Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
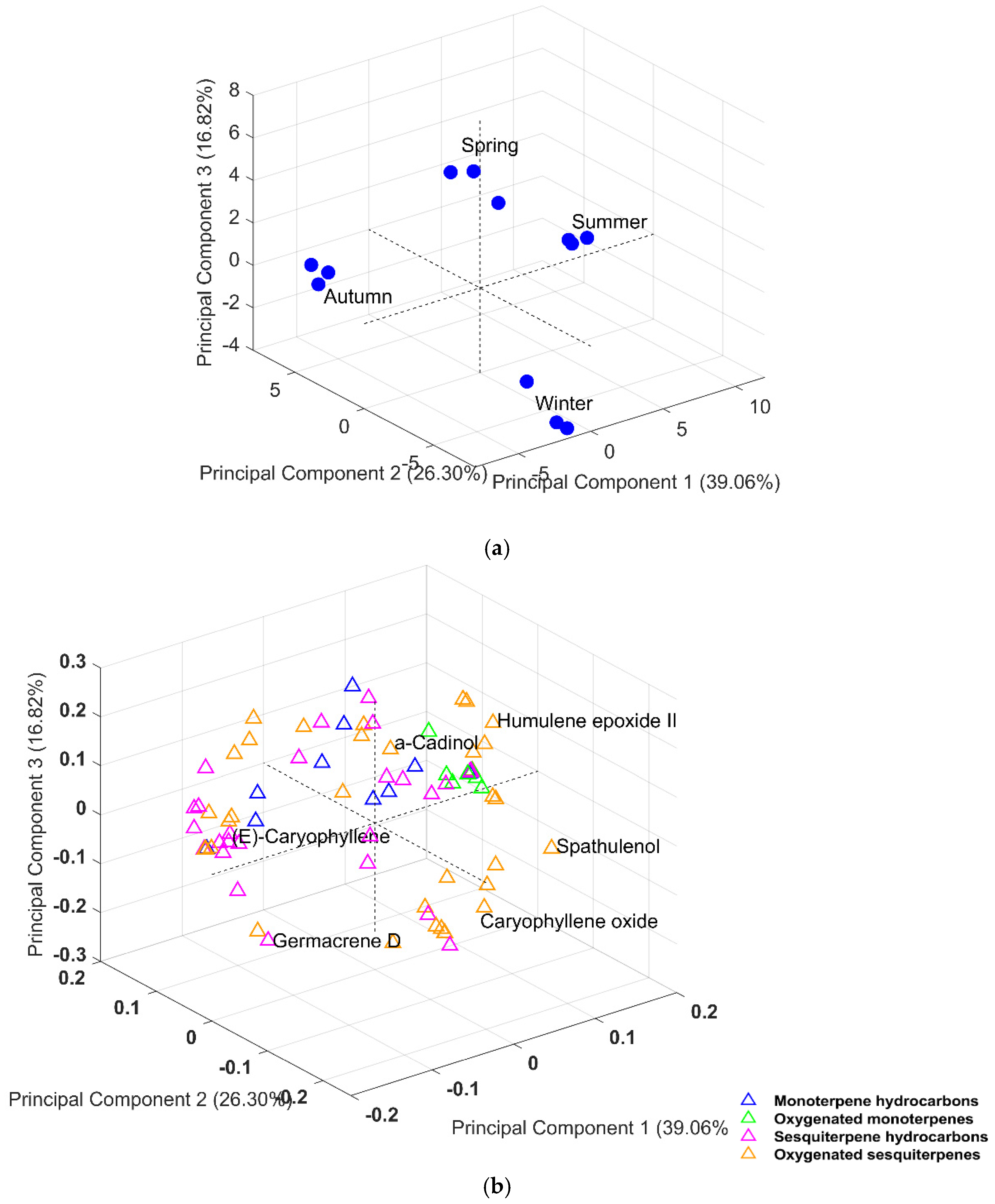
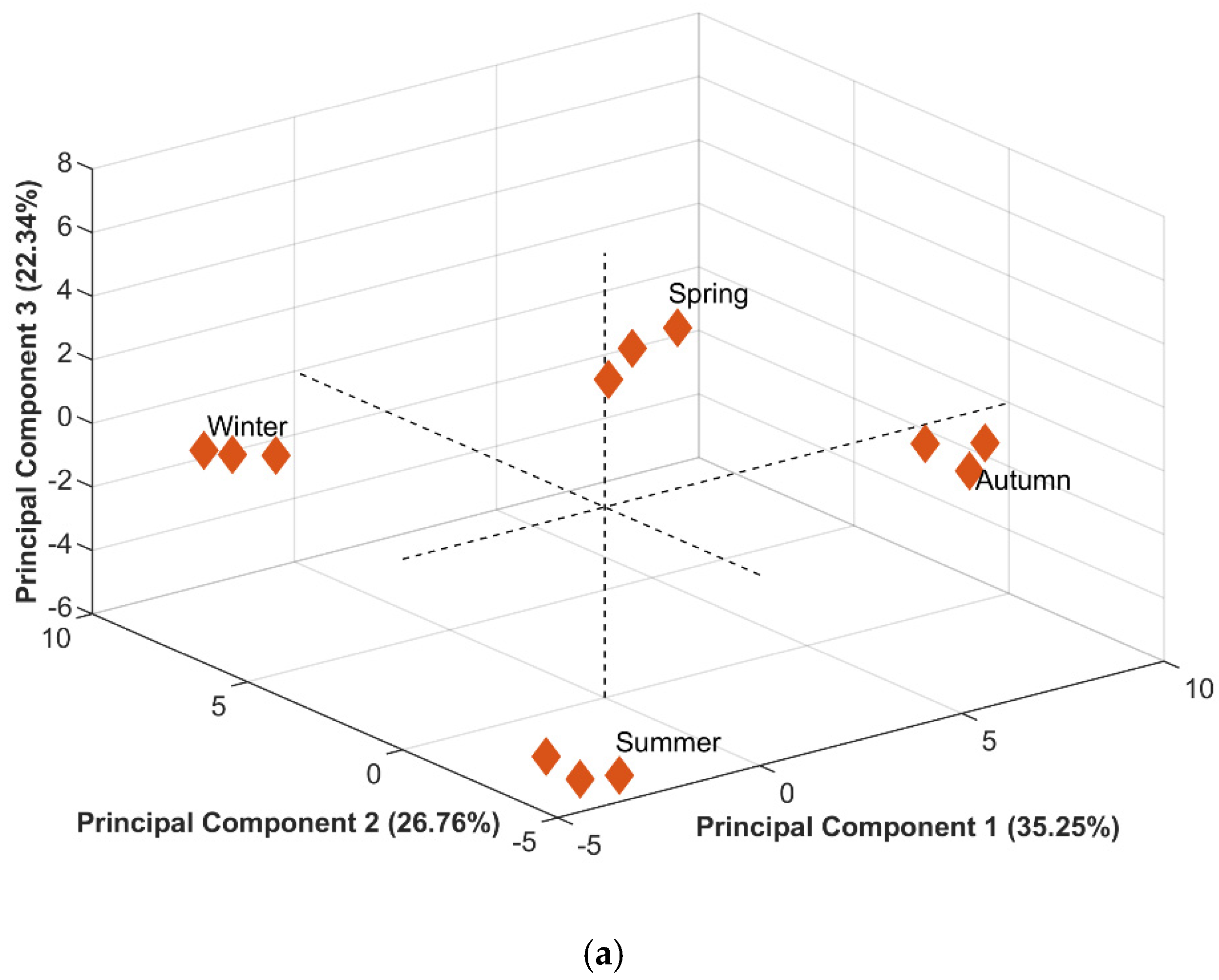
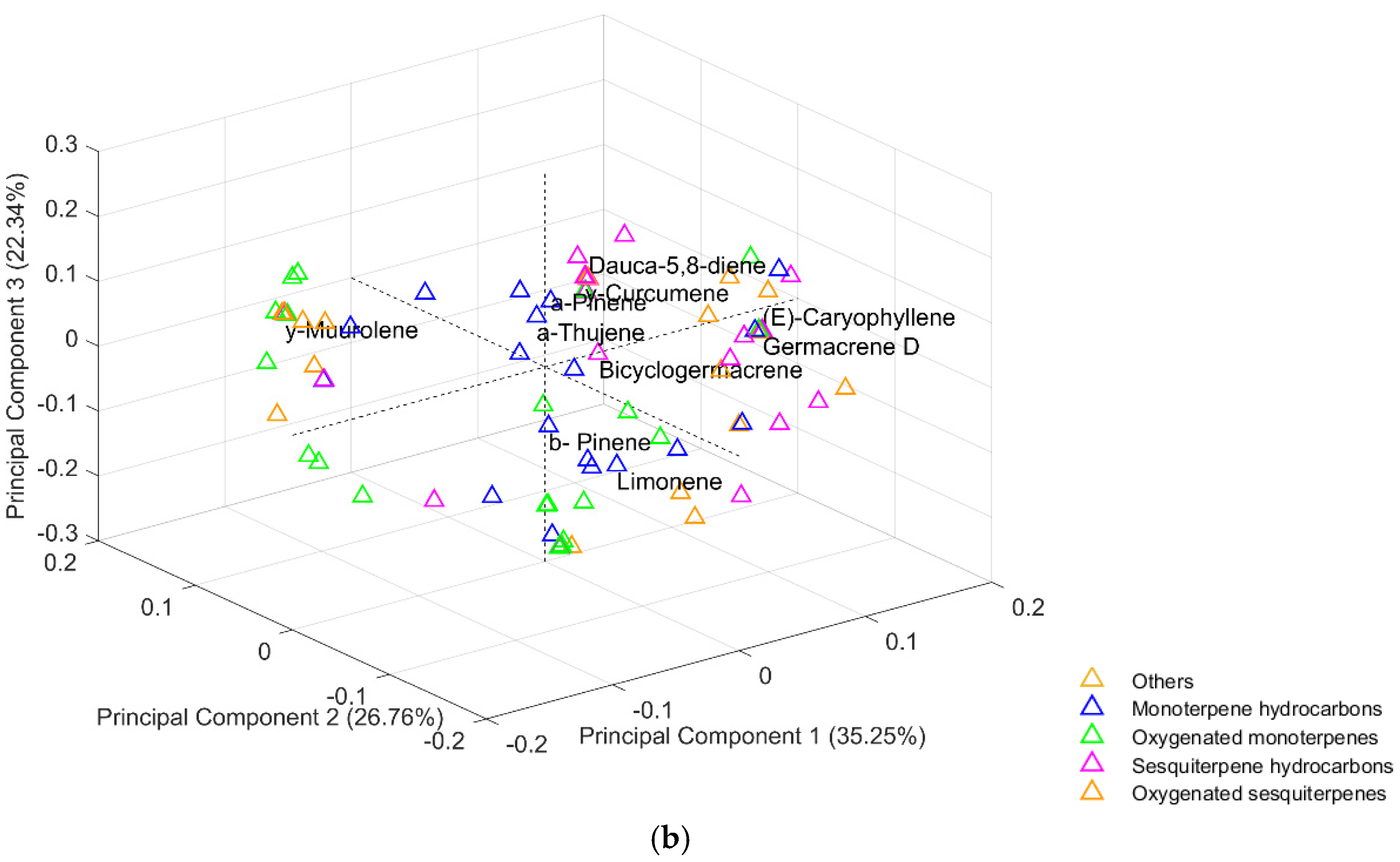
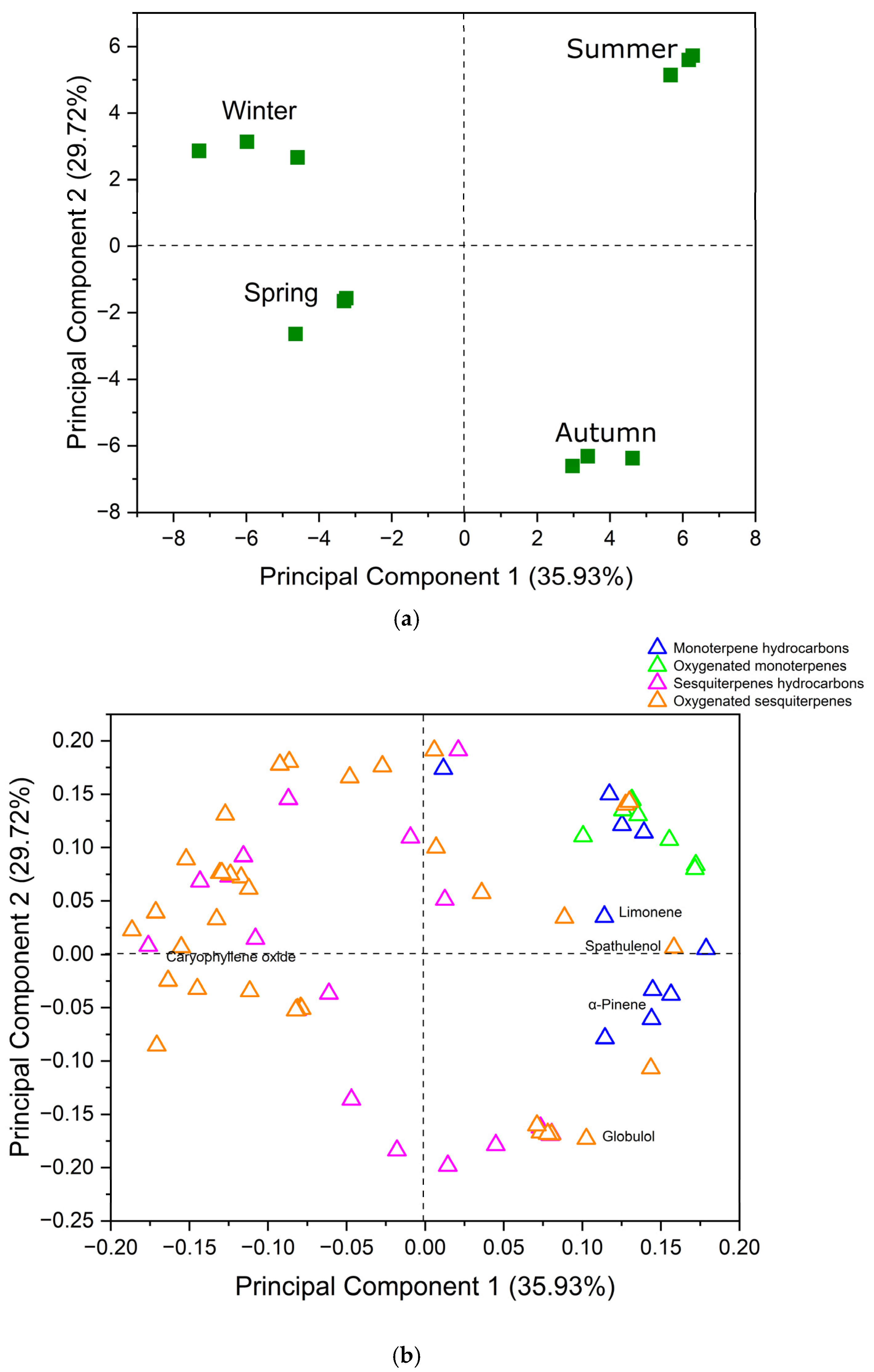
2.2. Principal Component Analysis (PCA)
2.3. Antimicrobial Evaluation
2.4. Anti-Proliferative Activity Evaluation
3. Materials and Methods
3.1. Plant Material
3.2. Isolation and Analysis of Essential Oil
Statistical Analysis
3.3. Biological Activity Screening
3.3.1. Antimicrobial Activity
3.3.2. Anti-Proliferative Activity Assay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grecco, S.D.S.; Ferreira, M.J.; Romoff, P.; Favero, O.A.; Lago, J.H.G. Phenolic Derivatives from Baccharis retusa DC. (Asteraceae). Biochem. Syst. Ecol. 2012, 42, 21–24. [Google Scholar] [CrossRef]
- Sayuri, V.A.; Romoff, P.; Fávero, O.A.; Ferreira, M.J.P.; Lago, J.H.G.; Buturi, F.O.S. Chemical Composition, Seasonal Variation, and Biosynthetic Considerations of Essential Oils from Baccharis microdonta and B. elaeagnoides (Asteraceae). Chem. Biodivers. 2010, 7, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Ascari, J.; de Oliveira, M.S.; Nunes, D.S.; Granato, D.; Scharf, D.R.; Simionatto, E.; Otuki, M.; Soley, B.; Heiden, G. Chemical Composition, Antioxidant and Anti-Inflammatory Activities of the Essential Oils from Male and Female Specimens of Baccharis punctulata (Asteraceae). J. Ethnopharmacol. 2019, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Minteguiaga, M.; Umpiérrez, N.; Fariña, L.; Falcão, M.A.; Xavier, V.B.; Cassel, E.; Dellacassa, E. Impact of Gas Chromatography and Mass Spectrometry Combined with Gas Chromatography and Olfactometry for the Sex Differentiation of Baccharis articulata by the Analysis of Volatile Compounds. J. Sep. Sci. 2015, 38, 3038–3046. [Google Scholar] [CrossRef]
- Minteguiaga, M.; Umpiérrez, N.; Xavier, V.; Lucas, A.; Mondin, C.; Fariña, L.; Cassel, E.; Dellacassa, E. Recent Findings in the Chemistry of Odorants from Four Baccharis Species and Their Impact as Chemical Markers. Chem. Biodivers. 2015, 12, 1339–1348. [Google Scholar] [CrossRef]
- Silva, F.G.; Oliveira, C.B.A.; Pinto, J.E.B.P.; Nascimento, V.E.; Santos, S.C.; Seraphin, J.C.; Ferri, P.H. Seasonal Variability in the Essential Oils of Wild and Cultivated Baccharis trimera. J. Braz. Chem. Soc. 2007, 18, 990–997. [Google Scholar] [CrossRef]
- Besten, M.A.; Jasinski, V.C.G.; Costa, Â.D.G.L.C.; Nunes, D.S.; Sens, S.L.; Wisniewski, A., Jr.; Simionatto, E.L.; Riva, D.; Dalmarco, J.B.; Granato, D. Chemical Composition Similarity between the Essential Oils Isolated from Male and Female Specimens of Each Five Baccharis Species. J. Braz. Chem. Soc. 2012, 23, 1041–1047. [Google Scholar] [CrossRef]
- Sosa, M.E.; Lancelle, H.G.; Tonn, C.E.; Andres, M.F.; Gonzalez-Coloma, A. Insecticidal and Nematicidal Essential Oils from Argentinean eupatorium and Baccharis spp. Biochem. Syst. Ecol. 2012, 43, 132–138. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; dos Santos Barbosa, C.R.; Muniz, D.F.; da Silva, A.C.A.; Rocha, J.E.; de Morais Oliveira-Tintino, C.D.; Ribeiro-Filho, J.; da Silva, L.E.; Confortin, C.; et al. GC-MS-FID and Potentiation of the Antibiotic Activity of the Essential Oil of Baccharis reticulata (Ruiz & Pav.) Pers. and α-Pinene. Ind. Crops Prod. 2020, 145, 112106. [Google Scholar] [CrossRef]
- Kurdelas, R.R.; López, S.; Lima, B.; Feresin, G.E.; Zygadlo, J.; Zacchino, S.; López, M.L.; Tapia, A.; Freile, M.L. Chemical Composition, Anti-Insect and Antimicrobial Activity of Baccharis darwinii Essential Oil from Argentina, Patagonia. Ind. Crops Prod. 2012, 40, 261–267. [Google Scholar] [CrossRef]
- Ferronatto, R.; Marchesan, E.D.; Pezenti, E.; Bednarski, F.; Onofre, S.B. Atividade Antimicrobiana de Óleos Essenciais Produzidos Por Baccharis dracunculifolia D.C. e Baccharis uncinella D.C. (Asteraceae). Rev. Bras. Farmacogn. 2007, 17, 224–230. [Google Scholar] [CrossRef]
- Ascari, J.; Sens, S.L.; Nunes, D.S.; Wisniewski, A.; Arbo, M.D.; Linck, V.M.; Lunardi, P.; Leal, M.B.; Elisabetsky, E. Sedative Effects of Essential Oils Obtained from Baccharis uncinella. Pharm. Biol. 2012, 50, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Pertot, I. Climate Change Impacts on Plant Pathogens and Plant Diseases. J. Crop Improv. 2014, 28, 99–139. [Google Scholar] [CrossRef]
