Antioxidant and In Vitro Hepatoprotective Activities of a Polyphenol-Rich Fraction from the Peel of Citrus lumia Risso (Rutaceae)
Abstract
:1. Introduction
2. Materials and Methods
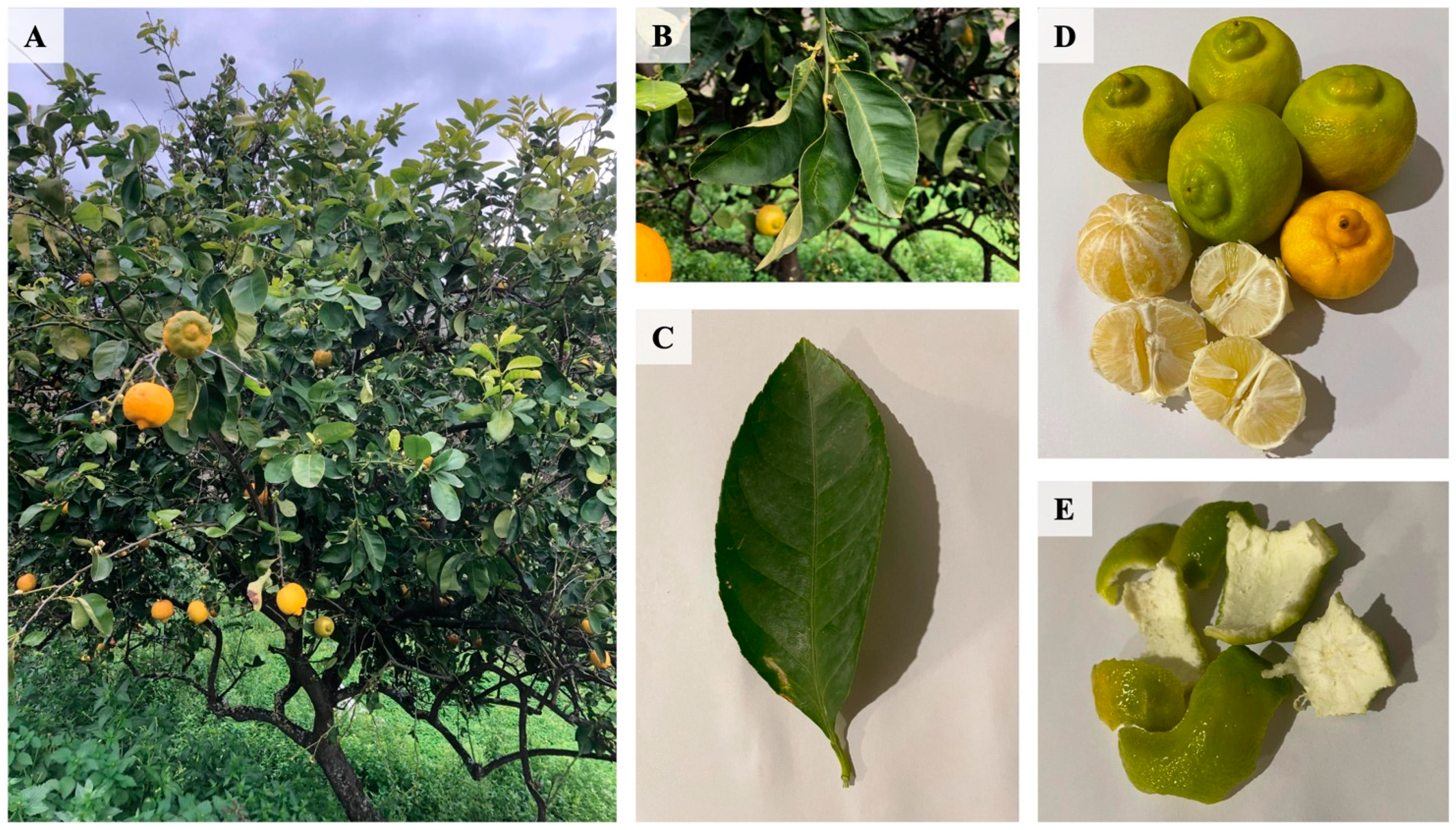
2.1. Plant Material
2.2. Extraction Procedure
2.3. Preparation of Citrus lumia Extracts Concentrated in Polyphenols
2.4. High Performance Liquid Chromatography (HPLC) Analysis
2.5. Total Flavonoid Content
2.6. Evaluation of Radicals Scavenging by Electron Paramagnetic Spectroscopy (EPR)
