Mechanism of Penoxsulam’s Effect on Chlorophyll Synthesis and the Metabolism of Foxtail Millet
Abstract
1. Introduction
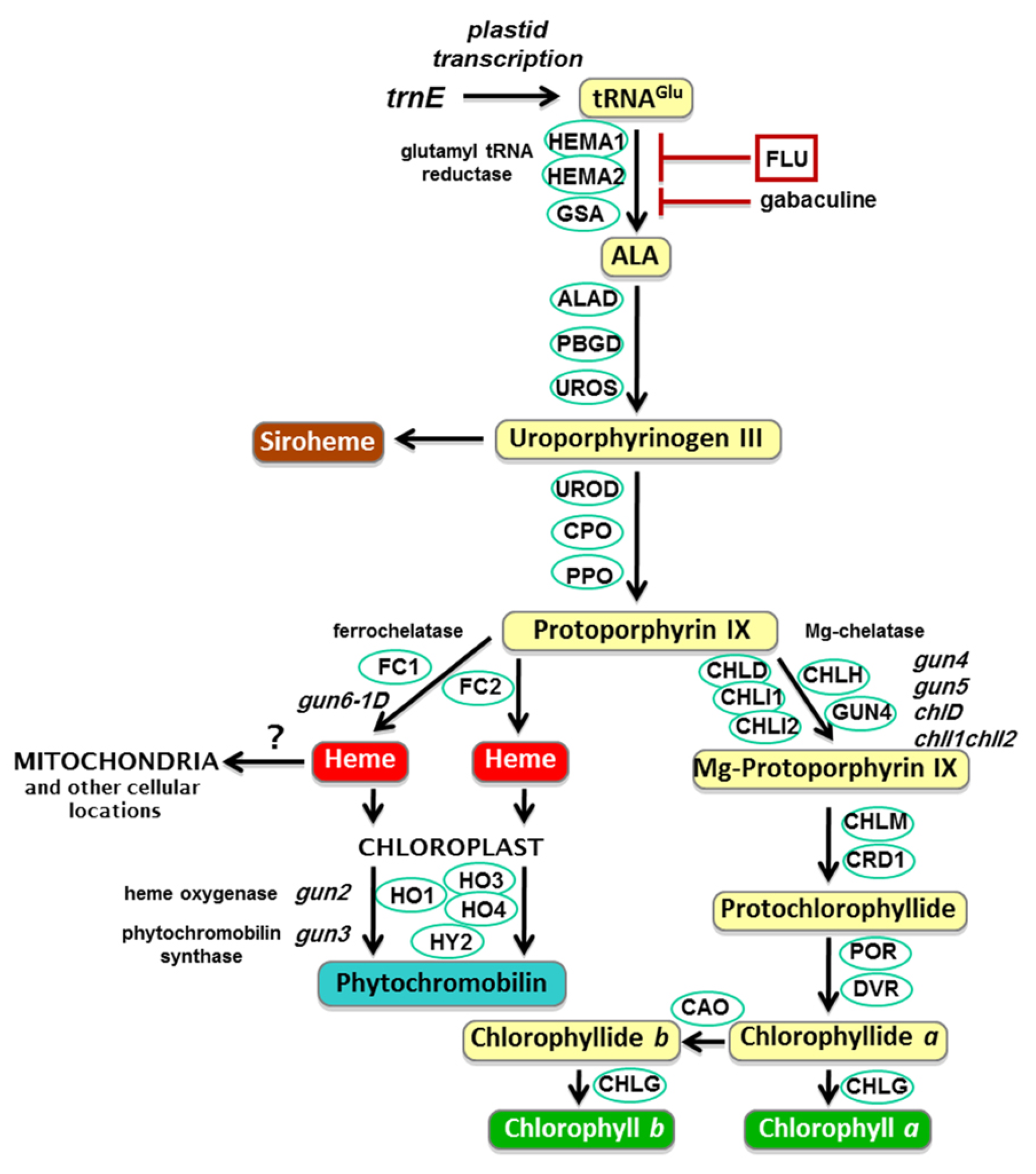
2. Results
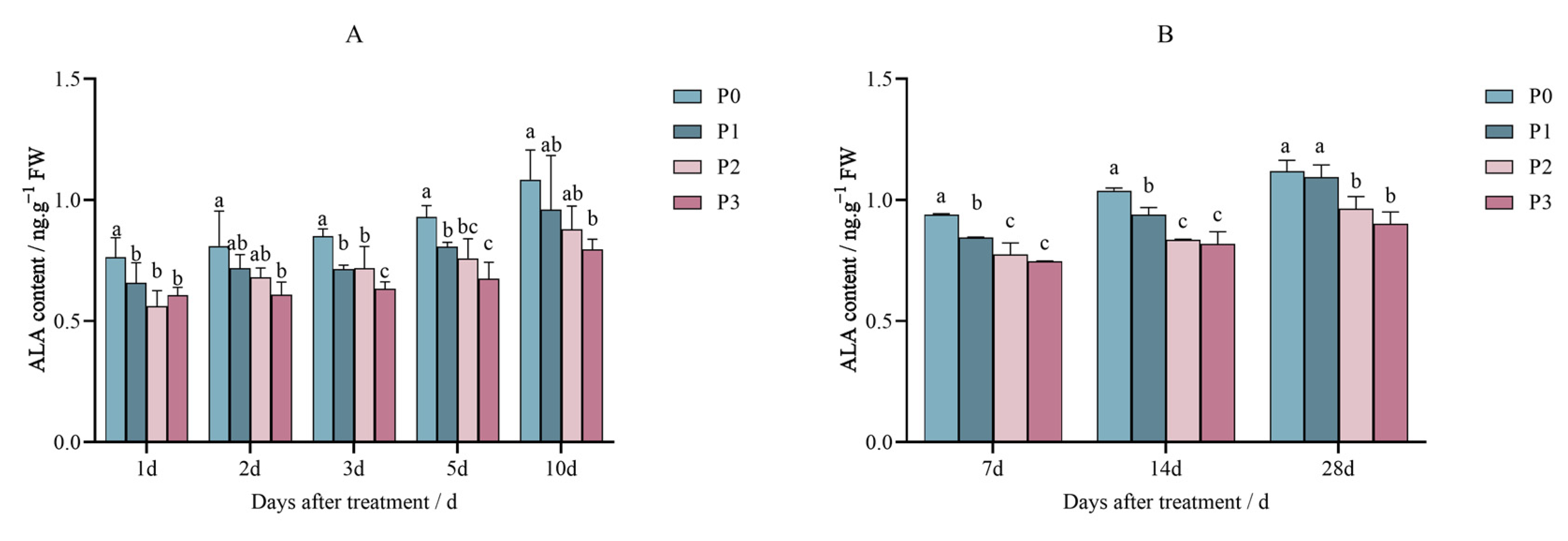
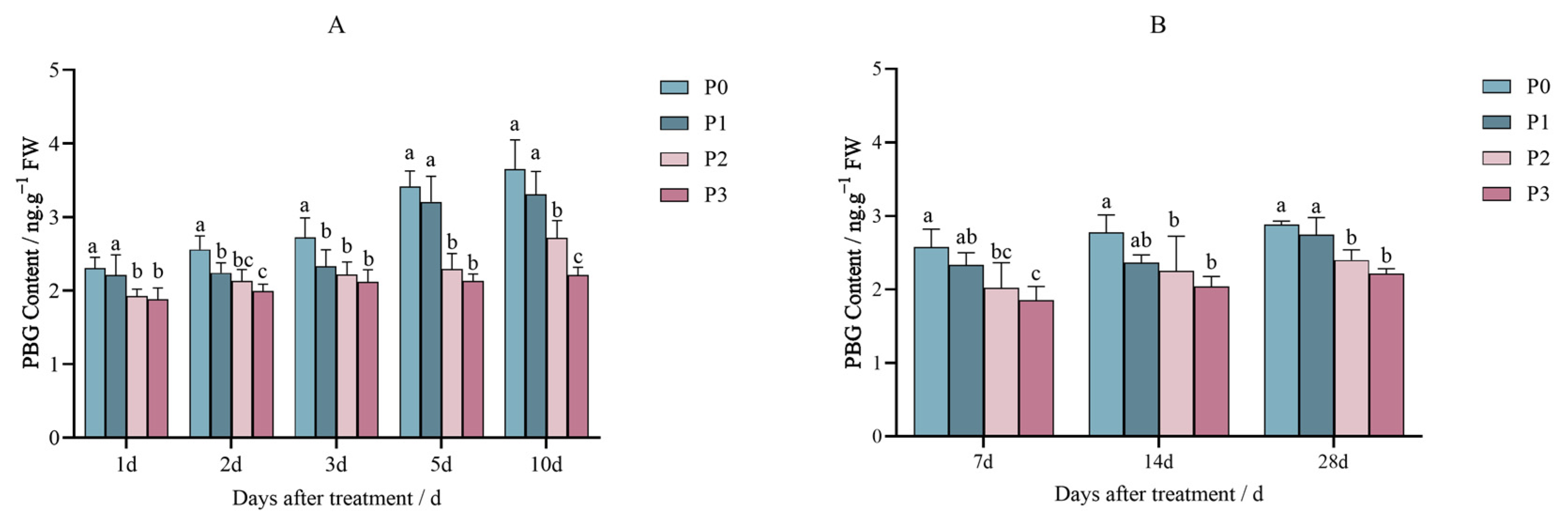
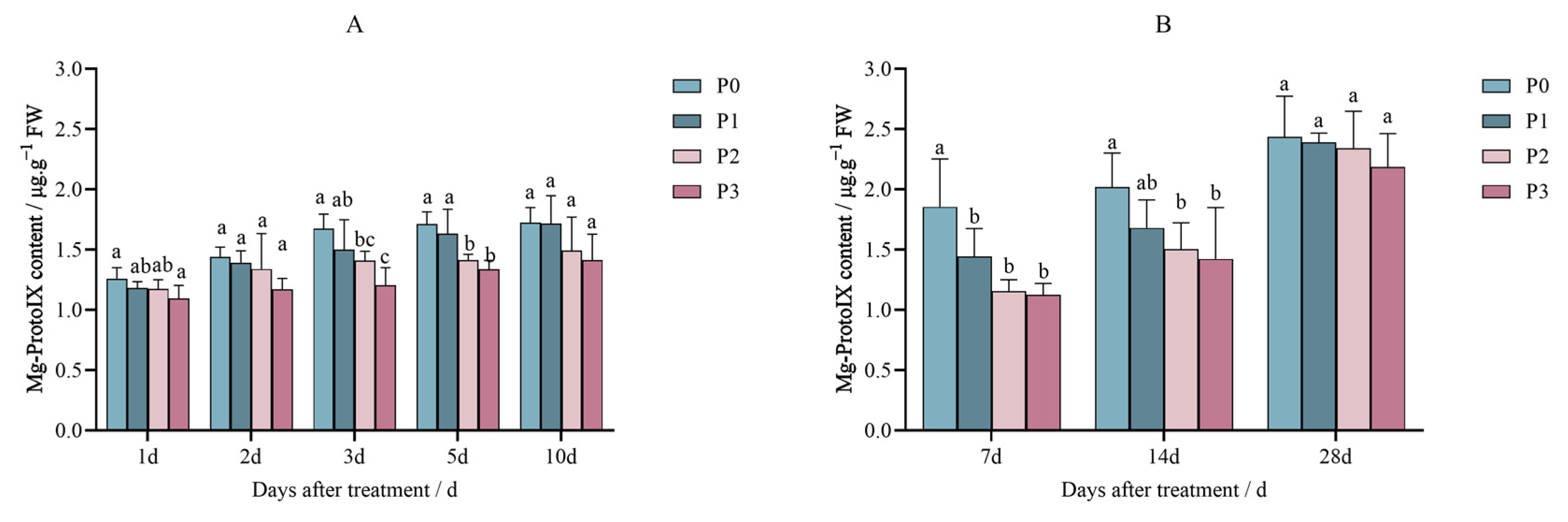
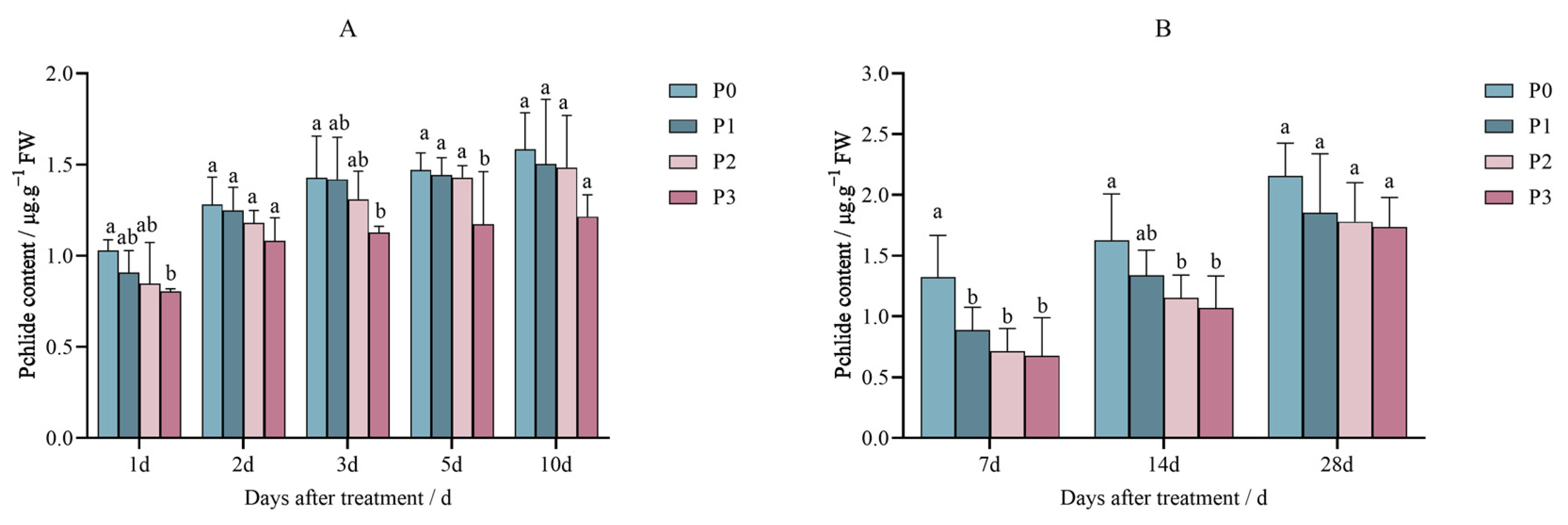