- Kalariya, K.A.; Mevada, R.R.; Meena, R.P.; Das, M. Biotic Stress Nexus: Integrating Various Physiological Processes in Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2024, 43, 100574. [Google Scholar] [CrossRef]
- Sartor, T.; Xavier, V.B.; Falcão, M.A.; Mondin, C.A.; Dos Santos, M.A.; Cassel, E.; Astarita, L.V.; Santarém, E.R. Seasonal Changes in Phenolic Compounds and in the Biological Activities of Baccharis dentata (Vell.) G.M. Barroso. Ind. Crops Prod. 2013, 51, 355–359. [Google Scholar] [CrossRef]
- Botrel, P.P.; Pinto, J.E.B.P.; Ferraz, V.; Bertolucci, S.K.V.; Figueiredo, F.C. Teor e Composição Química Do Óleo Essencial de Hyptis marrubioides Epl., Lamiaceae Em Função da Sazonalidade. Acta Sci. Agron. 2010, 32, 533–538. [Google Scholar] [CrossRef]
- Minteguiaga, M.; González, A.; Catalán, C.A.N.; Dellacassa, E. Relationship between Baccharis dracunculifolia DC. and B. microdonta DC. (Asteraceae) by Their Different Seasonal Volatile Expression. Chem. Biodivers. 2021, 18, e2100064. [Google Scholar] [CrossRef]
- Liebelt, D.J.; Jordan, J.T.; Doherty, C.J. Only a Matter of Time: The Impact of Daily and Seasonal Rhythms on Phytochemicals. Phytochem. Rev. 2019, 18, 1409–1433. [Google Scholar] [CrossRef]
- Xavier, V.B.; Vargas, R.M.F.; Cassel, E.; Lucas, A.M.; Santos, M.A.; Mondin, C.A.; Santarem, E.R.; Astarita, L.V.; Sartor, T. Mathematical Modeling for Extraction of Essential Oil from Baccharis spp. by Steam Distillation. Ind. Crops Prod. 2011, 33, 599–604. [Google Scholar] [CrossRef]
- De Sousa, J.P.B.; Jorge, R.F.; Leite, M.F.; Furtado, N.A.J.C.; Bastos, J.K.; Da Silva Filho, A.A.; Queiroga, C.L.; De Magalhães, P.M.; Soares, A.E.E. Seasonal Variation of the (E)-Nerolidol and Other Volatile Compounds within Ten Different Cultivated Populations of Baccharis dracunculifolia D.C. (Asteraceae). J. Essent. Oil Res. 2009, 21, 308–314. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Serafini, L.A.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential Oil of Baccharis uncinella DC. From Southern Brazil. Flavour. Fragr. J. 2001, 16, 286–288. [Google Scholar] [CrossRef]
- Agostini, F.; Santos, A.C.A.; Rossato, M.; Pansera, M.R.; Zattera, F.; Wasum, R.; Serafini, L.A. Estudo Do Óleo Essencial de Algumas Espécies Do Gênero Baccharis (Asteraceae) Do Sul Do Brasil. Rev. Bras. Farmacogn. 2005, 15, 215–219. [Google Scholar] [CrossRef]
- Fabiane, K.C.; Ferronatto, R.; Santos, A.C.d.; Onofre, S.B. Physicochemical Characteristics of the Essential Oils of Baccharis dracunculifolia and Baccharis uncinella D.C. (Asteraceae). Rev. Bras. Farmacogn. 2008, 18, 197–203. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Atti-Serafini, L.; Laguna, S.E.; Cassel, E.; Lorenzo, D.; Dellacassa, E. Essential Oil Variability in Baccharis uncinella DC and Baccharis dracunculifolia DC Growing Wild in Southern Brazil, Bolivia and Uruguay. Flavour. Fragr. J. 2008, 23, 99–106. [Google Scholar] [CrossRef]
- Lago, J.H.G.; Romoff, P.; Fávero, O.A.; Soares, M.G.; Baraldi, P.T.; Corrêa, A.G.; Souza, F.O. Composição Química Dos Óleos Essenciais Das Folhas de Seis Espécies Do Gênero Baccharis de “Campos de Altitude” Da Mata Atlântica Paulista. Quim. Nova 2008, 31, 727–730. [Google Scholar] [CrossRef]
- van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in Chemical Composition of Eucalyptus globulus Essential Oil under Phenological Stages and Evidence Synergism with Antimicrobial Standards. Ind. Crops Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Santos, V.M.C.S.; Pinto, M.A.S.; Bizzo, H.; Deschamps, C. Seasonal Variation of Vegetative Growth, Essential Oil Yield and Composition of Menthol Mint Genotypes at Southern Brazil. Biosci. J. 2012, 28, 790–798. [Google Scholar]
- Maciel Tomazzoli, M.; Do Amaral, W.; Raupp Cipriano, R.; Cerioni Belniaki, A.; de Cássia Tomasi, J.; Maia, B.H.L.N.S.; Deschamps, C. Chemical Analyses and Antioxidant Activity of the Essential Oils from Baccharis dracunculifolia DC. In Southern Brazil. J. Essent. Oil Res. 2024, 36, 342–352. [Google Scholar] [CrossRef]
- Ueno, A.K.; Barcellos, A.F.; Grecco, S.d.S.; Sartorelli, P.; Guadagnin, R.C.; Romoff, P.; Ferreira, M.J.P.; Tcacenco, C.M.; Lago, J.H.G. Sesquiterpenes, Diterpenes, Alkenyl p-Coumarates, and Flavonoid from the Aerial Parts of Baccharis retusa (Asteraceae). Biochem. Syst. Ecol. 2018, 78, 39–42. [Google Scholar] [CrossRef]