2.7. 2D and 3D Cell Culture
2.8. Treatments with C. lumia Polyphenols-Rich Extract
2.9. Cell Viability Assay
2.10. Quantification of Intracellular Neutral Lipid Content
2.11. Statistical Analysis
3. Results
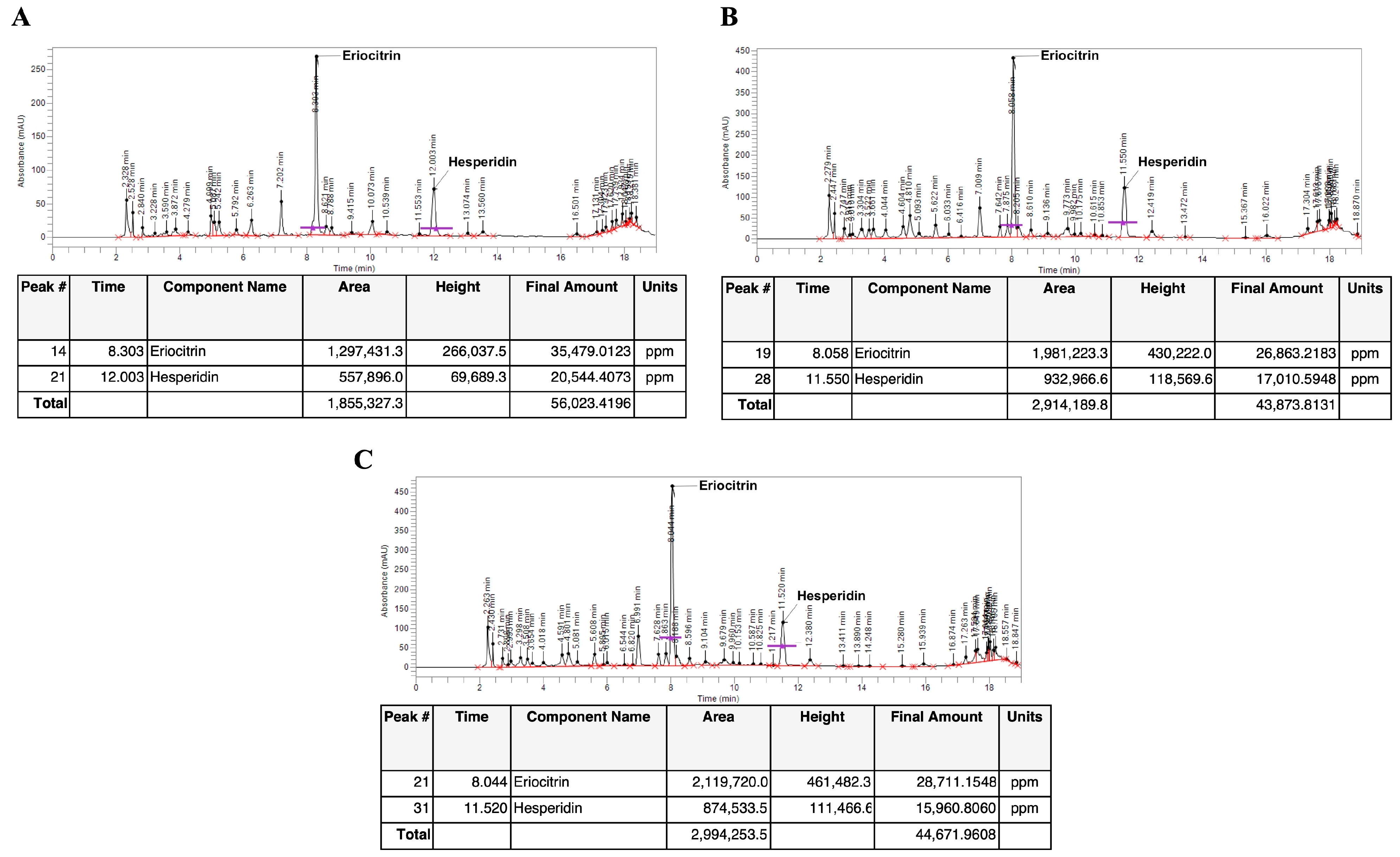
3.1. Identification and Quantification of Eriocitrin and Hesperidin in C. lumia Risso Peel Extracts
3.2. Analysis of Citrus lumia Risso Polyphenols-Rich Extracts
3.3. Characterization of Flavonoid Content in Citrus lumia Risso Peel Extracts
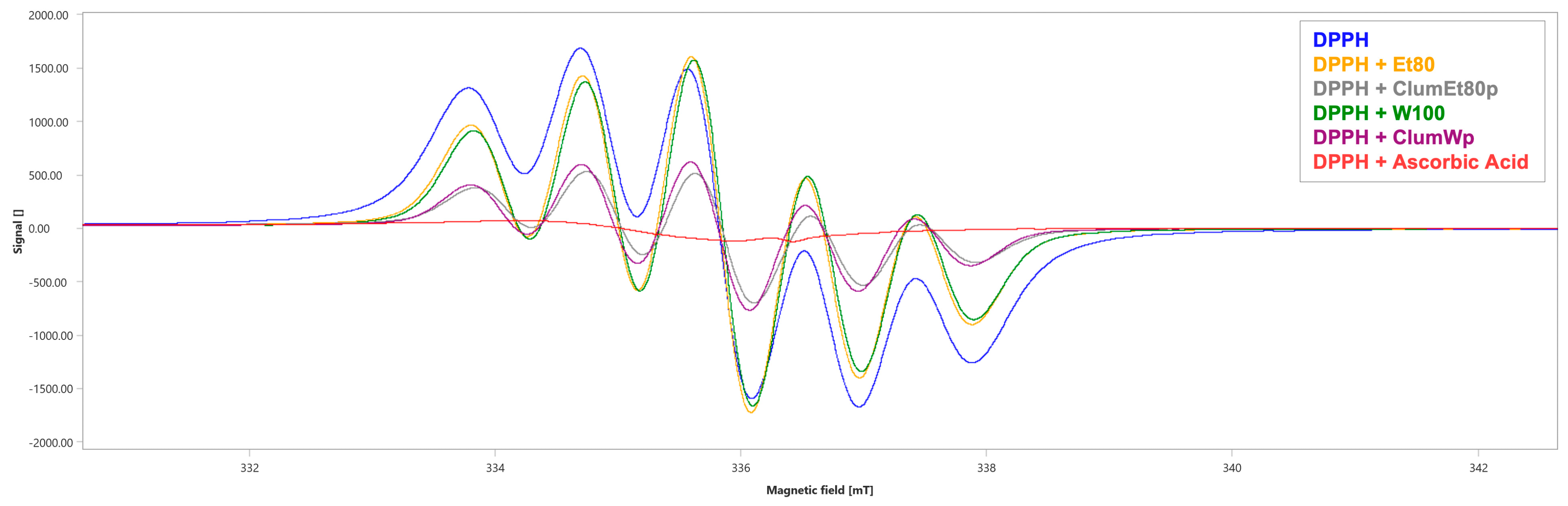