2.1. Effects of Penoxsulam on the Precursors of Chlorophyll Synthesis in Foxtail Millet
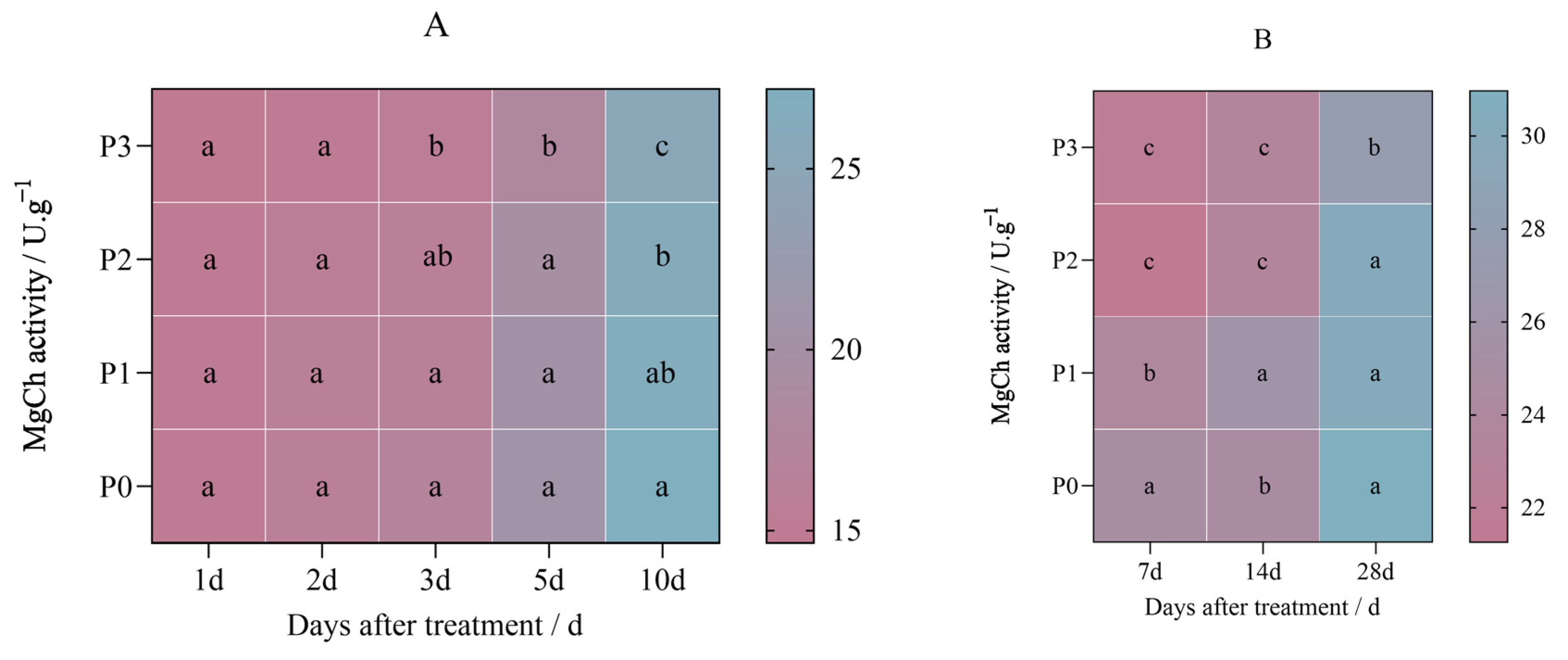
2.2. Effects of Penoxsulam on Chlorophyll Synthase in Foxtail Millet Leaves
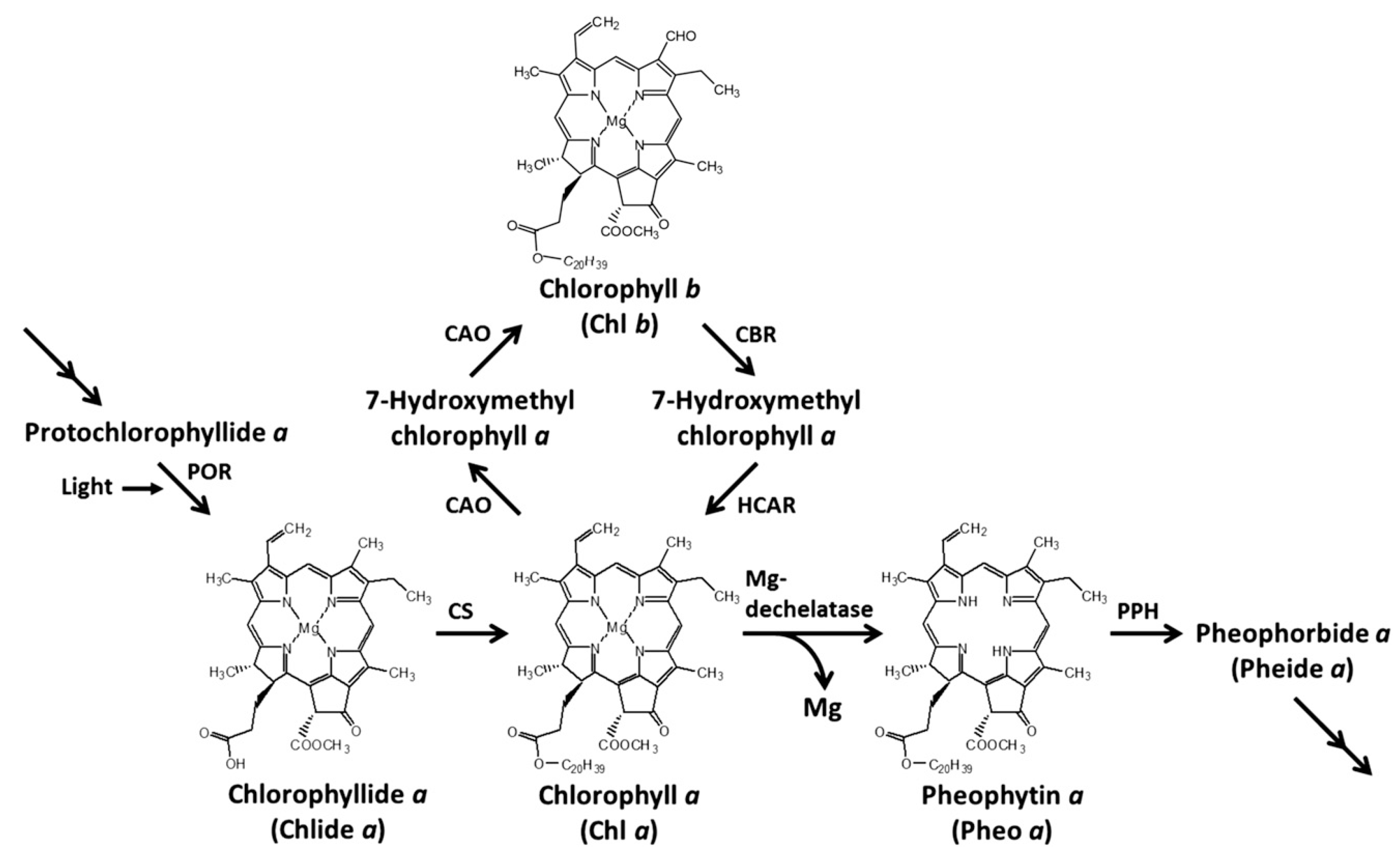
2.3. Effects of Penoxsulam on Chlorophyll Degrading Enzymes in Foxtail Millet