- Ueno, A.K.; Barcellos, A.F.; Costa-Silva, T.A.; Mesquita, J.T.; Ferreira, D.D.; Tempone, A.G.; Romoff, P.; Antar, G.M.; Lago, J.H.G. Antitrypanosomal Activity and Evaluation of the Mechanism of Action of Diterpenes from Aerial Parts of Baccharis retusa (Asteraceae). Fitoterapia 2018, 125, 55–58. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.T.; de Souza, M.T.; Bernardi, D.; de Melo, D.J.; Zarbin, P.H.G.; Zawadneak, M.A.C. Insecticidal and Oviposition Deterrent Effects of Essential Oils of Baccharis spp. and Histological Assessment against Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2021, 11, 3944. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.; Holl, D. Antimicrobial Natural Product Research: A Review from a South African Perspective for the Years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and Antimicrobial Activity of Essential Oils from Aromatic Plants Used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial Activity of Essential Oil of Baccharis dracunculifolia DC (Asteraceae) Aerial Parts at Flowering Period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; dos Santos Barbosa, C.R.; Muniz, D.F.; Rocha, J.E.; de Araújo Neto, J.B.; da Silva, M.M.C.; Silva Pereira, R.L.; da Silva, L.E.; do Amaral, W.; et al. Characterization and Antibacterial Activity of the Essential Oil Obtained from the Leaves of Baccharis coridifolia DC against Multiresistant Strains. Microb. Pathog. 2020, 145, 104223. [Google Scholar] [CrossRef]
- Bobek, V.B.; Cruz, L.S.; Oliveira, C.F.D.; Betim, F.C.M.; Swiech, J.N.D.; Folquitto, D.G.; Ito, C.A.S.; Budel, J.M.; Zanin, S.M.W.; Paula, J.D.F.P.D.; et al. Chemical Composition and Biological Activity of Baccharis erioclada DC. Essential Oil. Braz. J. Pharm. Sci. 2022, 58, e19118. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; LD Jayaweera, S.; A Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Borges, M.F.d.A.; Lacerda, R.d.S.; Correia, J.P.d.A.; de Melo, T.R.; Ferreira, S.B. Potential Antibacterial Action of α-Pinene. Med. Sci. Forum 2022, 12, 11. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, 12918. [Google Scholar] [CrossRef]
- Santana, C.B.; Souza, J.G.d.L.; Coracini, M.D.A.; Walerius, A.H.; Soares, V.D.; Costa, W.F.d.; Pinto, F.G.d.S. Chemical Composition of Essential Oil from Myrcia oblongata DC and Potencial Antimicrobial, Antioxidant and Acaricidal Activity against Dermanyssus gallinae (Degeer, 1778). Biosci. J. 2018, 34, 996–1009. [Google Scholar] [CrossRef]
- de Medeiros, J.P.; Rodrigues, S.A.; Sakumoto, K.; Ruiz, S.P.; Faria, M.G.I.; Gonçalves, J.E.; Piau Junior, R.; Glamočlija, J.; Soković, M.; Gonçalves, D.D.; et al. Bioactives of the Essential Oil from the Leaves of Eugenia pyriformis Cambess (Myrtaceae) on the Effects of Tobacco. Front. Pharmacol. 2024, 15, 1415659. [Google Scholar] [CrossRef]
- Budel, J.M.; Wang, M.; Raman, V.; Zhao, J.; Khan, S.I.; Rehman, J.U.; Techen, N.; Tekwani, B.; Monteiro, L.M.; Heiden, G.; et al. Essential Oils of Five Baccharis Species: Investigations on the Chemical Composition and Biological Activities. Molecules 2018, 23, 2620. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Fouche, G.; Cragg, G.M.; Pillay, P.; Kolesnikova, N.; Maharaj, V.J.; Senabe, J. In Vitro Anticancer Screening of South African Plants. J. Ethnopharmacol. 2008, 119, 455–461. [Google Scholar] [CrossRef]
- do Nascimento, K.F.; Moreira, F.M.F.; Santos, J.A.; Kassuya, C.A.L.; Croda, J.H.R.; Cardoso, C.A.L.; do Carmo Vieira, M.; Ruiz, A.L.T.G.; Foglio, M.A.; de Carvalho, J.E.; et al. Antioxidant, Anti-Inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium guineense Sw. and Spathulenol. J. Ethnopharmacol. 2018, 210, 351–358. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.d.; dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer Activity of Limonene: A Systematic Review of Target Signaling Pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- CLSI—Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. In Approved Standard M27-A3, 4th ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI—Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. In Approved Standard M07-A, 9th ed.; CLSI Document 9: Wayne, PA, USA, 2012. [Google Scholar]
