild |
| 223 | Annaba | 36°55′20′′ N | 7°43′54′′ E | 287 | Humid | Wild |
| 323 | Annaba | 36°55′23′′ N | 7°43′53′′ E | 294 | Humid | Wild |
| 106 | Bejaia | 36°34′59′′ N | 5°25′59′′ E | 289 | Humid | Wild |
| 206 | Bejaia | 36°35′07′′ N | 5°25′58′′ E | 340 | Humid | Wild |
| 107 | Biskra | 34°50′37′′ N | 5°44′04′′ E | 107 | Arid | Cultivated |
| 207 | Biskra | 34°50′36′′ N | 5°44′05′′ E | 108 | Arid | Cultivated |
| 109 | Blida | 36°31′54′′ N | 2°55′43′′ E | 112 | Sub-Humid | Cultivated |
| 209 | Blida | 36°31′54′′ N | 2°55′44′′ E | 113 | Sub-Humid | Cultivated |
| 109n | Blida | 36°31′54′′ N | 2°55′45′′ E | 113 | Sub-Humid | Cultivated |
| 209n | Blida | 36°31′52′′ N | 2°55′42′′ E | 113 | Sub-Humid | Cultivated |
| 134 | Bordj Bou Arréridj | 35°55′35′′ N | 4°44′59′′ E | 995 | Semi-Arid | Cultivated |
| 234 | Bordj Bou Arréridj | 35°55′27′′ N | 4°45′5′′ E | 982 | Semi-Arid | Wild |
| 110 | Bouïra | 36°35′28′′ N | 3°34′30′′ E | 105 | Semi-Arid | Wild |
| 210 | Bouïra | 36°35′43′′ N | 3°34′34′′ E | 108 | Semi-Arid | Wild |
| 135 | Boumerdès | 36°49′36′′ N | 3°50′21′′ E | 268 | Sub-Humid | Cultivated |
| 136 | El Taref | 36°39′21′′ N | 7°47′36′′ E | 35 | Sub-Humid | Cultivated |
| 336 | El Taref | 36°39′22′′ N | 7°47′45′′ E | 33 | Sub-Humid | Cultivated |
| 236 | El Taref * | 36°39′22′′ N | 7°47′45′′ E | 33 | Sub-Humid | Cultivated |
| 124 | Guelma | 36°18′28′′ N | 7°35′24′′ E | 812 | Sub-Humid | Wild |
| 224 | Guelma | 36°18′23′′ N | 7°35′14′′ E | 846 | Sub-Humid | Wild |
| 118 | Jijel | 36°39′49′′ N | 6°16′35′′ E | 62 | Humid | Wild |
| 218 | Jijel | 36°39′48′′ N | 6°16′41′′ E | 86 | Humid | Wild |
| 104 | Oum El Bouaghi | 35°52′02′′ N | 7°08′18′′ E | 901 | Semi-Arid | Cultivated |
| 204 | Oum El Bouaghi | 35°52′04′′ N | 7°08′17′′ E | 903 | Semi-Arid | Cultivated |
| 119 | Sétif | 36°32′31′′ N | 5°04′49′′ E | 931 | Semi-Arid | Wild |
| 121 | Skikda | 36°43′22′′ N | 7°16′29′′ E | 45 | Humid | Cultivated |
| 221 | Skikda | 36°43′22′′ N | 7°16′29′′ E | 45 | Humid | Cultivated |
| 321 | Skikda | 36°43′22′′ N | 7°16′29′′ E | 45 | Humid | Cultivated |
| 141 | Souk Ahras | 36°23′31″ N | 8°21′41″ E | 490 | Semi-Arid | Wild |
| 241 | Souk Ahras | 36°24′28” N | 8°21′55” E | 261 | Semi-Arid | Wild |
| 142 | Tipaza | 36°31′12′′ N | 2°29′13′′ E | 73 | Sub-Humid | Cultivated |
| 242 | Tipaza | 36°31′12′′ N | 2°29′13′′ E | 73 | Sub-Humid | Cultivated |