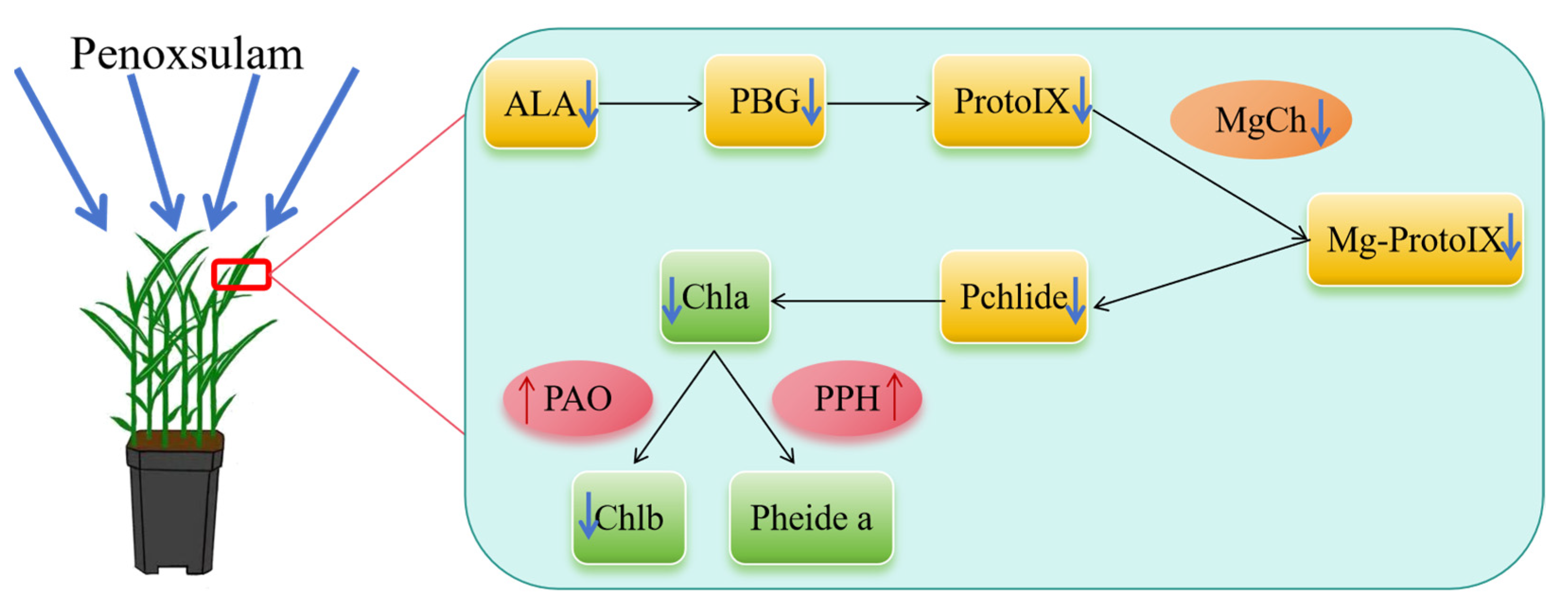
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Analysis of Test Indicators and Methods
- To prepare crude enzyme solution, 0.1 g of leaves were added with precooled 50 mM phosphate-buffered saline (pH 7.4), ground in an ice bath to homogenate and transferred into a 1.5 mL centrifuge tube to spin at 4 °C and 3000× g for 20 min. The obtained supernatant was the crude enzyme solution.
- Adding samples: Standard, sample, and control holes were established, and 50 μL of standard samples with different concentration gradients were added to the standard holes. Crude enzyme solution (10 μL) was added to the sample hole, followed by the addition of 40 μL of sample diluent. No reagent was added to the control hole. The samples were added directly to the bottom of the enzyme-labelled plate, shaken, and mixed evenly.
- Enzyme incubation: An enzyme-labelled reagent (100 μL) was added to each well except the control well, followed by incubation at 37 °C for 60 min.