- Sobreiro, M.A.; Della Torre, A.; de Araújo, M.E.M.B.; Canella, P.R.B.C.; de Carvalho, J.E.; Carvalho, P.d.O.; Ruiz, A.L.T.G. Enzymatic Hydrolysis of Rutin: Evaluation of Kinetic Parameters and Anti-Proliferative, Mutagenic and Anti-Mutagenic Effects. Life 2023, 13, 549. [Google Scholar] [CrossRef]
- de Souza, L.B.; Tinti, S.V.; Sousa, I.M.d.O.; Montanari, I.; da Costa, J.L.; de Carvalho, J.E.; Foglio, M.A.; Ruiz, A.L.T.G. Mentha aquatica L. Aerial Parts: In Vitro Anti-Proliferative Evaluation on Human Tumour and Non-Tumour Cell Lines. Nat. Prod. Res. 2022, 36, 3117–3123. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.G.; Della Torre, A.; Braga, L.E.d.O.; Bachiega, P.; Tinti, S.V.; de Carvalho, J.E.; Dionísio, A.P.; Ruiz, A.L.T.G. Yellow-Colored Extract from Cashew Byproduct—Nonclinical Safety Assessment. Regul. Toxicol. Pharmacol. 2020, 115, 104699. [Google Scholar] [CrossRef] [PubMed]
- Vaca Meza, E.T.; Vasquez-Kool, J.; Costilla Sánchez, N.I.; Vieira, A.; Rodrigues, R.A.F.; Sartoratto, A.; Flores Granados, A.d.P.; Marin Tello, C.L.; Ruiz, A.L.T.G. Chemical Composition and Anti-Proliferative Activity of Essential Oils from Some Medicinal Plants from Cachicadán, Región La Libertad, Perú. Nat. Prod. Res. 2024, 38, 2145–2150. [Google Scholar] [CrossRef] [PubMed]

| Nº | AI | Compound | Relative Content (%) b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baccharis calvescens | Baccharis retusa | Baccharis uncinella | ||||||||||||
| Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | |||
| 1 | 920 | α-Thujene | 0.3 ± 0.1 | 0.7 ± 0.2 | 1.1 ± 0.1 | 0.8 ± 0.3 | 5 ± 3 | 8.7 ± 1.6 | 5.0 ± 1.6 | 4.1 ± 1.2 | 0.8 ± 0.8 | 1.9 ± 0.3 | 2.4 ± 0.2 | 2.2 ± 0.5 |
| 2 | 928 | α-Pinene | 2 ± 1 | 2.2 ± 0.4 | 4.2 ± 0.4 | 2.6 ± 0.5 | 6 ± 3 | 11 ± 3.0 | 5.5 ± 1.5 | 5.2 ± 1.4 | 2.5 ± 1.9 | 4.7 ± 0.9 | 6.1 ± 0.4 | 5.8 ± 1.0 |
| 3 | 939 | α-Fenchene | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.2 | 0 | 0 | 0 | 0 | 0 |
| 4 | 966 | Sabinene | 0.2 ± 0.2 | 0.4 ± 0.2 | 0.3 ± 0.3 | 0.6 ± 0.2 | 1.9 ± 1.1 | 2.0 ± 1.3 | 4.0 ± 0.2 | 0.8 ± 0.2 | 0.6 ± 0.3 | 0.8 ± 0.2 | 1.2 ± 0.3 | 1.4 ± 0.4 |
| 5 | 971 | β-Pinene | 2 ± 1 | 3.2 ± 0.7 | 4.7 ± 0.6 | 4.4 ± 0.9 | 3.4 ± 1.6 | 5.9 ± 0.5 | 5.6 ± 0.1 | 2.7 ± 0.6 | 2 ± 1 | 3.6 ± 0.6 | 3.5 ± 0.2 | 3.8 ± 0.7 |
| 6 | 984 | Myrcene | 0 | 0 | 0.5 ± 0.1 | 0.9 ± 0.2 | 2.0 ± 0.7 | 2.4 ± 0.2 | 2.1 ± 0.1 | 1.7 ± 0.4 | 0.6 ± 0.2 | 0.8 ± 0.1 | 1.10 ± 0.03 | 0 |
| 7 | 999 | α-Phellandrene | 0 | 0 | 0 | 0 | 0.3 ± 0.3 | 0.7 ± 0.2 | 0.75 ± 0.04 | 0.7 ± 0.2 | 0 | 0 | 0 | 0 |
| 8 | 1003 | δ-3-Carene | 0 | 0 | 0 | 0 | 0.4 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | 1012 | α-Terpinene | 0 | 0 | 0.3 ± 0.3 | 0.2 ± 0.1 | 0.5 ± 0.2 | 1.3 ± 0.2 | 1.37 ± 0.03 | 0.8 ± 0.3 | 0.3 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.1 | 0.5 ± 0.1 |
| 10 | 1016 | p-Cymene | 0 | 0 | 0.4 ± 0.2 | 0 | 1.6 ± 0.4 | 1.3 ± 0.4 | 1.3 ± 0.6 | 0.6 ± 0.1 | 0.23 ± 0.03 | 0.18 ± 0.01 | 0.8 ± 0.1 | 0.7 ± 0.2 |
| 11 | 1022 | o-Cymene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.1 | 0 | 0 | 0 | 0 |
| 12 | 1025 | Limonene | 1.0 ± 0.4 | 2.9 ± 0.5 | 3.2 ± 0.4 | 2.4 ± 0.4 | 2.5 ± 0.7 | 6.4 ± 0.3 | 12 ± 0.0 | 3.7 ± 0.0 | 3.9 ± 0.9 | 5.3 ± 0.8 | 5.8 ± 0.4 | 4.8 ± 0.44 |
| 13 | 1036 | (Z)-β-Ocimene | 0 | 0 | 0 | 0 | 0 | 0.5 ± 0.2 | 0.6 ± 0.1 | 0 | 0 | 0 | 0 | 0 |
| 14 | 1044 | (E)-β-Ocimene | 0 | 0 | 0 | 0.9 ± 0.2 | 0.8 ± 0.3 | 0 | 0 | 0.5 ± 0.1 | 0 | 0 | 0 | 0 |
| 15 | 1052 | γ-Terpinene | 0.1 ± 0.1 | 0 | 0.5 ± 0.1 | 0.3 ± 0.1 | 1.0 ± 0.2 | 2.1 ± 0.5 | 1.6 ± 0.1 | 1.0 ± 0.9 | 0.6 ± 0.1 | 0.9 ± 0.2 | 1.4 ± 0.1 | 0.7 ± 0.1 |
| 16 | 1058 | cis-Sabinene hydrate | 0 | 0 | 0 | 0 | 0.3 ± 0.1 | 0 | 0.3 ± 0.1 | 0.4 ± 0.6 | 0 | 0 | 0 | 0 |
| 17 | 1078 | Mentha-2,4(8)-diene | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.1 | 0.9 ± 0.1 | 0 | 0 | 0 | 0 | 0 |
| 18 | 1082 | Terpinolene | 0 | 0 | 0 | 0 | 0.5 ± 0.1 | 0 | 0 | 0.6 ± 0.2 | 0.3 ± 0.3 | 0.2 ± 0.1 | 0.9 ± 0.1 | 0.24 ± 0.02 |
| 19 | 1086 | p-Cymenene | 0 | 0 | 0 | 0 | 0 | 0.20 ± 0.03 | 0 | 0.3 ± 0.1 | 0 | 0 | 0 | 0 |
| 20 | 1091 | Linalool | 0.1 ± 0.2 | 0 | 0.7 ± 0.1 | 0 | 0 | 0.3 ± 0.03 | 0 | 0.3 ± 0.1 | 0 | 0 | 0.64 ± 0.04 | 0 |
| 21 | 1092 | trans-Sabinene hydrate | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.1 | 0 | 0 | 0 | 0 | 0 |
| 22 | 1106 | Perillene | 0 | 0 | 0 | 0 | 0 | 0.2 ± 0.1 | 0.5 ± 0.04 | 0 | 0 | 0 | 0 | 0 |
| 23 | 1107 | trans-Thujone | 0 | 0 | 0 | 0 | 0 | 0 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 |