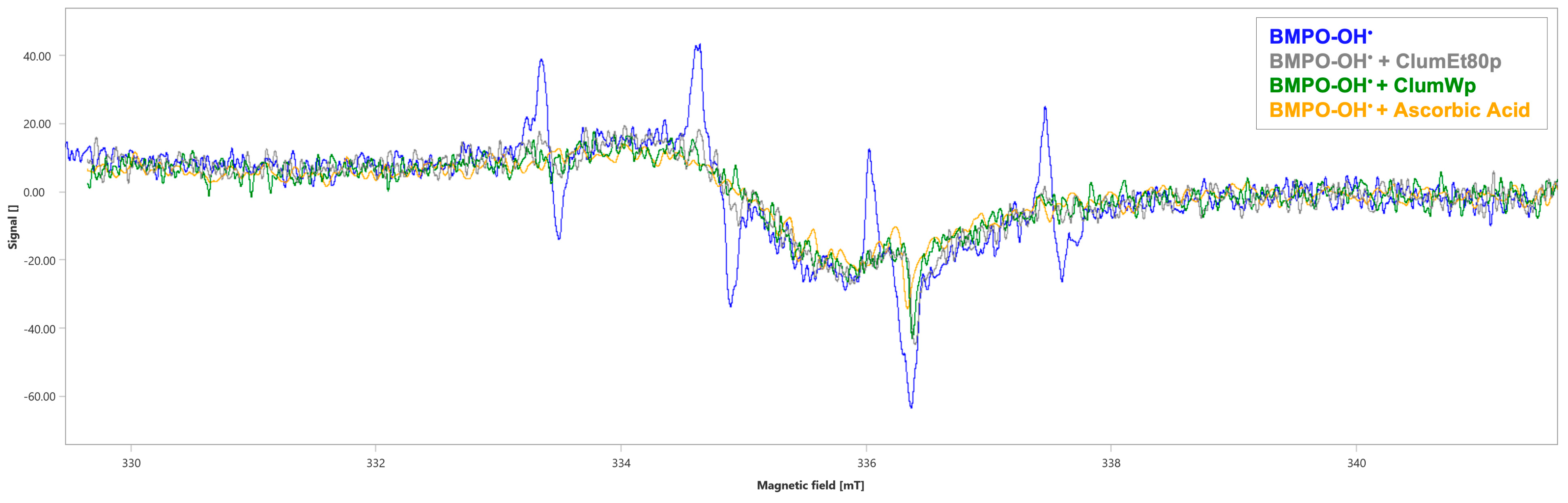
3.4. Evaluation of Radicals Scavenging Activity of Citrus lumia Risso Peel Extracts Through Electron Paramagnetic Spectroscopy (EPR)
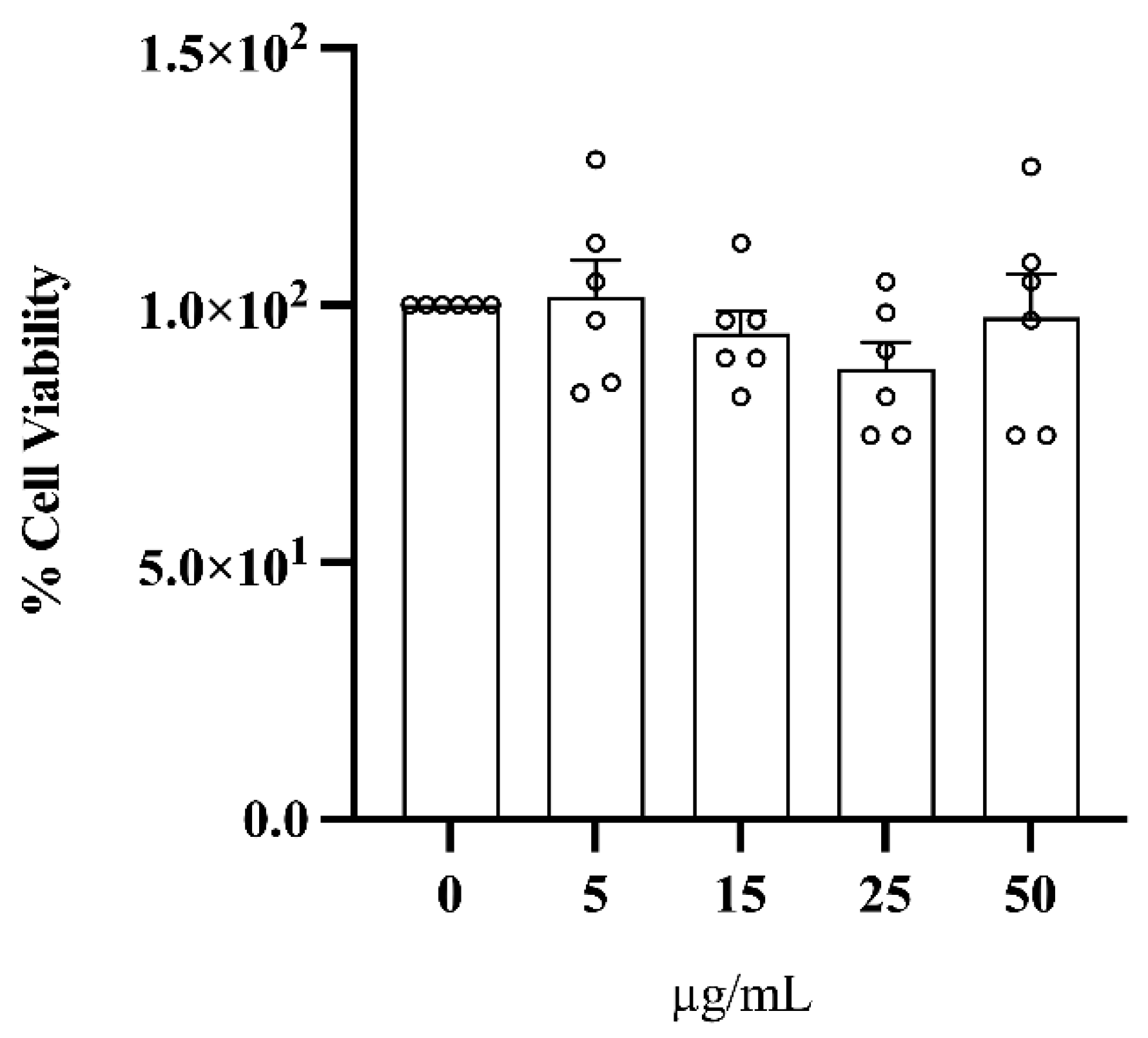
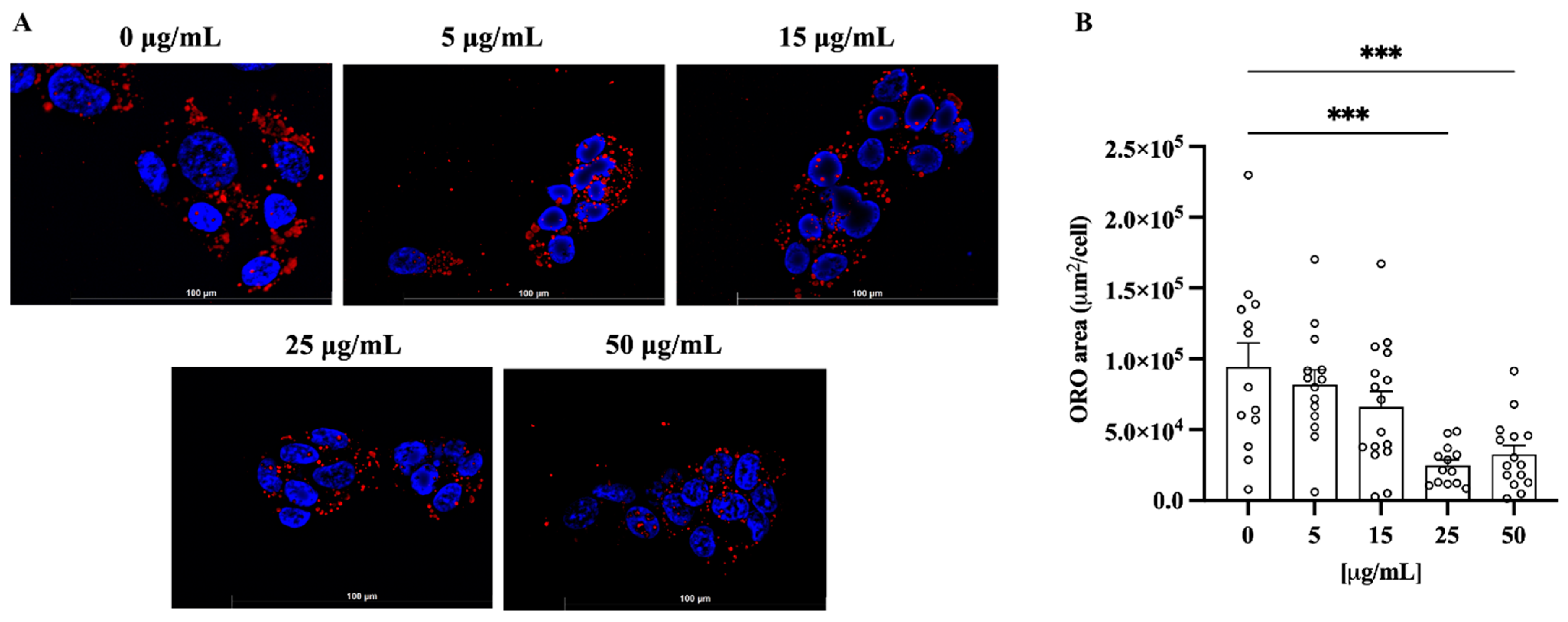
3.5. Treatment with C. lumia Polyphenols-Rich Extract Does Not Affect Cell Viability and Decreases Intracellular Lipids in HepG2 Cells
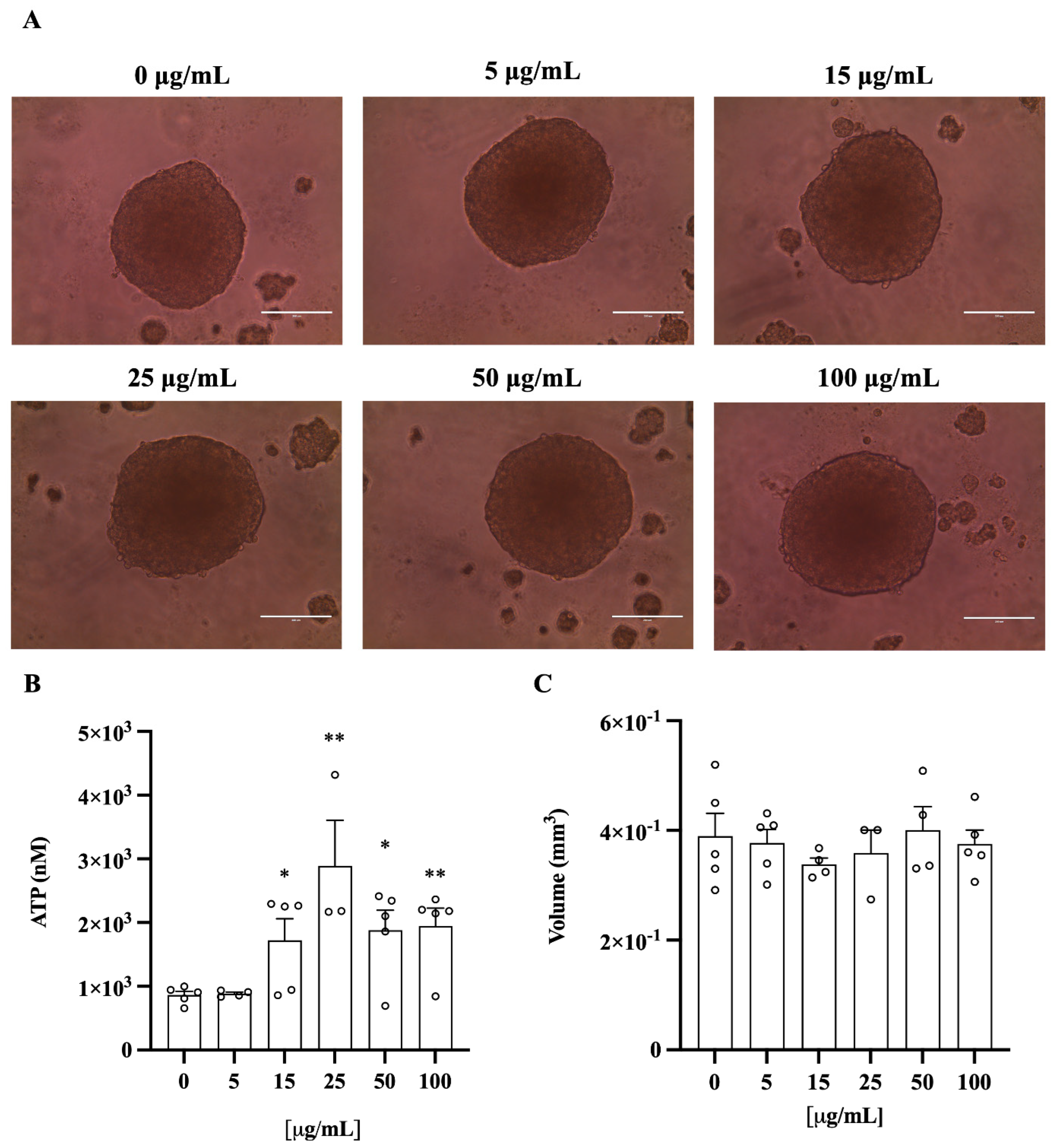
3.6. C. lumia Polyphenols-Rich Extract Decreases Intracellular Lipids in 3D HEPG2/LX2 