- Washing: The concentrated washing liquid was diluted 20 times into a one-time working liquid, the sealing film was removed and the liquid was discarded. Spin drying was performed, followed by the addition of washing liquid to wash for 30 s. This operation was repeated 5 times.
- Colour development: A and B colour development solutions (50 μL) were added in turn, shaken and mixed evenly and allowed to react at 37 °C for 15 min.
- Determination: Termination solution (50 μL) was added, and after the reaction was terminated (blue changed to yellow), the absorbance at 450 nm was determined by setting zero in the control hole.
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, P.R.; Pramitha, L.; Choudhary, P.; Singh, R.K.; Shukla, P.; Prasad, M.; Muthamilarasan, M. Multi-omics intervention in Setaria to dissect climate-resilient traits: Progress and prospects. Front. Plant Sci. 2022, 13, 892736. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses. Theor. Appl. Genet. 2015, 128, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Defelice, M. Green foxtail, Setaria viridis (L.) P. Beauv. Weed. Technol. 2002, 16, 253–257. [Google Scholar] [CrossRef]
- Pan, J.; Li, Z.; Wang, Q.; Garrell, A.K.; Liu, M.; Guan, Y.; Zhou, W.; Liu, W. Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018, 18, 315. [Google Scholar] [CrossRef] [PubMed]
- Ajithkumar, I.; Panneerselvam, R. ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. Under drought stress. Cell Biochem. Biophys. 2014, 68, 587–595. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, X.; Quan, Z.W.; Cheng, S.F.; Xu, X.; Pan, S.K.; Xie, M.; Zeng, P.; Yue, Z.; Wang, W.L.; et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat. Biotechnol. 2012, 30, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Masabni, J.; Baumann, P.; Isakeit, T.; Matocha, M.; Provin, T.; Liu, R.; Carson, K.; Bagavathiannan, M. Activated charcoal reduces pasture herbicide injury in vegetable crops. Crop. Prot. 2019, 117, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Weaver, S.E.; Hamill, A.S. Risks and reliability of using herbicides at below-labeled rates. Weed. Technol. 2000, 14, 106–115. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Chen, H.; Shao, B. Cellular response of freshwater green algae to perfluorooctanoic acid toxicity. Ecotoxicol. Environ. Saf. 2013, 88, 103–107. [Google Scholar] [CrossRef]
- Lin, L.; Chen, F.; Wang, J.; Liao, M.; Lv, X.; Wang, Z.; Li, H.; Deng, Q.; Xia, H.; Liang, D.; et al. Effects of living hyperaccumulator plants and their straws on the growth and cadmium accumulation of Cyphomandra betacea seedlings. Ecotoxicol. Environ. Saf. 2018, 155, 109–116. [Google Scholar] [CrossRef]
- Nasir, M.U.; Hussain, S.; Jabbar, S.; Rashid, F.; Khalid, N.; Mehmood, A. A review on the nutritional content, functional pro perties and medicinal potential of dates. Sci. Lett. 2015, 3, 17–22. [Google Scholar]
- Liu, C.; Shi, X.; Wu, F.; Ren, M.; Gao, G.; Wu, Q. Genome analyses provide insights into the evolution and adaptation of the eukaryotic Picophytoplankton Mychonastes homosphaera. BMC Genom. 2020, 21, 477. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 1, 163–190. [Google Scholar] [CrossRef]
- Field, K.J.; George, R.; Fearn, B.; Quick, W.P.; Davey, M.P. Best of both worlds: Simultaneous high-light and shade-tolerance adaptations within individual leaves of the living stone Lithops aucampiae. PLoS ONE 2013, 8, e75671. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.P.; Fang, J.G.; Zhang, J.H.; Ren, L.H.; Mao, Y.Z.; Li, B.; Zhang, M.L.; Liu, D.H.; Du, M.R. The impact of the herbicide atrazine on growth and photosynthesis of seagrass, Zostera marina (L.), seedlings. Mar. Pollut. Bull. 2011, 62, 1628–1631. [Google Scholar] [CrossRef]
- Teodorović, I.; Knežević, V.; Tunić, T.; Cučak, M.; Lečić, J.N.; Leovac, A.; Tumbas, I.I. Myriophyllum aquaticum versus Lemna minor: Sensitivity and recovery potential after exposure to atrazine. Environ. Toxicol. Chem. 2012, 31, 417–426. [Google Scholar] [CrossRef]
- Wang, Q.; Que, X.; Zheng, R.; Pang, Z.; Li, C.; Xiao, B. Phytotoxicity assessment of atrazine on growth and physiology of three emergent plants. Environ. Sci. Pollut. Res. 2015, 22, 9646–9657. [Google Scholar] [CrossRef]
- Nagata, N.; Tanaka, R.; Satoh, S.; Tanaka, A. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell 2005, 17, 233–240. [Google Scholar] [CrossRef]
- Czarnecki, O.; Grimm, B. Post-translational control of tetrapyrrole biosynthesis in plants, algae, and cyanobacteria. J. Exp. Bot. 2012, 63, 1675–1687. [Google Scholar] [CrossRef]