| 24 | 1112 | cis-p-Menth-2-en-1-ol | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.04 | 0 | 0 | 0 | 0.53 ± 0.04 | 0 |
| 25 | 1115 | trans-p-Mentha-2,8-dien-1-ol | 0 | 0 | 0 | 0 | 1 ± 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 26 | 1118 | α-Campholenal | 0 | 0 | 0 | 0 | 0.7 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 | 1135 | trans-Pinocarveol | 0.1 ± 0.2 | 0 | 1.7 ± 0.4 | 0 | 0.9 ± 0.1 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0.8 ± 0.1 | 0.33 ± 0.03 |
| 28 | 1136 | cis-Verbenol | 0 | 0 | 0 | 0 | 0.5 ± 0.1 | 0.2 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | 1138 | trans-Verbenol | 0 | 0 | 1.1 ± 0.1 | 0 | 1.7 ± 0.4 | 0 | 0.5 ± 0.2 | 0 | 0 | 0 | 0.3 ± 0.3 | 0 |
| 30 | 1158 | Isoborneol | 0 | 0 | 0 | 0 | 0 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 31 | 1160 | Pinocarvone | 0 | 0 | 1.3 ± 0.3 | 0 | 0.3 ± 0.1 | 0 | 0.4 ± 0.04 | 0 | 0 | 0 | 0.41 ± 0.04 | 0 |
| 32 | 1167 | Borneol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.1 | 0 | 0 | 0 | 0 |
| 33 | 1168 | p-Mentha-1,5-dien-8-ol | 0 | 0 | 0.7 ± 0.3 | 0 | 0.8 ± 0.2 | 0 | 0.7 ± 0.2 | 0 | 0 | 0 | 0.7 ± 0.2 | 0.16 ± 0.01 |
| 34 | 1173 | Terpinen-4-ol | 0.1 ± 0.2 | 0.6 ± 0.1 | 1.2 ± 0.3 | 0.4 ± 0.3 | 2.0 ± 0.4 | 2.8 ± 0.2 | 2.9 ± 0.3 | 2.4 ± 0.5 | 1.3 ± 0.3 | 1.5 ± 0.3 | 2.9 ± 0.3 | 1.4 ± 0.2 |
| 35 | 1175 | Naphthalene | 0 | 0 | 0 | 0 | 1.7 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 36 | 1179 | Cryptone | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 ± 0.2 | 0 | 0 | 0 | 0 | 0 |
| 37 | 1186 | α-Terpineol | 0 | 0 | 0.9 ± 0.2 | 0.2 ± 0.2 | 0.5 ± 0.2 | 1.3 ± 0.1 | 1.2 ± 0.02 | 0.7 ± 0.6 | 0.4 ± 0.1 | 0.5 ± 0.1 | 1.3 ± 0.2 | 0.5 ± 0.1 |
| 38 | 1190 | Myrtenol | 0 | 0 | 2.2 ± 0.5 | 0 | 0.9 ± 0.2 | 0 | 0.8 ± 0.2 | 0 | 0 | 0 | 0.7 ± 0.1 | 0.3 ± 0.1 |
| 39 | 1210 | trans-Carveol | 0 | 0 | 0.7 ± 0.2 | 0 | 0 | 0 | 0.2 ± 0.2 | 0 | 0 | 0 | 0.5 ± 0.1 | 0 |
| 40 | 1230 | Cumin aldehyde | 0 | 0 | 0 | 0 | 0 | 0 | 0.7 ± 0.1 | 0 | 0 | 0 | 0 | 0 |
| 41 | 1234 | Carvone | 0 | 0 | 0.4 ± 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 42 | 1264 | p-Menth-1-en-7-al | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 ± 0.3 | 0 | 0 | 0 | 0 | 0 |
| 43 | 1274 | α-Terpinen-7-al | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.1 | 0 | 0 | 0 | 0 | 0 |
| 44 | 1330 | δ-Elemene | 0 | 0 | 0 | 0 | 0.7 ± 0.1 | 0.8 ± 0.4 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0 | 0.2 ± 0.1 | 0.5 ± 0.1 | 0.25 ± 0.02 |
| 45 | 1371 | α-Copaene | 0.9 ± 0.4 | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.1 | 0 | 0 | 0.5 ± 0.1 | 1.0 ± 0.1 | 0.4 ± 0.2 | 0.5 ± 0.2 | 0 | 0.25 ± 0.02 |
| 46 | 1386 | β-Bourbonene | 0 | 2.3 ± 0.1 | 0 | 0.61 ± 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 47 | 1390 | β-Elemene | 0.2 ± 0.4 | 0 | 1.0 ± 0.1 | 4.2 ± 0.8 | 1.0 ± 0.2 | 1.1 ± 0.6 | 1.1 ± 0.1 | 1.8 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.1 ± 0.2 | 1.07 ± 0.04 |
| 48 | 1409 | (Z)-Caryophyllene | 0 | 0 | 2.5 ± 0.3 | 0 | 5.0 ± 1.4 | 5.0 ± 0.6 | 4.4 ± 0.1 | 0 | 2.9 ± 0.4 | 4.3 ± 0.2 | 2.3 ± 0.4 | 0 |
| 49 | 1412 | α-Gurjunene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 ± 0.1 | 0.9 ± 0.1 | 0.31 ± 0.03 |
| 50 | 1423 | (E)-Caryophyllene | 4 ± 2 | 4.7 ± 0.3 | 0 | 5.96 ± 0.06 | 0 | 0 | 0 | 9.1 ± 0.2 | 0 | 0 | 0 | 4.6 ± 0.2 |
| 51 | 1435 | γ-Elemene | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 |
| 52 | 1436 | α-Guaiene | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 53 | 1444 | Aromadendrene | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 ± 0.2 | 0.8 ± 0.1 | 0 | 0 | 0 | 0 |
| 54 | 1451 | α-Humulene | 0.8 ± 0.7 | 2.9 ± 0.3 | 1.8 ± 0.2 | 4.6 ± 0.3 | 0.8 ± 0.0 | 0.5 ± 0.2 | 0 | 1.2 ± 0.1 | 1.05 ± 0.04 | 1.3 ± 0.2 | 0.9 ± 0.1 | 1.4 ± 0.1 |
| 55 | 1460 | allo-Aromadendrene | 0.2 ± 0.3 | 0.4 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 56 | 1462 | cis-Cadina-1(6),4-diene | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.1 | 0 | 0 | 0.38 ± 0.04 | 0.5 ± 0.1 | 0 | 0 |
| 57 | 1464 | 9-epi-(E)-Caryophyllene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.0 ± 0.1 | 0 | 0.5 ± 0.1 | 0 | 0.22 ± 0.02 |
| 58 | 1465 | cis-Muurola-4(14),5-diene | 0 | 0 | 0.6 ± 0.1 | 0 | 0 | 0 | 3.3 ± 0.2 | 0 | 0 | 1.4 ± 0.3 | 0 | 0 |
| 59 | 1471 | Dauca-5,8-diene | 0.2 ± 0.4 | 0.31 ± 0.02 | 0.8 ± 0.3 | 0.2 ± 0.2 | 0 | 5 ± 2 | 0 | 0.1 ± 0.1 | 0 | 0 | 0 | 0 |
