Effects of Arbuscular Mycorrhizal Fungi on the Growth and Nutrient Uptake in Wheat Under Low Potassium Stress
Abstract
:1. Introduction
2. Results
2.1. AMF Colonization Under Low Potassium Stress
2.2. AMF Improve Wheat Growth
2.3. AMF Affect the Antioxidant Defense of Wheat Seedlings
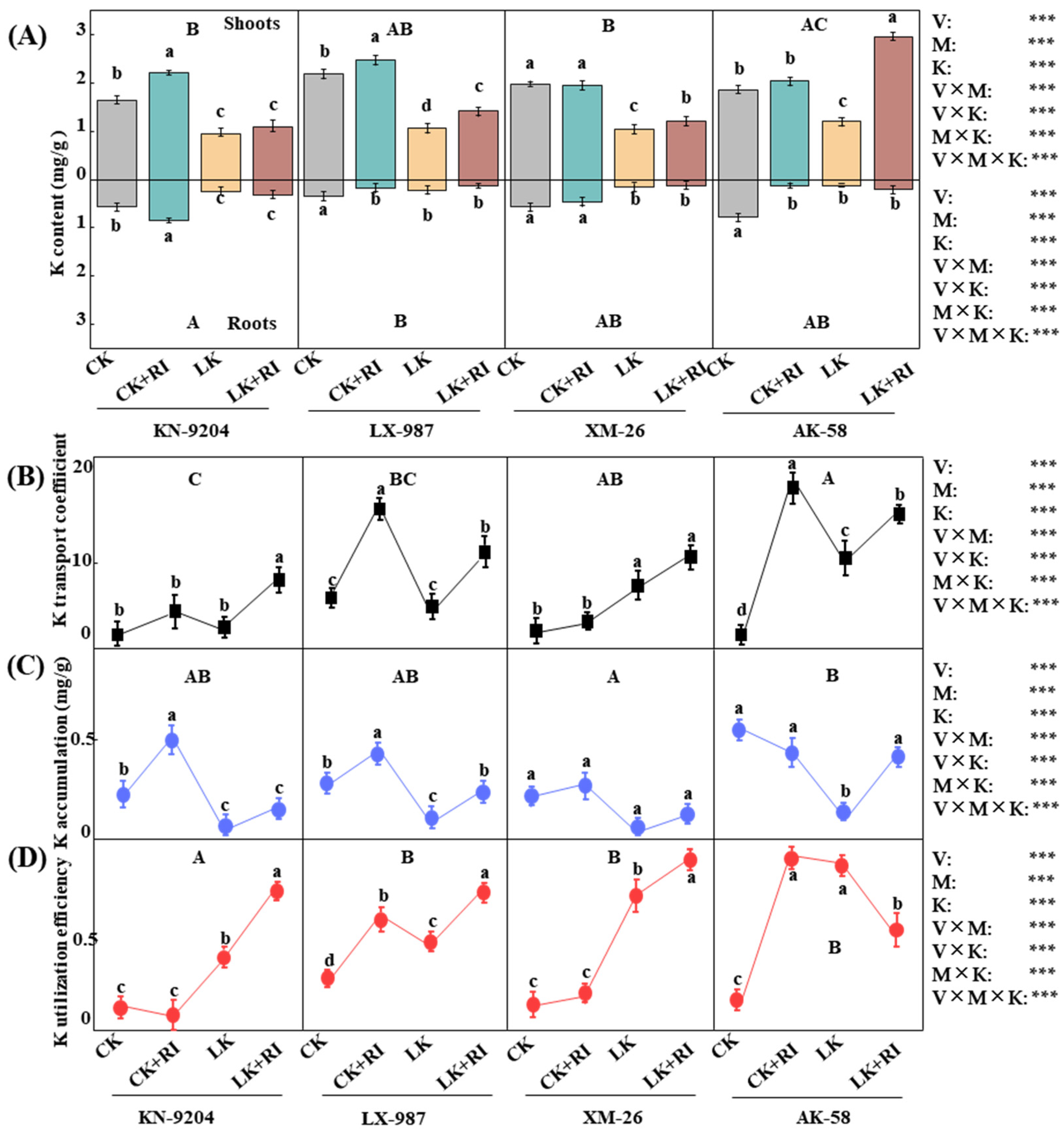
2.4. AMF Enhance the Uptake and Transport of K and Several Mineral Elements
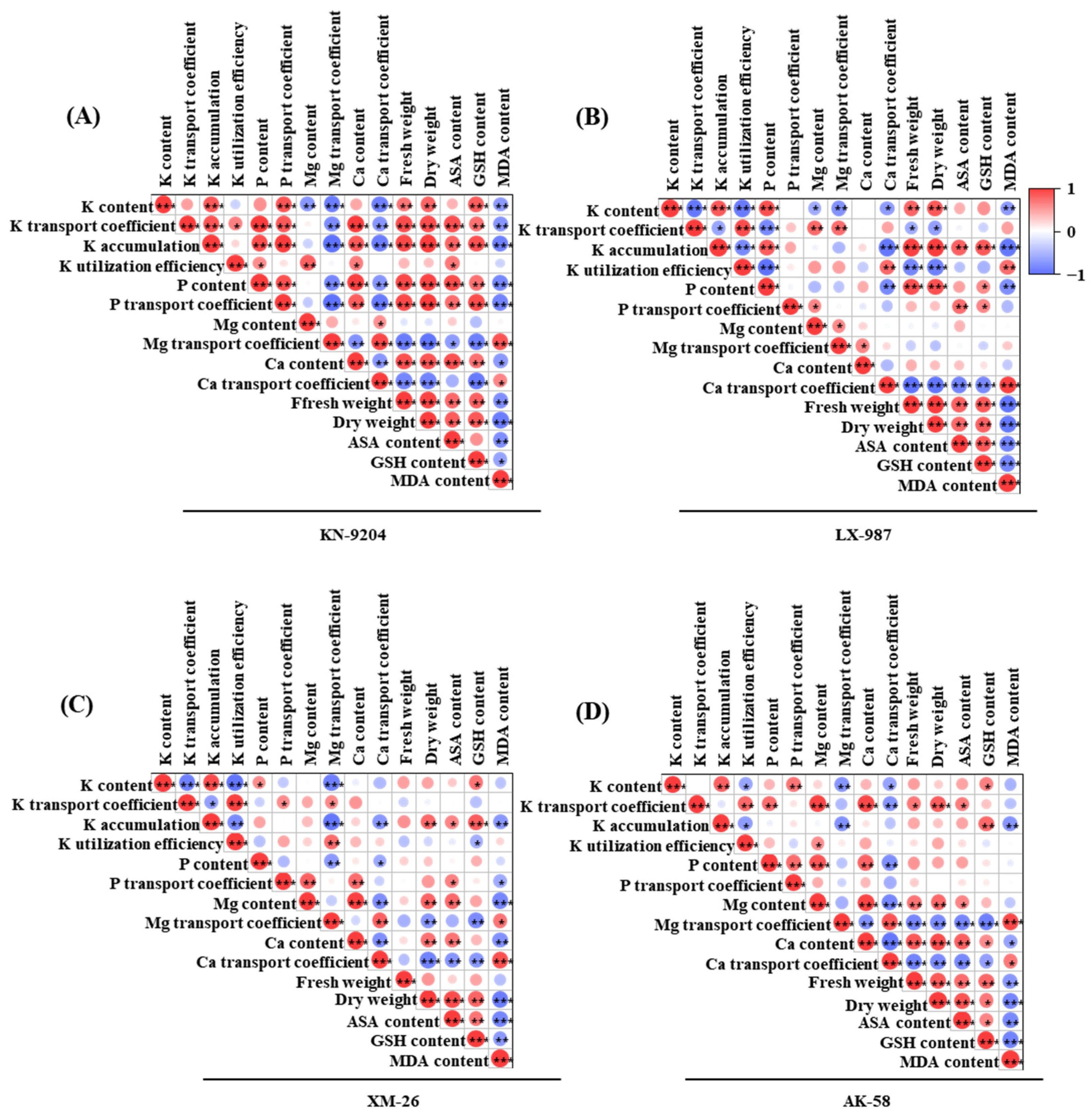
2.5. Correlation Analysis Between AMF and Various Indices in Four Wheat Seedlings
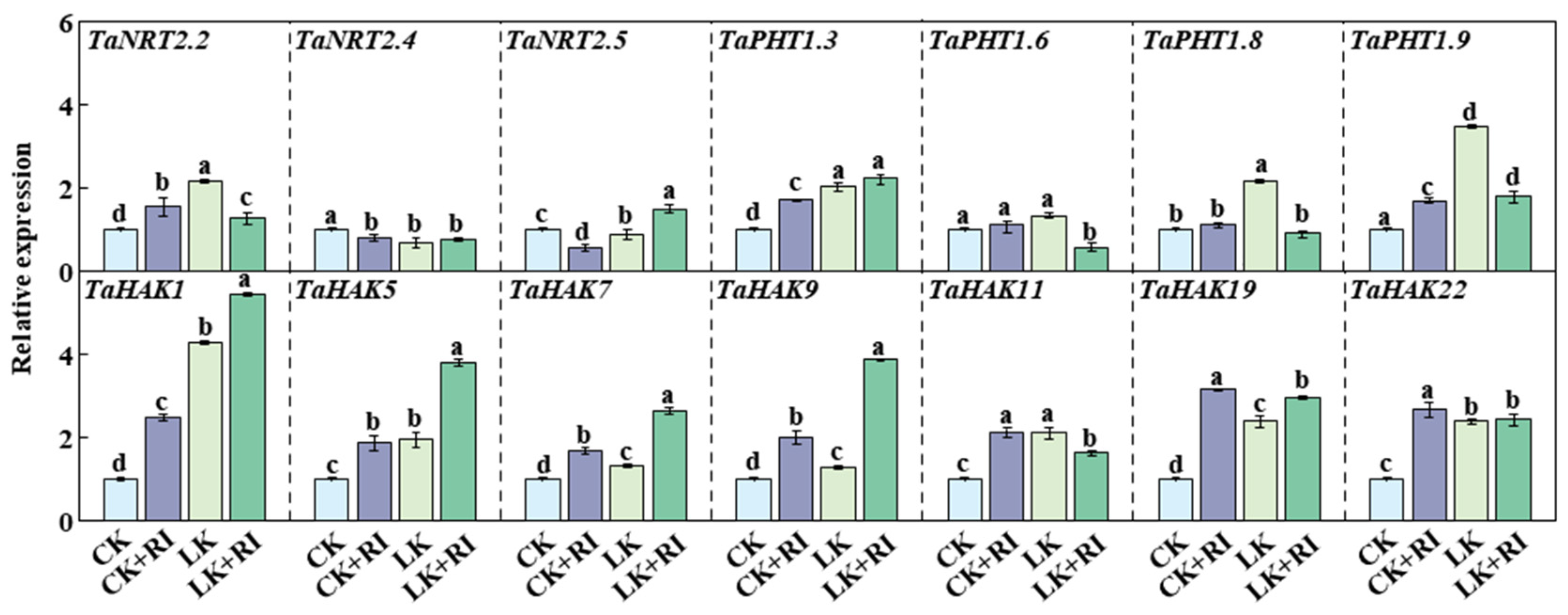
2.6. AMF Affect the Expression Levels of Transporter Genes for K and Other Elements
2.7. Analyses of AMF’s Effects on Changes in Various Indices in KN-9204
3. Discussion
3.1. AMF Promote Wheat Growth by Regulating the Antioxidant Systems
3.2. AMF Improve Wheat Growth by Metabolizing K and Other Mineral Elements
3.3. AMF Affect Wheat Growth by Affecting the Expression of Genes Related to Mineral Element Absorption and Transport
4. Materials and Methods
4.1. Experimental Material and Experimental Design
4.2. Determination of Mycorrhizal Infection Rate
4.3. Determination of the Contents of ASA, GSH, and MDA and the H2O2 Staining
4.4. Determination of Mineral Elements
4.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1
| Gene Name | Primer Sequence (5′-3′) |
|---|---|
| qPCR-TaNRT2.2-F | GCTGCCTGTCGCTCTTGT |
| qPCR-TaNRT2.2-R | CCTTGTTCTTCTCCTCCTCC |
| qPCR-TaNRT2.4-F | TGGACTCGGAGAACAAGG |
| qPCR-TaNRT2.4-R | AGACAAAGCAGGTGAAGAAGG |
| qPCR-TaNRT2.5-F | CTGAAGAGCGCCCATACTACGT |
| qPCR-TaNRT2.5-R | CAGCATAAAGCACCCTCCAAA |
| qPCR-TaPHT1.3-F | CTGCTCACCTACTACTGGCGG |
| qPCR-TaPHT1.3-R | CCCGAAGTCGTTGGCTCC |
| qPCR-TaPHT1.6-F | TTTTTTATGGTCGGAGAGCGTT |
| qPCR-TaPHT1.6-R | CAGCCCTAATTAACCTGGACAACT |
| qPCR-TaPHT1.8-F | GATCTTCAGGGACATCAAGTGGATC |
| qPCR-TaPHT1.8-R | TGAACCCGAGGAACTGGATGG |
| qPCR-TaPHT1.9-F | GAGACCGGCTACTCACGGG |
| qPCR-TaPHT1.9-R | CTAAGCTTCGATGCCATCGTC |
| qPCR-TaHAK1-F | GTTCGAGTCATCCACACCTC |
| qPCR-TaHAK1-R | CACCACACAGATCCCGTAAG |
| qPCR-TaHAK5-F | ACAGGAACCGAAGCAATGTTTG |
| qPCR-TaHAK5-R | GATATGCAGCCTGTCCCATGTA |
| qPCR-TaHAK7-F | CCGGGCTGATCATGAGAGAC |
| qPCR-TaHAK7-R | GTTGATCTGCTCCTCCTCGG |
| qPCR-TaHAK9-F | TCTACAAGAGCACCTTCGCC |
| qPCR-TaHAK9-R | GAGACGTACTTGAGGAGCGG |
| qPCR-TaHAK11-F | CGAGCCTTGGTCTAGTCAGG |
| qPCR-TaHAK11-R | ACCGGGACTGTGTAGACTGG |
| qPCR-TaHAK19-F | AAGACCAGGATGATCCCCAA |
| qPCR-TaHAK19-R | TGCCGAGGATGGTAATTGTG |
| qPCR-TaHAK22-F | GTGAAGCGCTACAAGTACGA |
| qPCR-TaHAK22-R | GATCTTCTCGATGAGGTGCG |
Appendix A.2
| Varieties | Treatments | P Content in Shoots (mg/g) | P Content in Roots (mg/g) | P Translocation Coefficient | Mg Content in Shoots (mg/g) | Mg Content in Roots (mg/g) | Mg Translocation Coefficient | Ca Content in Shoots (mg/g) | Ca Content in Roots (mg/g) | Ca Translocation Coefficient |
|---|---|---|---|---|---|---|---|---|---|---|
| KN-9204 | CK | 0.10 ± 0.00 c | 0.08 ± 0.01 a | 1.22 ± 0.01 d | 0.14 ± 0.01 a | 0.10 ± 0.00 a | 1.34 ± 0.02 b | 0.32 ± 0.02 a | 0.43 ± 0.01 b | 0.75 ± 0.01 b |