- Masuda, T.; Fujita, Y. Regulation and evolution of chlorophyll metabolism. Photochem. Photobiol. Sci. 2008, 7, 1131–1149. [Google Scholar] [CrossRef]
- Chekunova, E.M.; Yaronskaya, E.B.; Yartseva, N.V.; Averina, N.G. New factors regulating magnesium chelatase in the green alga Chlamydomonas reinhardtii. Russ. J. Plant Physiol. 2014, 61, 169–177. [Google Scholar] [CrossRef]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant. Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Willows, R.D. Biosynthesis of chlorophylls from protoporphyrin IX. Nat. Prod. Rep. 2003, 20, 327–341. [Google Scholar] [CrossRef]
- Masuda, T.; Fusada, N.; Oosawa, N.; Takamatsu, K.; Yamamoto, Y.Y.; Ohto, M.; Nakamura, K.; Goto, K.; Shibata, D.; Shirano, Y.; et al. Functional analysis of isoforms of NADPH: Protochlorophyllide oxidoreductase (POR), PORB and PORC, in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 963–974. [Google Scholar] [CrossRef]
- Terry, M.J.; Smith, A.G. A model for tetrapyrrole synthesis as the primary mechanism for plastid-to-nucleus signaling during chloroplast biogenesis. Front. Plant. Sci. 2013, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Schelbert, S.; Park, S.Y.; Han, S.H.; Lee, B.D.; Andrès, C.B.; Kessler, F.; Hörtensteiner, S.; Paek, N.C. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. Plant Cell 2012, 24, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Chlorophyll degradation during senescence. Annu. Rev. Plant. Biol. 2006, 57, 55–77. [Google Scholar] [CrossRef]
- Hauenstein, M.; Christ, B.; Das, A.; Aubry, S.; Hörtensteiner, S. A role for TIC55 as a hydroxylase of phyllobilins, the products of chlorophyll breakdown during plant senescence. Plant Cell 2016, 28, 2510–2527. [Google Scholar] [CrossRef]
- Hörtensteiner, S. Update on the biochemistry of chlorophyll breakdown. Plant. Mol. Biol. 2013, 82, 505–517. [Google Scholar] [CrossRef]
- Pruzinská, A.; Tanner, G.; Anders, I.; Roca, M.; Hörtensteiner, S. Chlorophyll breakdown: Pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc. Natl. Acad. Sci. USA 2003, 100, 15259–15264. [Google Scholar] [CrossRef]
- Schelbert, S.; Aubry, S.; Burla, B.; Agne, B.; Kessler, F.; Krupinska, K.; Hörtensteiner, S. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. Plant Cell 2009, 21, 76–785. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, M.; Liang, N.; Yan, H.; Wei, Y.; Xu, X.; Liu, J.; Xu, Z.; Chen, F.; Wu, G. Molecular cloning and function analysis of the stay green gene in rice: Molecular cloning and function analysis of the rice stay green gene. Plant J. 2007, 52, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, L.; Zhang, L.; Pan, X.; Hu, X. Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015, 15, 303. [Google Scholar] [CrossRef]
- Dei, M. Benzyladenine-induced stimulation of 5-aminolevulinic acid accumulation under various light intensities in levulinic acid-treated cotyledons of etiolated cucumber. Physiol. Plant 1985, 64, 153–160. [Google Scholar] [CrossRef]
- Shimoda, Y.; Ito, H.; Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s Green Cotyledon Gene, Encodes Magnesium-Dechelatase. Plant Cell 2016, 28, 2147–2160. [Google Scholar] [CrossRef]
- Woodson, J.D. Chloroplast stress signals: Regulation of cellular degradation and chloroplast turnover. Curr. Opin. Plant Biol. 2019, 52, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Pereira, R.; Martins, M.; Tamagnini, P.; Serôdio, J.; Moutinho-Pereira, J.; Cunha, A.; Fidalgo, F. Glyphosate-dependent effects on photosynthesis of Solanum lycopersicum L.—An ecophysiological, ultrastructural and molecular approach. J. Hazard. Mater. 2020, 398, 122871. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Zhang, L.G.; Huang, L.; Qi, X.; Wen, Y.Y.; Dong, S.Q.; Song, X.E.; Wang, H.F.; Guo, P.Y. Photosynthetic and physiological responses of foxtail millet (Setaria italica L.) to low-light stress during grain-filling stage. Photosynthetica 2017, 55, 491–500. [Google Scholar] [CrossRef]
- Guo, M.J.; Bai, Y.Q.; Gao, P.; Shen, J.; Dong, S.Q.; Yuan, X.Y.; Guo, P.Y. Effect of MCPA on leaf senescence and endogenous hormones content in leaves of foxtail millet seedlings. Scientia. Agric. Sin. 2020, 53, 513–526. [Google Scholar]
- Cornah, J.E.; Terry, M.J.; Smith, A.G. Green or red: What stops the traffic in the tetrapyrrole pathway? Trends Plant Sci. 2003, 8, 224–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, Q.; Ding, C.; Huang, Y.; Liao, J.; Chen, T.; Feng, S.; Zhou, L.; Zhang, Z.; Chen, Y.; et al. Effect of low temperature on chlorophyll biosynthesis and chloroplast biogenesis of rice seedlings during greening. Int. J. Mol. Sci. 2020, 21, 1390. [Google Scholar] [CrossRef]