| 60 | 1477 | trans-Cadina-1(6),4-diene | 0 | 0 | 0 | 1.2 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 61 | 1478 | γ-Gurjunene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.4 | 0 | 0 |
| 62 | 1479 | γ-Muurolene | 0.6 ± 0.5 | 0.6 ± 0.1 | 0.7 ± 0.6 | 1.8 ± 0.3 | 5.4 ± 2.1 | 0 | 0 | 0 | 1.3 ± 0.4 | 0.1 ± 0.1 | 0.6 ± 0.1 | 0 |
| 63 | 1481 | γ-Himachalene | 0 | 0 | 0.2 ± 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 64 | 1483 | γ-Curcumene | 0 | 0 | 0 | 0 | 0 | 6.1 ± 0.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| 65 | 1485 | α-Amorphene | 0 | 0 | 0.4 ± 0.3 | 0 | 0 | 0.7 ± 0.6 | 0 | 0 | 0 | 0.5 ± 0.1 | 0 | 0 |
| 66 | 1486 | Germacrene D | 5 ± 1 | 1.6 ± 0.1 | 1.4 ± 0.2 | 6.6 ± 0.3 | 0 | 0 | 0 | 11.7 ± 0.7 | 0 | 0 | 0 | 0 |
| 67 | 1487 | cis-Eudesma-6,11-diene | 0.6 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 68 | 1489 | β-Selinene | 0.2 ± 0.1 | 0.9 ± 0.2 | 0 | 1.63 ± 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 69 | 1492 | trans-Muurola-4(14),5-diene | 0.2 ± 0.3 | 0.4 ± 0.1 | 0 | 1.05 ± 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 70 | 1497 | γ-Amorphene | 0.7 ± 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 71 | 1499 | Viridiflorene | 0 | 1.6 ± 0.1 | 0 | 1 ± 2 | 0 | 0 | 0 | 0 | 0.6 ± 0.2 | 0 | 0 | 0 |
| 72 | 1500 | Bicyclogermacrene | 2.7 ± 0.9 | 1.4 ± 0.4 | 0 | 5 ± 2 | 5 ± 1 | 0 | 2.8 ± 0.4 | 8.3 ± 0.1 | 1.1 ± 0.1 | 0 | 1.4 ± 0.3 | 0 |
| 73 | 1501 | α-Muurolene | 0.7 ± 0.1 | 0.6 ± 0.1 | 0 | 0.3 ± 0.6 | 0 | 0 | 0 | 0 | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.1 | 2.78 ± 0.04 |
| 74 | 1510 | Germacrene A | 0 | 0 | 0 | 1.18 ± 0.09 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 75 | 1511 | δ-Amorphene | 0 | 0 | 1.8 ± 0.3 | 0 | 1.0 ± 0.1 | 0 | 1.2 ± 0.1 | 0.6 ± 0.0 | 0 | 1.8 ± 0.2 | 1.3 ± 0.1 | 0 |
| 76 | 1512 | trans-Cycloisolongifol-5-ol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.7 ± 0.3 | 0 | 0 | 0 |
| 77 | 1514 | γ-Cadinene | 0.1 ± 0.2 | 0.3 ± 0.3 | 0 | 0.8 ± 0.1 | 0 | 0 | 0 | 1.0 ± 0.1 | 0.5 ± 0.1 | 0.1 ± 0.2 | 0 | 0 |
| 78 | 1518 | Cubebol | 0.3 ± 0.6 | 0.06 ± 0.04 | 0 | 0.9 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 79 | 1519 | (Z)-γ-Bisabolene | 1 ± 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 80 | 1521 | δ-Cadinene | 2 ± 1 | 2.5 ± 0.2 | 0 | 3.9 ± 0.1 | 0 | 1.4 ± 0.1 | 0 | 2.4 ± 0.2 | 2.0 ± 0.5 | 0 | 0 | 0.74 ± 0.05 |
| 81 | 1537 | trans-Cadina-1,4-diene | 0 | 0 | 0 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 82 | 1542 | α-Cadinene | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.30 ± 0.0 | 0 | 0 | 0 | 0 |
| 83 | 1548 | α-Calacorene | 0.3 ± 0.3 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0 | 0 | 0 | 0.30 ± 0.0 | 0 | 0 | 0 | 0 |
| 84 | 1551 | Elemol | 0 | 0 | 0 | 1.2 ± 0.2 | 0 | 0 | 0 | 0 | 0.08 ± 0.14 | 0 | 0 | 1.29 ± 0.09 |
| 85 | 1554 | Germacrene B | 0 | 0 | 0 | 0 | 0 | 0.9 ± 0.5 | 0.9 ± 0.0 | 1.3 ± 0.4 | 0 | 0 | 0 | 0 |
| 86 | 1560 | (E)-Nerolidol | 0 | 0 | 1.3 ± 0.1 | 1 ± 0 | 0.40 ± 0.4 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.8 | 0.4 ± 0.0 |
| 87 | 1565 | β-Calacorene | 0 | 0.4 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 88 | 1566 | Maaliol | 0 | 0 | 0 | 0 | 0 | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.2 ± 0.3 | 0.9 ± 0.1 | 0 | 0 |
| 89 | 1567 | Palustrol | 0 | 0 | 0 | 0.6 ± 0.0 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 90 | 1572 | Longipinanol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.1 |
| 91 | 1577 | Spathulenol | 15 ± 3.0 | 10 ± 1.0 | 13 ± 1.0 | 2.9 ± 0.2 | 10 ± 3 | 9 ± 3 | 8.1 ± 0.4 | 4.3 ± 0.5 | 11 ± 1 | 11.0 ± 0.6 | 15 ± 1 | 14.6 ± 0.8 |
| 92 | 1578 | Caryophyllene oxide | 12 ± 2.0 | 7 ± 1 | 5 ± 1 | 3.2 ± 0.2 | 7 ± 4 | 4 ± 1 | 2.5 ± 0.5 | 0 | 5 ± 2 | 6.6 ± 1.8 | 0 | 0 |
| 93 | 1582 | Thujopsan-2α-ol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.29 ± 0.04 | 0 | 0 |
| 94 | 1585 | allo-Hedycaryol | 0 | 0 | 0 | 0.7 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 95 | 1590 | Globulol | 1.1 ± 0.1 | 0 | 1.2 ± 0.04 | 0 | 1.0 ± 0.2 | 1.2 ± 0.2 | 0 | 3.9 ± 0.5 | 0 | 2.8 ± 0.3 | 1.9 ± 0.3 | 5.6 ± 0.4 |
| 96 | 1593 | Viridiflorol | 0 | 0.3 ± 0.6 | 0 | 1.04 ± 0.06 | 0 | 0 | 0 | 1.8 ± 0.3 | 0 | 0 | 0 | 0 |
| 97 | 1594 | Salvial-4(14)-en-1-one | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.2 ± 0.2 | 0 | 0 | 0 |
| 98 | 1597 | cis-dihydro-Mayurone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 ± 0.4 | 0 | 0 |