| CK + RI | 0.13 ± 0.00 a | 0.07 ± 0.00 a | 1.93 ± 0.02 b | 0.13 ± 0.00 b | 0.11 ± 0.01 a | 1.11 ± 0.01 d | 0.25 ± 0.01 b | 0.42 ± 0.02 b | 0.59 ± 0.02 d | |
| LK | 0.09 ± 0.00 c | 0.05 ± 0.01 b | 1.73 ± 0.02 c | 0.14 ± 0.01 a | 0.11 ± 0.02 a | 1.31 ± 0.00 c | 0.28 ± 0.03 b | 0.32 ± 0.01 c | 0.88 ± 0.00 a | |
| LK + RI | 0.11 ± 0.01 b | 0.06 ± 0.01 b | 1.94 ± 0.01 a | 0.15 ± 0.01 a | 0.10 ± 0.03 a | 1.52 ± 0.02 a | 0.31 ± 0.01 a | 0.49 ± 0.02 a | 0.65 ± 0.01 c | |
| LX-987 | CK | 0.13 ± 0.01 c | 0.06 ± 0.00 b | 2.18 ± 0.00 b | 0.12 ± 0.01 b | 0.09 ± 0.02 b | 1.40 ± 0.01 c | 0.29 ± 0.02 c | 0.36 ± 0.01 b | 0.82 ± 0.02 c |
| CK + RI | 0.20 ± 0.00 a | 0.07 ± 0.01 a | 2.92 ± 0.03 a | 0.11 ± 0.00 b | 0.12 ± 0.01 a | 0.95 ± 0.03 d | 0.17 ± 0.03 d | 0.63 ± 0.00 a | 0.27 ± 0.03 d | |
| LK | 0.09 ± 0.02 d | 0.07 ± 0.00 a | 1.35 ± 0.00 d | 0.17 ± 0.01 a | 0.08 ± 0.02 b | 2.10 ± 0.02 a | 0.35 ± 0.00 b | 0.29 ± 0.01 d | 1.22 ± 0.02 a | |
| LK + RI | 0.15 ± 0.01 b | 0.08 ± 0.01 a | 1.96 ± 0.03 c | 0.18 ± 0.02 a | 0.11 ± 0.03 a | 1.65 ± 0.01 b | 0.39 ± 0.01 a | 0.33 ± 0.02 c | 1.18 ± 0.02 b | |
| XM-26 | CK | 0.12 ± 0.00 a | 0.08 ± 0.01 a | 1.65 ± 0.02 d | 0.12 ± 0.01 c | 0.13 ± 0.02 a | 0.95 ± 0.00 c | 0.31 ± 0.02 b | 0.59 ± 0.01 c | 0.52 ± 0.01 b |
| CK + RI | 0.11 ± 0.01 a | 0.05 ± 0.01 b | 2.07 ± 0.01 b | 0.14 ± 0.02 b | 0.14 ± 0.01 a | 0.99 ± 0.01 d | 0.34 ± 0.01 a | 0.70 ± 0.03 b | 0.48 ± 0.01 c | |
| LK | 0.10 ± 0.00 b | 0.05 ± 0.00 b | 1.87 ± 0.01 c | 0.15 ± 0.01 b | 0.09 ± 0.01 b | 1.74 ± 0.02 a | 0.37 ± 0.2 a | 0.46 ± 0.02 d | 0.79 ± 0.02 a | |
| LK + RI | 0.12 ± 0.01 a | 0.06 ± 0.01 b | 2.10 ± 0.00 a | 0.17 ± 0.01 a | 0.13 ± 0.02 a | 1.30 ± 0.03 b | 0.37 ± 0.03 a | 0.84 ± 0.01 a | 0.45 ± 0.03 c | |
| AK-58 | CK | 0.10 ± 0.00 c | 0.05 ± 0.01 b | 1.84 ± 0.02 b | 0.11 ± 0.01 a | 0.09 ± 0.03 b | 1.16 ± 0.01 b | 0.23 ± 0.01 b | 0.23 ± 0.01 c | 1.01 ± 0.01 b |
| CK + RI | 0.12 ± 0.01 b | 0.07 ± 0.00 a | 1.58 ± 0.03 d | 0.12 ± 0.00 a | 0.13 ± 0.01 a | 0.94 ± 0.02 d | 0.28 ± 0.02 a | 0.64 ± 0.02 b | 0.43 ± 0.01 c | |
| LK | 0.12 ± 0.00 b | 0.07 ± 0.01 a | 1.78 ± 0.01 c | 0.13 ± 0.02 a | 0.09 ± 0.02 b | 1.50 ± 0.01 a | 0.28 ± 0.03 a | 0.25 ± 0.03 c | 1.13 ± 0.01 a | |
| LK + RI | 0.18 ± 0.02 a | 0.06 ± 0.01 a | 2.90 ± 0.00 a | 0.13 ± 0.01 a | 0.13 ± 0.01 a | 0.98 ± 0.00 c | 0.18 ± 0.01 c | 0.72 ± 0.03 a | 0.26 ± 0.01 d | |
| KN-9204 | A | A | A | A | A | A | A | A | A | |
| LX-987 | B | A | A | A | A | A | A | A | A | |
| XM-26 | AB | A | A | A | A | B | A | B | A | |
| AK-58 | AB | A | A | A | A | A | A | A | A | |
| Measurement Index | V | M | K | V × M | V × K | M × K | V × M × K |
|---|---|---|---|---|---|---|---|
| P content in shoots | *** | *** | *** | *** | *** | *** | *** |
| P content in Roots | ns | ns | *** | *** | *** | *** | *** |
| P transport coefficient | ns | *** | ns | *** | *** | *** | *** |
| Mg content in shoots | ns | ns | ns | ns | ns | ns | ns |
| Mg content in roots | ns | *** | * | *** | ** | ** | ** |