Spheroids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United States Department of Agriculture Foreign Agricultural Service. Citrus: World Markets and Trade; Foreign Agricultural Service/USDA: Washington, DC, USA, January 2025. [Google Scholar]
- Kalita, B.; Roy, A.; Annamalai, A.; Ptv, L. A molecular perspective on the taxonomy and journey of Citrus domestication. Perspect. Plant Ecol. Evol. Syst. 2021, 53, 125644. [Google Scholar] [CrossRef]
- Raimondo, F.M.; Mazzola, P. Le lumie di Sicilia: Note storiche e botaniche. Quad. Bot. Amb. Appl. 2017, 26, 43–50. [Google Scholar]
- Risso, A. Citrus lumia, Citre lumie. In Histoire Naturelle des Principales Productions de l’Europe Méridionale et Particulièrement de Celles des Environs de Nice et des Alpes Maritimes; Chez F.-G. Levrault: Paris, France, 1826; pp. 414–418. [Google Scholar]
- Available online: https://powo.science.kew.org/taxon/771964-1?_gl=1*v18ktp*_ga*Mjc1MjMyMDYwLjE3NDI2NDYyNzU.*_ga_ZVV2HHW7P6*MTc0Mzk0NTA5NS40LjEuMTc0Mzk0NTQyMC4wLjAuMA..#synonyms (accessed on 1 April 2025).