| 99 | 1604 | Rosifoliol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 ± 0.2 | 0 | 0 | 0 | 0 |
| 100 | 1605 | Ledol | 1.9 ± 0.8 | 0 | 0.99 ± 0.02 | 1.2 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 101 | 1607 | Humulene epoxide II | 2.2 ± 0.3 | 4 ± 1 | 5.7 ± 0.4 | 1.4 ± 0.1 | 1.1 ± 0.2 | 0 | 0.6 ± 0.0 | 0 | 3.2 ± 0.2 | 2.1 ± 0.3 | 1.9 ± 0.1 | 2.2 ± 0.3 |
| 102 | 1614 | cis-Isolongifolanone | 0 | 1.6 ± 0.3 | 0 | 0.61 ± 0.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.2 | 0 |
| 103 | 1617 | Junenol | 0.5 ± 0.1 | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 104 | 1619 | 1,10-di-epi-Cubenol | 0 | 0 | 1.51 ± 0.03 | 0 | 0 | 0 | 0 | 0.8 ± 0.2 | 0 | 1.4 ± 0.8 | 0 | 0 |
| 105 | 1620 | 10-epi-γ-Eudesmol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 ± 0.3 | 0 | 0 | 0 |
| 106 | 1630 | 1-epi-Cubenol | 1 ± 1 | 2.3 ± 0.1 | 0 | 2.7 ± 0.3 | 0 | 0 | 0 | 0.7 ± 0.2 | 2.4 ± 0.3 | 1.0 ± 0.1 | 1.67 ± 0.03 | 0 |
| 107 | 1633 | γ-Eudesmol | 0 | 0 | 0 | 1.6 ± 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 108 | 1636 | cis-Cadin-4-en-7-ol | 2 ± 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 ± 0.2 | 0 | 0 |
| 109 | 1639 | epi-α-Cadinol | 0 | 0 | 0 | 0 | 0 | 1.8 ± 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 110 | 1640 | allo-Aromadendrene epoxide | 0 | 0 | 0 | 0 | 1.1 ± 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 111 | 1642 | allo-Aromadendrene epoxide | 0.3 ± 0.1 | 0 | 1.1 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.03 ± 0.06 | 3.5 ± 0.5 |
| 112 | 1643 | Caryophylla-4(12),8(13)-dien-5β-ol | 0 | 1 ± 1 | 2.2 ± 0.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 113 | 1644 | epi-α-Muurolol | 0.9 ± 0.5 | 4 ± 2 | 1.4 ± 0.1 | 3.6 ± 0.3 | 0.8 ± 0.1 | 0 | 2.2 ± 0.4 | 3.9 ± 0.9 | 4.2 ± 1.3 | 3.2 ± 0.3 | 1.4 ± 0.1 | 0 |
| 114 | 1645 | α-Muurolol | 1.0 ± 0.7 | 1.8 ± 0.2 | 1.3 ± 0.1 | 2.1 ± 0.3 | 0 | 0 | 0.9 ± 0.4 | 0.6 ± 0.6 | 1.9 ± 0.2 | 1.9 ± 0.1 | 1.3 ± 0.1 | 0 |
| 115 | 1646 | Cubenol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 ± 1.3 | 0 | 0 | 0 |
| 116 | 1650 | β-Eudesmol | 0 | 1.3 ± 0.5 | 1.1 ± 0.1 | 1.6 ± 0.2 | 0.9 ± 0.3 | 0 | 0 | 0 | 2.0 ± 0.1 | 1.8 ± 0.1 | 0 | 1.3 ± 0.2 |
| 117 | 1653 | α-Cadinol | 1.6 ± 0.9 | 6.4 ± 0.7 | 0 | 0 | 1.6 ± 1.4 | 1.8 ± 0.3 | 2.0 ± 1.0 | 3.3 ± 0.8 | 3.4 ± 0.3 | 3.6 ± 0.1 | 1.9 ± 0.1 | 1.6 ± 0.2 |
| 118 | 1655 | allo-himachalol | 0 | 0 | 0 | 0 | 0 | 1.1 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 119 | 1659 | Selin-11-en-4α-ol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.71 ± 0.04 | 0 | 0 |
| 120 | 1665 | 14-hydroxy-(Z)-Caryophyllene | 0.5 ± 0.4 | 0 | 2.3 ± 0.1 | 0 | 0 | 0 | 1.6 ± 0.2 | 0.1 ± 0.1 | 2.2 ± 2.1 | 2 ± 1 | 0 | 0.9 ± 0.1 |
| 121 | 1668 | (Z)-α-Santalol | 0 | 3.5 ± 0.7 | 4.3 ± 0.7 | 1.2 ± 0.2 | 0 | 0 | 0 | 0 | 0.8 ± 1.4 | 1 ± 1 | 1.43 ± 0.02 | 0 |
| 122 | 1671 | Guaia-3,10(14)-dien-11-ol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.1 ± 0.3 |
| 123 | 1674 | Khusinol | 1.4 ± 1.1 | 0 | 1.1 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 124 | 1680 | Elemol acetate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.1 | 0 |
| 125 | 1682 | Germacra-4(15),5,10(14)-trien-1α-ol | 0.7 ± 0.3 | 1.4 ± 0.1 | 0.9 ± 0.3 | 0 | 0 | 0.35 ± 0.04 | 0 | 0.7 ± 0.4 | 3.3 ± 0.6 | 2.1 ± 0.2 | 0 | 0.4 ± 0.1 |
| 126 | 1689 | Eudesma-4(15),7-dien-1β-ol | 0.6 ± 0.1 | 0 | 0 | 0.9 ± 0.2 | 0 | 0 | 0.7 ± 0.2 | 0.5 ± 0.2 | 1.2 ± 0.2 | 0 | 1.2 ± 0.0 | 0 |
| 127 | 1690 | Shyobunol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 ± 0.2 | 0 | 0 | 0 |
| 128 | 1705 | Amorpha-4,9-dien-2-ol | 0.9 ± 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.3 | 0.2 ± 0.2 | 0.6 ± 0.1 | 0.6 ± 0.1 |
| 129 | 1712 | cis-Thujopsenal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.9 ± 0.2 | 0 | 0.6 ± 0.1 | 0 |
| 130 | 1714 | Longifolol | 0.3 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 131 | 1733 | Curcumenol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.4 ± 0.1 | 0 | 0 |
| 132 | 1737 | Isobicyclogermacrenal | 0 | 0.7 ± 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 ± 0.1 | 1.1 ± 0.1 | 0 | 0 |
| 133 | 1743 | γ-Costol | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.1 | 1.4 ± 0.2 |
| 134 | 1751 | Cyclocolorenone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.2 | 0 | 0.7 ± 0.2 | 0 |