| Mg translocation coefficient | ** | *** | *** | *** | *** | ns | ns |
| Ca content in shoots | ns | ns | *** | * | *** | * | *** |
| Ca content in roots | ** | *** | *** | *** | *** | * | *** |
| Ca translocation coefficient | ns | *** | *** | *** | *** | ns | *** |
Appendix B

References
- Wang, Y.; Chen, Y.F.; Wu, W.H. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2020, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Guillaume, T. Potassium dependency of enzymes in plant primary metabolism. Plant Physiol. Bioch. 2021, 166, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.X.; Song, X.; Cai, S.L.; Wang, P.Y.; Lu, G.D.; Yu, L.; Zhang, C.; Wu, Z.J. Overexpression of OsHAK5 potassium transporter enhances virus resistance in rice (Oryza sativa). Mol. Plant Pathol. 2022, 23, 1107–1121. [Google Scholar] [CrossRef]
- Yin, L.J.; Liao, Y.X.; Mou, X. Delayed sowing can improve potassium utilization efficiency and grain potassium concentration in winter wheat. Agriculture 2024, 14, 678. [Google Scholar] [CrossRef]
- Run, Y.H.; Cheng, X.Y.; Dou, W.; Dong, Y.; Zhang, Y.N.; Li, B.B.; Liu, T.F.; Xu, H.X. Wheat potassium transporter TaHAK13 mediates K+ absorption and maintains potassium homeostasis under low potassium stress. Front. Plant Sci. 2022, 13, 3389. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.K.; Liang, Y.Y.; Han, Y.L.; Han, Y.; Tan, J.F. High potassium application rate increased grain yield of shading-stressed winter wheat by improving photosynthesis and photosynthate translocation. Front. Plant Sci. 2020, 11, 3389. [Google Scholar] [CrossRef]
- Wang, C.W.; Xie, Y.K.; Tan, Z.X. Soil potassium depletion in global cereal croplands and its implications. Sci. Total Environ. 2024, 907, 167875. [Google Scholar] [CrossRef]
- Lu, D.J.; Dong, Y.H.; Chen, X.Q.; Wang, H.Y.; Zhou, J.M. Comparison of potential potassium leaching associated with organic and inorganic potassium sources in different arable soils in China. Pedosphere 2022, 32, 330–338. [Google Scholar] [CrossRef]
- Babar, S.; Baloch, A.; Qasim, M.; Wang, J.Y.; Wang, X.L.; Li, Y.X.; Khalid, S.; Jiang, C.C. Unearthing the soil-bacteria nexus to enhance potassium bioavailability for global sustainable agriculture: A mechanistic preview. Microbiol. Res. 2024, 288, 127885. [Google Scholar] [CrossRef]
- Duan, S.L.; Feng, G.; Limpens, E.; Bonfante, P.; Xie, X.N.; Zhang, L. Cross-kingdom nutrient exchange in the plant-arbuscular mycorrhizal fungus-bacterium continuum. Nat. Rev. Microbiol. 2024, 22, 773–790. [Google Scholar] [CrossRef]
- Sun, J.H.; Rong, Z.; Yang, L.; Zhu, Q.M.; Yuan, Y.B.; Feng, Z.P.; Li, L.M.; Li, N.X.; Zhang, L.; Guo, S.X. Effects of AMF inoculation on the growth, photosynthesis and root physiological morphology of root-pruned Robinia pseudoacacia seedlings. Tree Physiol. 2024, 44, 1. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.Z.; Choudhury, T.R.; Mridha, M.A.U. Arbuscular mycorrhizal fungi enhance biomass growth; mineral content; and antioxidant activity in tomato plants under drought stress. J. Food Qual. 2023, 2023, 581608. [Google Scholar] [CrossRef]