- Linu, C.; Girija, T. Physiological response of rice to herbicide application. Indian. J. Weed. Sci 2020, 52, 270–275. [Google Scholar]
- Netherland, M.D.; Lembi, C.A.; Glomski, L.M. Potential for selective activity of the ALS Inhibitors penoxsulam, bispyribac-sodium, and imazamox on algae responsible for harmful blooms. J. Aquat. Plant Manag. 2009, 47, 147. [Google Scholar]
- Tanaka, R.; Kobayashi, K.; Masuda, T. Tetrayrrole metabolism in Arabidopsis thaliana. Arab. Book. 2011, 9, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Yoo, J.H.; Yoo, S.C.; Cho, S.H.; Koh, H.J.; Seo, H.S.; Paek, N.C. Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant. Mol. Biol. 2006, 62, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.H.; Satoh, K.J.; Kikuchi, S.S.; Kim, S.C.; Ko, S.M.; Kang, H.G.; Jeon, J.S.; Kim, C.S.; Park, Y.I. Mitochondrial activity in illuminated leaves of chlorophyll deficient mutant rice (OsCHLH) seedlings. Plant. Biotechnol. Rep. 2010, 4, 281–291. [Google Scholar] [CrossRef]
- Fukura, K.; Tanaka, A.; Tanaka, R.; Ito, H. Enrichment of chlorophyll catabolic enzymes in grana margins and their cooperation in catabolic reactions. J. Plant. Physiol. 2021, 266, 153535. [Google Scholar] [CrossRef]
- Dey, D.; Dhar, D.; Fortunato, H.; Obata, D.; Tanaka, A.; Tanaka, R.; Basu, S.; Ito, H. Insights into the structure and function of the ratelimiting enzyme of chlorophyll degradation through analysis of a bacterial Mg-dechelatase homolog. Comput. Struct. Biotechnol. J. 2021, 19, 5333–5347. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, G.; Wen, W.; Ma, X.; Xu, B.; Huang, B. Functional characterization and hormonal regulation of the PHEOPHYTINASE gene LpPPH controlling leaf senescence in perennial ryegrass. J. Exp. Bot. 2016, 67, 935–945. [Google Scholar] [CrossRef]
- Pruzinská, A.; Tanner, G.; Aubry, S.; Anders, I.; Moser, S.; Müller, T.; Ongania, K.H.; Kräutler, B.; Youn, J.Y.; Liljegren, S.J.; et al. Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant. Physiol. 2005, 139, 52–63. [Google Scholar] [CrossRef]
- Roca, M.; James, C.; Pruzinská, A.; Hörtensteiner, S.; Thomas, H.; Ougham, H. Analysis of the chlorophyll catabolism pathway in leaves of an introgression senescence mutant of Lolium temulentum. Phytochemistry 2004, 65, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ghandchi, F.P.; Caetano-Anolles, G.; Clough, S.J.; Ort, D.R. Investigating the control of chlorophyll degradation by genomic correlation mining. PLoS ONE 2016, 11, e0162327. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Chen, T.; Xi, R.; Gao, S.; Li, G.; Zhou, X.; Song, X.; Ma, Y.; Hu, C.; Yuan, X. Effects of penoxsulam on photosynthetic characteristics and safety evaluation of foxtail millet. Agronomy 2024, 14, 641. [Google Scholar] [CrossRef]
- Bogorad, L. Porphyrin synthesis. Methods Enzymol. 1962, 5, 885–895. [Google Scholar]
- Rebeiz, C.; Mattheis, J.; Smith, B.; Rebeiz, C.; Dayton, D. Chloroplast biogenesis: Biosynthesis and accumulation of protochlorophyll by isolated etioplasts and developing chloroplasts. Arch. Biochem. Biophys. 1975, 171, 549–567. [Google Scholar] [CrossRef]



| Variety | Treatments | Application Rate/g a.i. Ha−1 |
|---|---|---|
| Jingu 21 | P0 | 0 |
| P1 | 15 | |
| P2 | 30 | |
| P3 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; An, Q.; Zhang, T.; Yan, S.; Li, J.; Song, X.; Zhao, J.; Li, X.; Hu, C.; Dong, S. Mechanism of Penoxsulam’s Effect on Chlorophyll Synthesis and the Metabolism of Foxtail Millet. Plants 2025, 14, 1211. https://doi.org/10.3390/plants14081211
Chen T, An Q, Zhang T, Yan S, Li J, Song X, Zhao J, Li X, Hu C, Dong S. Mechanism of Penoxsulam’s Effect on Chlorophyll Synthesis and the Metabolism of Foxtail Millet. Plants. 2025; 14(8):1211. https://doi.org/10.3390/plants14081211
Chicago/Turabian StyleChen, Tingting, Qi An, Ting Zhang, Siyu Yan, Jiaxing Li, Xie Song, Juan Zhao, Xiaorui Li, Chunyan Hu, and Shuqi Dong. 2025. "Mechanism of Penoxsulam’s Effect on Chlorophyll Synthesis and the Metabolism of Foxtail Millet" Plants 14, no. 8: 1211. https://doi.org/10.3390/plants14081211
APA StyleChen, T., An, Q., Zhang, T., Yan, S., Li, J., Song, X., Zhao, J., Li, X., Hu, C., & Dong, S. (2025). Mechanism of Penoxsulam’s Effect on Chlorophyll Synthesis and the Metabolism of Foxtail Millet. Plants, 14(8), 1211. https://doi.org/10.3390/plants14081211