| 342 | Tipaza | 36°31′12′′ N | 2°29′12′′ E | 73 | Sub-Humid | Cultivated |
| 115 | Tizi Ouzou | 36°45′35′′ N | 4°22′12′′ E | 339 | Humid | Wild |
| 215 | Tizi Ouzou | 36°45′33′′ N | 4°22′15′′ E | 347 | Humid | Wild |
| 315 | Tizi Ouzou | 36°45′33′′ N | 4°22′15′′ E | 347 | Humid | Wild |
| 415 | Tizi Ouzou | 36°53′54′′ N | 4°17′51′′ E | 86 | Humid | Wild |
| Nuclear SSR | Alleles | Genotypes | No. of Obs. | He | Ho | PIC | Simpson Index (D) |
|---|---|---|---|---|---|---|---|
| CTTT7 | 2 | 2 | 38 | 0.19 | 0.21 | 0.17 | 0.2 |
| AT15 | 8 | 11 | 35 | 0.63 | 0.51 | 0.59 | 0.62 |
| 2GA12 | 4 | 7 | 38 | 0.45 | 0.39 | 0.40 | 0.46 |
| AT9 | 5 | 9 | 38 | 0.61 | 0.24 | 0.55 | 0.62 |
| GCT6 | 3 | 5 | 37 | 0.36 | 0.38 | 0.31 | 0.37 |
| TA5TG6 | 3 | 4 | 38 | 0.37 | 0.37 | 0.33 | 0.36 |
| TA7 | 2 | 4 | 38 | 0.43 | 0.45 | 0.33 | 0.42 |
| TTA7 | 7 | 9 | 38 | 0.60 | 0.82 | 0.52 | 0.59 |
| Chloroplastic SSR | Alleles | No. of Obs. | Simpson Index (D) |
|---|---|---|---|
| ccSA-ndhd | 3 | 36 | 0.85 |
| rpl32-TrnL | 2 | 38 | 0.85 |
| PsbD-Trn | 3 | 38 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smaili, O.; Luca, L.P.; Scollo, F.; Chebouti-Meziou, N.; Catalano, C.; Di Guardo, M.; Distefano, G.; Nicolosi, E.; Gentile, A.; La Malfa, S. Comprehensive Genetic and Morphological Analysis of Algerian Carob (Ceratonia siliqua L.) Accessions. Plants 2025, 14, 990. https://doi.org/10.3390/plants14070990
Smaili O, Luca LP, Scollo F, Chebouti-Meziou N, Catalano C, Di Guardo M, Distefano G, Nicolosi E, Gentile A, La Malfa S. Comprehensive Genetic and Morphological Analysis of Algerian Carob (Ceratonia siliqua L.) Accessions. Plants. 2025; 14(7):990. https://doi.org/10.3390/plants14070990
Chicago/Turabian StyleSmaili, Oussama, Leonardo Paul Luca, Francesco Scollo, Nadjiba Chebouti-Meziou, Chiara Catalano, Mario Di Guardo, Gaetano Distefano, Elisabetta Nicolosi, Alessandra Gentile, and Stefano La Malfa. 2025. "Comprehensive Genetic and Morphological Analysis of Algerian Carob (Ceratonia siliqua L.) Accessions" Plants 14, no. 7: 990. https://doi.org/10.3390/plants14070990
APA StyleSmaili, O., Luca, L. P., Scollo, F., Chebouti-Meziou, N., Catalano, C., Di Guardo, M., Distefano, G., Nicolosi, E., Gentile, A., & La Malfa, S. (2025). Comprehensive Genetic and Morphological Analysis of Algerian Carob (Ceratonia siliqua L.) Accessions. Plants, 14(7), 990. https://doi.org/10.3390/plants14070990