- Molaei, P.; Barzegar, T.; Fazli, M. Nutrient content amelioration in red lettuce growing in nutrient deficient soils via arbuscular mycorrhizal fungi. Plant Soil 2024, 508, 483–497. [Google Scholar] [CrossRef]
- Navarro, J.M.; Morte, A. Arbuscular mycorrhizal fungi as biofertilizers to increase the plant quality of sour-orange seedlings. Agronomy 2024, 14, 230. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, Y.; Xia, R.X.; Wu, Q.S.; Hashem, A.; Abd-Allah, E.F. Interactions between root hair development and arbuscular mycorrhizal fungal colonization in trifoliate orange seedlings in response to P levels. Agriculture 2024, 14, 763. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L.X. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 01068. [Google Scholar] [CrossRef]
- Li, J.W.; Han, T.F.; Liu, K.L.; Shen, Z.; Daba, N.A.; Tadesse, K.A.; Khan, M.N.; Shah, A.; Wang, Z.F.; Zhang, H.M. Optimizing potassium and nitrogen fertilizer strategies to mitigate greenhouse gas emissions in global agroecosystems. Sci. Total Environ. 2024, 916, 170270. [Google Scholar] [CrossRef]
- Brouns, F.; Aan, R.G.; Shewry, P.; Rustgi, S.; Jonkers, D. Adverse reactions to wheat or wheat components. Compr. Rev. Food Sci. F. 2019, 18, 1437–1452. [Google Scholar] [CrossRef]
- Das, D.; Sahoo, J.; Raza, M.B.; Barman, M.; Das, R. Ongoing soil potassium depletion under intensive cropping in india and probable mitigation strategies. Agron. Sustain. Dev. 2022, 42, 4. [Google Scholar] [CrossRef]
- Huang, S.; Gill, S.; Ramzan, M.; Ahmad, M.Z.; Danish, S.; Huang, P.; Al Obaid, S.; Alharbi, S.A. Uncovering the impact of AM fungi on wheat nutrient uptake; ion homeostasis; oxidative stress; and antioxidant defense under salinity stress. Sci. Rep. 2023, 13, 8249. [Google Scholar] [CrossRef]
- Wahab, A.; Muhammad, M.; Munir, A.; Abdi, G.; Zaman, W.; Ayaz, A.; Khizar, C.; Reddy, S.P.P. Role of arbuscular mycorrhizal fungi in regulating growth; enhancing productivity; and potentially influencing ecosystems under abiotic and biotic Stresses. Plants 2023, 12, 3102. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, T.W.; Jansa, J. Arbuscular mycorrhiza: Advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant Soil 2023, 489, 41–88. [Google Scholar] [CrossRef]
- Fang, C.; Xie, J.J.; Yang, X.Y.; Yang, R.Y.; Fransson, P.; Mohamed, S.S.; Wei, M.; Yang, H.H. Arbuscular mycorrhizal fungi drive soil nitrogen transformation under wheat varieties with different nitrogen utilization efficiencies. Rhizosphere 2023, 27, 100775. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, F.L. Arbuscular mycorrhiza improve growth; nitrogen uptake; and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Ingraffia, R.; Saia, S.; Giovino, A.; Amato, G.; Badagliacca, G.; Giambalvo, D.; Martinelli, F. Addition of high C:N crop residues to a P-limited substrate constrains the benefits of arbuscular mycorrhizal symbiosis for wheat P and N nutrition. Mycorrhiza 2021, 31, 441–454. [Google Scholar] [CrossRef]