- WFO (2025): Citrus lumia Risso. Available online: http://www.worldfloraonline.org/taxon/wfo-0000608115 (accessed on 1 April 2025).
- Samanta, S.; Banerjee, J.; Ahmed, R.; Dash, S.K. Potential Benefits of Bioactive Functional Components of Citrus Fruits for Health Promotion and Disease Prevention. In Recent Advances in Citrus Fruits; Singh Purewal, S., Punia Bangar, S., Kaur, P., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Cautela, D.; De Sio, F.; Balestrieri, M.L.; Casale, R.; Laratta, B.; Castaldo, D.; Pastore, A.; Servillo, L.; D’Onofrio, N. Amino acids, betaines and related ammonium compounds in Neapolitan limmo, a Mediterranean sweet lime, also known as lemoncetta Locrese. J. Sci. Food Agric. 2021, 101, 981–988. [Google Scholar] [CrossRef]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Bashir, M.; Nisar, M.F.; Wan, C.C. Eriocitrin: A review of pharmacological effects. Biomed. Pharmacother.=Biomed. Pharmacother. 2022, 154, 113563. [Google Scholar] [CrossRef]
- Sivaslioglu, A.; Goktas, Z. A comprehensive review on the impact of hesperidin and its aglycone hesperetin on metabolic dysfunction-associated steatotic liver disease and other liver disorders. Nutr. Res. Rev. 2024, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Sookoian, S. From NAFLD to MASLD: Updated naming and diagnosis criteria for fatty liver disease. J. Lipid Res. 2024, 65, 100485. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Tumilaar, S.G.; Hardianto, A.; Dohi, H.; Kurnia, D. A Comprehensive Review of Free Radicals, Oxidative Stress, and Antioxidants: Overview, Clinical Applications, Global Perspectives, Future Directions, and Mechanisms of Antioxidant Activity of Flavonoid Compounds. J. Chem. 2024, 2024, 5594386. [Google Scholar] [CrossRef]
- Morant-Ferrando, B.; Jimenez-Blasco, D.; Alonso-Batan, P.; Agulla, J.; Lapresa, R.; Garcia-Rodriguez, D.; Yunta-Sanchez, S.; Lopez-Fabuel, I.; Fernandez, E.; Carmeliet, P.; et al. Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition. Nat. Metab. 2023, 5, 1290–1302. [Google Scholar] [CrossRef]
- Tirosh, A.; Tuncman, G.; Calay, E.S.; Rathaus, M.; Ron, I.; Tirosh, A.; Yalcin, A.; Lee, Y.G.; Livne, R.; Ron, S.; et al. Intercellular Transmission of Hepatic ER Stress in Obesity Disrupts Systemic Metabolism. Cell Metab. 2021, 33, 319–333.e6. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Macrì, R.; Cardamone, A.; Tucci, L.; Serra, M.; Lupia, C.; Maurotti, S.; Mare, R.; Nucera, S.; Guarnieri, L.; et al. Salvia rosmarinus Spenn. (Lamiaceae) Hydroalcoholic Extract: Phytochemical Analysis, Antioxidant Activity and In Vitro Evaluation of Fatty Acid Accumulation. Plants 2023, 12, 3306. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Pujia, R.; Maurotti, S.; Greco, S.; Cardamone, A.; Coppoletta, A.R.; Bonacci, S.; Procopio, A.; Pujia, A. Assessment of Mediterranean Citrus Peel Flavonoids and Their Antioxidant Capacity Using an Innovative UV-Vis Spectrophotometric Approach. Plants 2023, 12, 4046. [Google Scholar] [CrossRef] [PubMed]
- Settino, M.; Maurotti, S.; Tirinato, L.; Greco, S.; Coppoletta, A.R.; Cardamone, A.; Musolino, V.; Montalcini, T.; Pujia, A.; Mare, R. Zibibbo Grape Seeds’ Polyphenolic Profile: Effects on Bone Turnover and Metabolism. Pharmaceuticals 2024, 17, 1418. [Google Scholar] [CrossRef]
- Cardamone, A.; Coppoletta, A.R.; Macrì, R.; Nucera, S.; Ruga, S.; Scarano, F.; Mollace, R.; Mollace, A.; Maurotti, S.; Micotti, E.; et al. Targeting leptin/CCL3-CCL4 axes in NAFLD/MAFLD: A novel role for BPF in counteracting thalamic inflammation and white matter degeneration. Pharmacol. Res. 2024, 209, 107417. [Google Scholar] [CrossRef]