| 135 | 1752 | β-Acoradienol | 0 | 0.5 ± 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 136 | 1774 | 14-oxy-α-Muurolene | 0.5 ± 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 137 | 1777 | Squamulosone | 0 | 0.7 ± 0.2 | 0.9 ± 0.1 | 0 | 0 | 0 | 0 | 0 | 1.0 ± 0.3 | 0.5 ± 0.1 | 0 | 0.4 ± 0.4 |
| 138 | 1779 | α-Costol | 0.4 ± 0.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Samples | S. aureus | E. coli | P. aeruginosa | S. choleraesuis | C. albicans | B. subtilis | S. epidermidis |
|---|---|---|---|---|---|---|---|
| Baccharis uncinella | |||||||
| Spring | 0.062 | 0.125 | * | 0.125 | 0.062 | 0.125 | * |
| Summer | 0.125 | 0.125 | * | 0.25 | 0.062 | 0.062 | * |
| Autumn | 0.125 | 0.125 | * | 0.25 | 0.125 | 0.125 | * |
| Winter | 0.125 | 0.25 | * | 0.50 | 0.25 | 0.125 | * |
| Baccharis retusa | |||||||
| Spring | 0.125 | 0.25 | * | 0.50 | 0.25 | 0.125 | * |
| Summer | 0.125 | 0.25 | * | 0.50 | 0.50 | 0.125 | * |
| Autumn | 0.125 | 0.25 | * | 0.50 | 0.50 | 0.125 | * |
| Baccharis calvescens | |||||||
| Spring | 0.125 | 0.25 | * | 0.50 | 0.50 | 0.125 | * |
| Summer | 0.25 | 0.25 | * | 0.50 | 0.125 | 0.125 | * |
| Autumn | 0.25 | 0.50 | * | 0.50 | 1.0 | 0.125 | * |
| Thymus vulgaris [34] | 1.0 | * | * | 0.6 | 2.0 | 0.5 | * |
| Chloramphenicol | 0.008 | 0.004 | 0.062 | 0.004 | 0.004 | 0.008 | |
| Nystatin | 0.002 | ||||||
| Samples b | TGI (µg/mL) a | MeanTGI | TGI (µg/mL) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| U251 | UACC-62 | MCF-7 | NCI-ADR/Res | 786-0 | NCI-H460 | OVCAR-03 | HT29 | K562 | HaCaT | ||
| Doxorubicin | 10 * | 0.35 ± 0.06 | 4 * | 16 * | 0.8 ± 0.1 | 0.17 ± 0.09 | 2 ± 1 | >25 | 1.5 * | > 6.6 | 1.9 * |
| Baccharis uncinella | |||||||||||
| Autumn | 56 ± 2 | 62 ± 11 | 60 ± 2 | 73 ± 13 | 67 ± 4 | 61 ± 9 | 40 ± 4 | 72 ± 5 | 22 ± 1 | 57.0 | 57 ± 5 |
| Winter | 99 ± 24 | 82 ± 42 | 157 ± 24 | >250 | 52 * | 112 * | 19 * | 134 ± 37 | 52 * | >106.3 | 83 ± 22 |
| Spring | 43 * | 44 ± 21 | 50 ± 18 | 53 * | 38 * | 56 * | 10 * | 50 * | 3 ± 1 | 38.6 | 34 * |
| Summer | 47 ± 1 | 41 ± 5 | 24 ± 5 | 61 ± 5 | 49 ± 5 | 50 ± 2 | 25 ± 2 | 28 ± 3 | 19 ± 7 | 38.2 | 69.2 ± 0.3 |
| Baccharis retusa | |||||||||||
| Autumn | 70 ± 29 | 42 * | 58 * | 72 * | 65 ± 23 | 48 * | 67 ± 11 | 50 * | 8.5 * | 53.4 | 77 * |
| Winter | 30.5 ± 0.8 | 10 ± 3 | 31 ± 2 | 44 ± 7 | 38 ± 3 | 25 ± 3 | 17 * | 28 ± 4 | 7.2 ± 0.2 | 25.6 | 29 ± 3 |
| Spring | 55 ± 11 | 46 ± 19 | 56 ± 7 | 58 ± 4 | 70 ± 3 | 57 ± 13 | 4 * | 56 ± 20 | 18 ± 6 | 46.7 | 60 ± 3 |
| Summer | 37 * | 17 * | 9 * | 31 * | 17 * | 23 * | >250 | 15 * | 2 * | 44.6 | 36 * |
| Baccharis calvescens | |||||||||||
| Autumn | 15 * | 15 ± 3 | 13 ± 2 | 34 ± 7 | 16 ± 7 | 16 ± 8 | 16 ± 7 | 20 ± 4 | n.t. | 18.1 | n.t. |
| Winter | 14 ± 2 | 8 ± 2 | 1.3 ± 0.4 | 33 * | 15 ± 7 | 9 ± 1 | 2.4 ± 0.1 | 6 ± 3 | n.t. | 11.1 | n.t. |
| Spring | 6.6 ± 0.9 | 4 ± 1 | <0.25 | 28 * | 10 ± 3 | 6 ± 1 | <0.25 | 4 ± 1 | n.t. | <7.4 | n.t. |
| Summer | 47 ± 2 | 41 ± 6 | 24 ± 8 | 61 ± 7 | 49 ± 7 | 50 ± 2 | 25 ± 2 | 46 ± 2 | n.t. | 42.9 | n.t. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dlugoviet, T.F.; Ferriani, A.P.; Hendges, A.P.P.K.; Camargo, R.G.; Duarte, M.C.T.; Duarte, R.M.T.; Ruiz, A.L.T.G.; Nagata, N.; Marques, F.A.; Sales Maia, B.H.L.N. Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae). Plants 2025, 14, 1311. https://doi.org/10.3390/plants14091311
Dlugoviet TF, Ferriani AP, Hendges APPK, Camargo RG, Duarte MCT, Duarte RMT, Ruiz ALTG, Nagata N, Marques FA, Sales Maia BHLN. Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae). Plants. 2025; 14(9):1311. https://doi.org/10.3390/plants14091311
Chicago/Turabian StyleDlugoviet, Tânia F., Aurea P. Ferriani, Ana Paula P. Klein Hendges, Rebeca G. Camargo, Marta C. T. Duarte, Renata M. T. Duarte, Ana Lúcia Tasca Gois Ruiz, Noemi Nagata, Francisco A. Marques, and Beatriz H. L. N. Sales Maia. 2025. "Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae)" Plants 14, no. 9: 1311. https://doi.org/10.3390/plants14091311
APA StyleDlugoviet, T. F., Ferriani, A. P., Hendges, A. P. P. K., Camargo, R. G., Duarte, M. C. T., Duarte, R. M. T., Ruiz, A. L. T. G., Nagata, N., Marques, F. A., & Sales Maia, B. H. L. N. (2025). Seasonal Chemical Variability and Antimicrobial, Anti-Proliferative Potential of Essential Oils from Baccharis uncinella, B. retusa, and B. calvescens (Asteraceae). Plants, 14(9), 1311. https://doi.org/10.3390/plants14091311