- Xue, J.W.; Guo, L.L.; Li, L.L.; Zhang, Z.W.; Huang, M.; Cai, J.; Wang, X.; Zhong, Y.X.; Dai, T.B.; Jiang, D.; et al. Effects of arbuscular mycorrhizal fungi on uptake; partitioning and use efficiency of nitrogen in wheat. Field Crop Res. 2024, 306, 109244. [Google Scholar] [CrossRef]
- Yuan, J.; Shi, K.; Zhou, X.Y.; Wang, L.; Xu, C.; Zhang, H.; Zhu, G.P.; Si, C.C.; Wang, J.D.; Zhang, Y.C. Interactive impact of potassium and arbuscular mycorrhizal fungi on the root morphology and nutrient uptake of sweet potato (Ipomoea batatas L.). Front. Microbiol. 2023, 13, 2022. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Han, X.; Ren, W.; Zhang, H.Q.; Tang, M. Arbuscular mycorrhizal fungi improve Lycium barbarum potassium uptake by activating the expression of LbHAK. Plants 2024, 13, 1244. [Google Scholar] [CrossRef]
- Marro, N.; Grilli, G.; Soteras, F.; Caccia, M.; Longo, S.; Cofré, N.; Borda, V.; Burni, M.; Janoušková, M.; Urcelay, C. The effects of arbuscular mycorrhizal fungal species and taxonomic groups on stressed and unstressed plants: A global meta-analysis. New Phytol. 2022, 235, 320–332. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, G.Z.; Li, G.W.; Yuan, S.S.; Wang, C.Y.; Xie, Y.X.; Guo, T.C.; Kang, G.Z.; Wang, D.W. TaPHT1;9-4B and its transcriptional regulator TaMYB4-7D contribute to phosphate uptake and plant growth in bread wheat. New Phytol. 2021, 231, 1968–1983. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Liu, X.D.; Mao, W.W.; Zhang, X.R.; Chen, S.L.; Zhan, K.H.; Bi, H.H.; Xu, H.X. Genome-wide identification and analysis of HAK/KUP/KT potassium transporters gene family in wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2018, 19, 3969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Wang, M.Q.; Li, Y.; Wu, A.P.; Huang, J.Y. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Fan, X.; Zheng, W.; Gao, Z.; Yin, C.; Li, T.; Liang, Y. Silicon alleviates salt stress-induced potassium deficiency by promoting potassium uptake and translocation in rice (Oryza sativa L.). J Plant Physiol. 2021, 258–259, 153379. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ishikawa, T. Chemistry and metabolism of ascorbic acid in plants. In Ascorbic Acid in Plant Growth; Hossain, M., Munné-Bosch, S., Burritt, D., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Development and Stress Tolerance; Springer: Cham, Switzerland, 2017; pp. 1–23. [Google Scholar]
- Ishikawa, T.; Maruta, T.; Yoshimura, K.; Smirnoff, N. Biosynthesis and regulation of ascorbic acid in plants. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D.K., Palma, J.M., Corpas, F.J., Eds.; Springer: Cham, Switzerland, 2018; pp. 163–179. [Google Scholar]
- Zhang, B.; Shi, F.; Zheng, X.; Pan, H.Y.; Wen, Y.Q.; Song, F.Q. Effects of AMF compound inoculants on growth; ion homeostasis; and salt tolerance-related gene expression in Oryza sativa L. under salt treatments. Rice 2023, 16, 18. [Google Scholar] [CrossRef]