- Pennisi, G.; Maurotti, S.; Ciociola, E.; Jamialahmadi, O.; Bertolazzi, G.; Mirarchi, A.; Bergh, P.O.; Scionti, F.; Mancina, R.M.; Spagnuolo, R.; et al. ANGPTL3Downregulation Increases Intracellular Lipids by Reducing Energy Utilization. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1086–1097. [Google Scholar] [CrossRef]
- Kumari, S.; Bhowal, R.; Suprasanna, P. Sustainable Approaches for Biodiversity and Bioprospecting of Citrus. Sustainability 2023, 15, 7731. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Durmus, N.; Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Recovery, Bioactivity, and Utilization of Bioactive Phenolic Compounds in CitrusPeel. Food Sci. Nutr. 2024, 12, 9974–9997. [Google Scholar] [CrossRef]
- Langgut, D. The Citrus Route Revealed: From Southeast Asia into the Mediterranean. HortSci. Horts 2017, 52, 814–822. [Google Scholar] [CrossRef]
- Sawalha, S.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Quantification of main phenolic compounds in sweet and bitter orange peel using CE–MS/MS. Food Chem. 2009, 116, 567–574. [Google Scholar] [CrossRef]
- Cautela, D.; Balestrieri, M.L.; Savini, S.; Sannino, A.; Ferrari, G.; Servillo, L.; De Masi, L.; Pastore, A.; Castaldo, D. The Ancient Neapolitan Sweet Lime and the Calabrian Lemoncetta Locrese Belong to the Same Citrus Species. Molecules 2019, 25, 113. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.M.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 119, 407–416. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germanò, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; Di Gristina, E.; Mastracci, L.; Grillo, F.; Cornara, L.; Trombetta, D. Pharmacognostic approach to evaluate the micromorphological, phytochemical and biological features of Citrus lumia seeds. Food Chem. 2022, 375, 131855. [Google Scholar] [CrossRef] [PubMed]
- Musolino, V.; Gliozzi, M.; Scarano, F.; Bosco, F.; Scicchitano, M.; Nucera, S.; Carresi, C.; Ruga, S.; Zito, M.C.; Maiuolo, J.; et al. Bergamot Polyphenols Improve Dyslipidemia and Pathophysiological Features in a Mouse Model of Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2020, 10, 2565. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Musolino, V.; Macrì, R.; Cardamone, A.; Serra, M.; Coppoletta, A.R.; Tucci, L.; Maiuolo, J.; Lupia, C.; Scarano, F.; Carresi, C.; et al. Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants 2022, 12, 27. [Google Scholar] [CrossRef]
- Mateus, A.R.S.; Marino-Cortegoso, S.; Barros, S.C.; Sendón, R.; Barbosa, L.; Pena, A.; Sanches-Silva, A. Citrus by-products: A dual assessment of antioxidant properties and food contaminants towards circular economy. Innov. Food Sci. Emerg. Technol. 2024, 95, 103737. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Fang, Z.; Lu, S. Hesperetin acts as a potent xanthine oxidase inhibitor: New evidence from its reactive oxygen suppression and enzyme binding. Int. J. Biol. Macromol. 2025, 306, 141429. [Google Scholar] [CrossRef] [PubMed]
- Eggler, A.L.; Gay, K.A.; Mesecar, A.D. Molecular mechanisms of natural products in chemoprevention: Induction of cytoprotective enzymes by Nrf2. Mol. Nutr. Food Res. 2008, 52 (Suppl. S1), S84–S94. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Li, N. Research Progress on Natural Products Alleviating Liver Inflammation and Fibrosis via NF-κB Pathway. Chem. Biodivers. 2025, e202402248. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef]
- Kadenbach, B. Complex IV—The regulatory center of mitochondrial oxidative phosphorylation. Mitochondrion 2021, 58, 296–302. [Google Scholar] [CrossRef]
- Popov, L.D. Mitochondrial biogenesis: An update. J. Cell. Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef]