- Chen, X.; Aili, Y.; Ma, X.; Wang, H.O.; Dawuti, M. Mycorrhizal fungal colonization promotes apparent growth and physiology of Alhagi sparsifolia seedlings under salt or drought stress at vulnerable developmental stage. Plant Growth Regul. 2024, 102, 267–278. [Google Scholar] [CrossRef]
- Keyes, S.; Van Veelen, A.; McKay Fletcher, D.; Scotson, C.; Koebernick, N.; Petroselli, C.; Williams, K.; Ruiz, S.; Cooper, L.; Mayon, R.; et al. Multimodal correlative imaging and modelling of phosphorus uptake from soil by hyphae of mycorrhizal fungi. New Phytol. 2022, 234, 688–703. [Google Scholar] [CrossRef]
- Jiang, F.Y.; Zhang, L.; Zhou, J.C.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230, 304–315. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, G.; Declerck, S. Signal beyond nutrient; fructose; exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 2018, 12, 2339–2351. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wu, J.; Xie, K.; Li, X. LjAMT2;2 promotes ammonium nitrogen transport during arbuscular mycorrhizal fungi symbiosis in Lotus japonicus. Int. J. Mol. Sci. 2022, 23, 9522. [Google Scholar] [CrossRef] [PubMed]
- Rozmoš, M.; Bukovská, P.; Hršelová, H.; Kotianová, M.; Dudáš, M.; Gančarčíková, K.; Jansa, J. Organic nitrogen utilisation by an arbuscular mycorrhizal fungus is mediated by specific soil bacteria and a protist. ISME J. 2021, 16, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Maghrebi, M.; Novero, M.; Votta, C.; Mazzarella, T.; Buffoni, B.; Astolfi, S.; Vigani, G. Arbuscular mycorrhizal symbiosis differentially affects the nutritional status of two durum wheat genotypes under drought conditions. Plants 2022, 11, 804. [Google Scholar] [CrossRef]
- Xu, Y.J.; Yan, Y.X.; Zhou, T.Y.; Chun, J.H.; Tu, Y.C.; Yang, X.Y.; Qin, J.; Ou, L.; Ye, L.; Liu, F. Genome-wide transcriptome and gene family analysis reveal candidate genes associated with potassium uptake of maize colonized by arbuscular mycorrhizal fungi. BMC Plant Biol. 2024, 24, 838. [Google Scholar] [CrossRef]
- Han, X.; Zhou, Y.H.; Li, Y.P.; Ren, W.; Liu, K.K.; Zhang, W.R.; Zhang, H.Q.; Tang, M. LbKAT3 may assist in mycorrhizal potassium uptake; and overexpression of LbKAT3 may promote potassium; phosphorus; and water transport from arbuscular mycorrhizal fungi to the host plant. Front. Plant Sci. 2023, 14, 1161220. [Google Scholar] [CrossRef]
- Breuillin-Sessoms, F.; Floss, D.S.; Gomez, S.K.; Pumplin, N.; Ding, Y.; Levesque-Tremblay, V.; Noar, R.D.; Daniels, D.A.; Bravo, A.; Eaglesham, J.B.; et al. Suppression of arbuscule degeneration in Medicago truncatula phosphate transporter4 mutants is dependent on the ammonium transporter 2 family protein AMT2;3. Plant Cell 2015, 27, 1352–1366. [Google Scholar] [CrossRef]
- Guether, M.; Balestrini, R.; Hannah, M.; He, J.; Udvardi, M.K.; Bonfante, P. Genome-wide reprogramming of regulatory networks; transport; cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol. 2009, 182, 200–212. [Google Scholar] [CrossRef]
- Shao, Y.D.; Hu, X.C.; Wu, Q.S.; Yang, T.Y.; Srivastava, A.K.; Zhang, D.J.; Gao, X.B.; Kuča, K. Mycorrhizas promote P acquisition of tea plants through changes in root morphology and P transporter gene expression. S. Afr. J. 2021, 137, 455–462. [Google Scholar] [CrossRef]
- Wu, T.; Li, P.; Zipori, I.; Mao, J.H.; Li, R.B.; Li, Y.P.; Li, Y.J.; Jing, Y.B.; Chen, H.Y. Arbuscular mycorrhizal fungi enhanced the growth; phosphorus uptake and Pht expression of olive (Olea europaea L.) plantlets. Peer J. 2022, 10, e13813. [Google Scholar] [CrossRef]
- Rong, Z.L.; Lei, A.Q.; Wu, Q.S.; Srivastava, A.K.; Hasnem, A.; Abd-Allah, E.F.; Kuča, K.; Yang, T.Y. Serendipita indica promotes P acquisition and growth in tea seedlings under P deficit conditions by increasing cytokinins and indoleacetic acid and phosphate transporter gene expression. Front. Plant Sci. 2023, 14, 1146182. [Google Scholar] [CrossRef]
- Chen, X.; Bai, Y.; Lin, Y.; Liu, H.; Han, F.; Chang, H.; Li, M.; Liu, Q. Genome-wide identification and characterization of the PHT1 gene family and its response to mycorrhizal symbiosis in salvia miltiorrhiza under phosphate Stress. Genes 2024, 15, 589. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Yan, M.; Li, G.; Liu, M.; Zhao, P.; Zhang, Z.; Zhang, Q.; Zhu, X.; Wang, J.; Yu, Y.; et al. Comparative physiological and transcriptome analysis between potassium-deficiency tolerant and sensitive sweet potato genotypes in response to potassium-deficiency stress. BMC Genom. 2024, 25, 61. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Peng, X.Q.; Xuan, H.G.; Wei, L.T.; Yang, Y.Y.; Guo, T.C.; Kang, G.Z. Proteomic analysis of leaves and roots of common wheat (Triticum aestivum L.) under copper-stress conditions. J. Proteome Res. 2013, 12, 4846–4861. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, H.Q.; Nie, Z.J.; Tao, Z.K.; Peng, H.Y.; Shi, H.Z.; Zhao, P.; Liu, H.G. Combined application of arbuscular mycorrhizal fungi and selenium fertilizer increased wheat biomass under cadmium stress and shapes rhizosphere soil microbial communities. BMC Plant Biol. 2024, 24, 359. [Google Scholar] [CrossRef]
- Elmes, R.P.; Mosse, B. Vesicular-arbuscular endomycorrhizal inoculum production. II. Experiments with maize (Zea mays) and other hosts in nutrient flow culture. Can J Bot. 1984, 62, 1531–1536. [Google Scholar] [CrossRef]
- Vierheilig, H.; Coughlan, A.P.; Wyss, U.; Piché, Y. Ink and vinegar; a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microb. 1998, 64, 12. [Google Scholar] [CrossRef]
- Plenchette, C.; Fortin, J.A.; Furlan, V. Growth responses of several plant species to mycorrhizae in a soil of moderate P-fertility. Plant Soil 1983, 70, 199–209. [Google Scholar] [CrossRef]
- Li, G.Z.; Wu, Y.F.; Liu, G.Y.; Xiao, X.H.; Wang, P.F.; Gao, T.; Xu, M.J.; Han, Q.X.; Wang, Y.H.; Guo, T.C.; et al. Large-scale proteomics combined with transgenic experiments demonstrates an important role of jasmonic acid in potassium deficiency response in wheat and rice. Mol. Cell. Proteom. 2017, 16, 1889–1905. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio Protoc. 2012, 2, e263. [Google Scholar] [CrossRef]