- Fukuchi, Y.; Hiramitsu, M.; Okada, M.; Hayashi, S.; Nabeno, Y.; Osawa, T.; Naito, M. Lemon Polyphenols Suppress Diet-induced Obesity by Up-Regulation of mRNA Levels of the Enzymes Involved in beta-Oxidation in Mouse White Adipose Tissue. J. Clin. Biochem. Nutr. 2008, 43, 201–209. [Google Scholar] [CrossRef]
- Mirarchi, A.; Mare, R.; Musolino, V.; Nucera, S.; Mollace, V.; Pujia, A.; Montalcini, T.; Romeo, S.; Maurotti, S. Bergamot Polyphenol Extract Reduces Hepatocyte Neutral Fat by Increasing Beta-Oxidation. Nutrients 2022, 14, 3434. [Google Scholar] [CrossRef] [PubMed]
- McLeod, M.; Chang, M.C.; Rushin, A.; Ragavan, M.; Mahar, R.; Sharma, G.; Badar, A.; Giacalone, A.; Glanz, M.E.; Malut, V.R.; et al. Detecting altered hepatic lipid oxidation by MRI in an animal model of MASLD. Cell Rep. Med. 2024, 5, 101714. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Della Pepa, G.; Sabatini, S.; Vidal Puig, A.; Gastaldelli, A. Lipid metabolism in MASLD and MASH: From mechanism to the clinic. JHEP Rep. Innov. Hepatol. 2024, 6, 101185. [Google Scholar] [CrossRef] [PubMed]


| Sample | ABS341nm | TFC (mg/mL) | Mean ± SD (mg/mL) |
|---|---|---|---|
| Et50 | 0.126 | 4.216 | 4.630 ± 0.508 |
| 0.141 | 5.196 | ||
| 0.130 | 4.477 | ||
| Et80 | 0.115 | 3.497 | 3.932 ± 0.399 |
| 0.127 | 4.281 | ||
| 0.123 | 4.020 | ||
| W100 | 0.118 | 3.693 | 4.586 ± 1.173 |
| 0.125 | 4.150 | ||
| 0.152 | 5.915 | ||
| ClumEt50p | 0.287 | 14.739 | 16.394 ± 1.440 a |
| 0.323 | 17.092 | ||
| 0.327 | 17.353 | ||
| ClumEt80p | 0.273 | 13.824 | 17.680 ± 3.352 b |
| 0.366 | 19.902 | ||
| 0.357 | 19.314 | ||
| ClumWp | 0.370 | 20.163 | 18.355 ± 1.607 c |
| 0.323 | 17.092 | ||
| 0.334 | 17.810 |
| Sample | Spectral Area (∫, a.u. ± SD) | % Scavenging |
|---|---|---|
| DPPH | 3356 ± 0.9 | |
| DPPH + Et80 | 3330 ± 0.7 | 0.77% |
| DPPH + W100 | 3228 ± 1.25 | 3.81% |
| DPPH + ClumEt80p | 1228 ± 1.26 a, b | 63.41% |
| DPPH + ClumWp | 1386 ± 1.02 a, c | 58.70% |
| DPPH + Ascorbic acid | 199 ± 0.87 a | 94.07% |
| Sample | Spectral Area (∫, a.u. ± SD) | % Scavenging |
|---|---|---|
| BMPO-OH• | 107 ± 2.24 | |
| BMPO-OH• + ClumEt80p | 64 ± 2.42 a | 40.19% |
| BMPO-OH• + ClumWp | 61 ± 2.78 a | 42.99% |
| BMPO-OH• + Ascorbic acid | 48 ± 1.66 a | 55.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musolino, V.; Cardamone, A.; Mare, R.; Coppoletta, A.R.; Lorenzo, F.; Noto, F.R.; Galluccio, A.; Tucci, L.; Lupia, C.; Carresi, C.; et al. Antioxidant and In Vitro Hepatoprotective Activities of a Polyphenol-Rich Fraction from the Peel of Citrus lumia Risso (Rutaceae). Plants 2025, 14, 1209. https://doi.org/10.3390/plants14081209
Musolino V, Cardamone A, Mare R, Coppoletta AR, Lorenzo F, Noto FR, Galluccio A, Tucci L, Lupia C, Carresi C, et al. Antioxidant and In Vitro Hepatoprotective Activities of a Polyphenol-Rich Fraction from the Peel of Citrus lumia Risso (Rutaceae). Plants. 2025; 14(8):1209. https://doi.org/10.3390/plants14081209
Chicago/Turabian StyleMusolino, Vincenzo, Antonio Cardamone, Rosario Mare, Anna Rita Coppoletta, Francesca Lorenzo, Francesca Rita Noto, Angelo Galluccio, Luigi Tucci, Carmine Lupia, Cristina Carresi, and et al. 2025. "Antioxidant and In Vitro Hepatoprotective Activities of a Polyphenol-Rich Fraction from the Peel of Citrus lumia Risso (Rutaceae)" Plants 14, no. 8: 1209. https://doi.org/10.3390/plants14081209
APA StyleMusolino, V., Cardamone, A., Mare, R., Coppoletta, A. R., Lorenzo, F., Noto, F. R., Galluccio, A., Tucci, L., Lupia, C., Carresi, C., Marrelli, M., Maurotti, S., Gliozzi, M., Montalcini, T., Pujia, A., & Mollace, V. (2025). Antioxidant and In Vitro Hepatoprotective Activities of a Polyphenol-Rich Fraction from the Peel of Citrus lumia Risso (Rutaceae). Plants, 14(8), 1209. https://doi.org/10.3390/plants14081209