- Ma, L.L.; Qing, C.Y.; Zhang, M.Y.; Zou, C.Y.; Pan, G.T.; Shen, Y.U. GWAS with a PCA uncovers candidate genes for accumulations of microelements in maize seedlings. Physiol. Plantarum. 2021, 172, 2170–2180. [Google Scholar] [CrossRef]
- Yang, Z.J.; Wu, X.H.; Chen, L.H.; Huang, L.M.; Chen, Y.; Wu, J.; El-Kassaby, Y.A.; Grossnickle, S.C.; Feng, J.L. Fertilization regulates accumulation and allocation of biomass and nutrients in phoebe bournei seedlings. Agriculture 2021, 11, 1187. [Google Scholar] [CrossRef]
- Wang, J.D.; Hou, P.F.; Zhu, G.P.; Dong, Y.; Hui, Z.; Ma, H.B.; Xu, X.J.; Nin, Y.W.; Ai, Y.H.; Zhang, Y.C. Potassium partitioning and redistribution as a function of K-use efficiency under K deficiency in sweet potato (Ipomoea batatas L.). Field Crop Res. 2017, 211, 147–154. [Google Scholar] [CrossRef]
- Li, G.Z.; Liu, J.; Chen, S.J.; Wang, P.F.; Liu, H.T.; Dong, J.; Zheng, Y.X.; Xie, Y.X.; Wang, C.Y.; Guo, T.C.; et al. Melatonin promotes potassium deficiency tolerance by regulating HAK1 transporter and its upstream transcription factor NAC71 in wheat. J. Pineal Res. 2021, 70, e12727. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Z.; Liu, J.; Wang, Y.Y.; Han, A.Q.; Liu, H.T.; Guo, T.C.; Han, Q.X.; Kang, G.Z. TaWRKY24-1D, interacts with TaERFL1a, regulates DHAR-mediated ASA-GSH biosynthesis to enhance drought tolerance in wheat. Plant Growth Regul. 2024, 104, 713–725. [Google Scholar] [CrossRef]
- Eltigani, A.; Müller, A.; Ngwene, B.; George, E. Physiological and morphological responses of Okra (Abelmoschus esculentus L.) to rhizoglomus irregulare Inoculation under ample water and drought Stress conditions are variety dependent. Plants 2022, 11, 89. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, A.-Q.; Chen, S.-B.; Zhang, D.-D.; Liu, J.; Zhang, M.-C.; Wang, B.; Xiao, Y.; Liu, H.-T.; Guo, T.-C.; Kang, G.-Z.; et al. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Nutrient Uptake in Wheat Under Low Potassium Stress. Plants 2025, 14, 1288. https://doi.org/10.3390/plants14091288
Han A-Q, Chen S-B, Zhang D-D, Liu J, Zhang M-C, Wang B, Xiao Y, Liu H-T, Guo T-C, Kang G-Z, et al. Effects of Arbuscular Mycorrhizal Fungi on the Growth and Nutrient Uptake in Wheat Under Low Potassium Stress. Plants. 2025; 14(9):1288. https://doi.org/10.3390/plants14091288
Chicago/Turabian StyleHan, An-Qi, Shuai-Bo Chen, Dan-Dan Zhang, Jin Liu, Meng-Chuan Zhang, Bin Wang, Yue Xiao, Hai-Tao Liu, Tian-Cai Guo, Guo-Zhang Kang, and et al. 2025. "Effects of Arbuscular Mycorrhizal Fungi on the Growth and Nutrient Uptake in Wheat Under Low Potassium Stress" Plants 14, no. 9: 1288. https://doi.org/10.3390/plants14091288
APA StyleHan, A.-Q., Chen, S.-B., Zhang, D.-D., Liu, J., Zhang, M.-C., Wang, B., Xiao, Y., Liu, H.-T., Guo, T.-C., Kang, G.-Z., & Li, G.-Z. (2025). Effects of Arbuscular Mycorrhizal Fungi on the Growth and Nutrient Uptake in Wheat Under Low Potassium Stress. Plants, 14(9), 1288. https://doi.org/10.3390/plants14091288






