Abstract
HD-Zip (homeodomain-leucine zipper) transcription factors play a crucial role in plant growth, development, and stress response; however, the HD-Zip gene family of Coix lacryma-jobi L. has not been identified. In this study, a total of 40 HD-Zip gene family members were identified in the genome of Coix. According to phylogenetic analysis, the Coix HD-Zip gene was divided into four subfamilies (I–IV), of which the HD-Zip I subfamily can be further divided into five branches. Moreover, HD-Zip members of the same subfamily usually share similar gene structures and conserved motifs. The transcription factor binding site enrichment analysis showed that there are many motifs for binding with transcription factors such as ERF (Ethylene responsive factor), MYB (v-myb avian myeloblastosis viral oncogene homolog), and ARF (Auxin Response Factor) in the promoter region of the ClHDZ genes. The results of qPCR (Quantitative Polymerase Chain Reaction) and expression profile analysis showed that ClHD-Zip I genes showed different levels of expression under different stress treatments. Among them, ClHDZ4 was located in the nucleus, and its expression pattern was significantly upregulated under salt, drought, and high-temperature stress. In addition, ectopic expression of ClHDZ4 enhanced the growth of yeast strains under drought, salt, or high-temperature treatment. In summary, these results laid a foundation for further research on the resistance function of the Coix HD-Zip gene.
1. Introduction
Transcription factors (TFs) are important regulatory factors that control gene expression in plants and play an important regulatory function against various adversity stresses [1]. The homeodomain-leucine zipper (HD-Zip) TFs are unique to plants and play an important role in plant adversity signaling and adaptation. The DNA-binding structural domain of HD-Zip TFs consists of a homeodomain (HD) and leucine zipper (LZ) [2]. The HD is encoded by HB and generally contains 60 or 61 amino acids for specific interaction with DNA, whereas the LZ is located downstream of HD and is involved in heterodimerization of proteins [3]. Based on variations in protein structure and function, the HD-Zip TF family can be divided into four subfamilies, namely HD-Zip I, HD-Zip II, HD-Zip III, and HD-Zip IV (Figure 1) [4]. HD-Zip I and HD-Zip II contain only the basic HD and LZ domains, while HD-Zip III and HD-Zip IV also have a START domain associated with steroid binding [5,6]. In addition, HD-Zip III subfamily proteins contain a specific MEKHLA domain at the C-terminal [7].
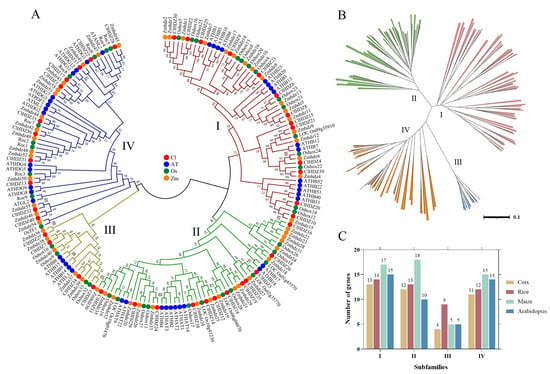
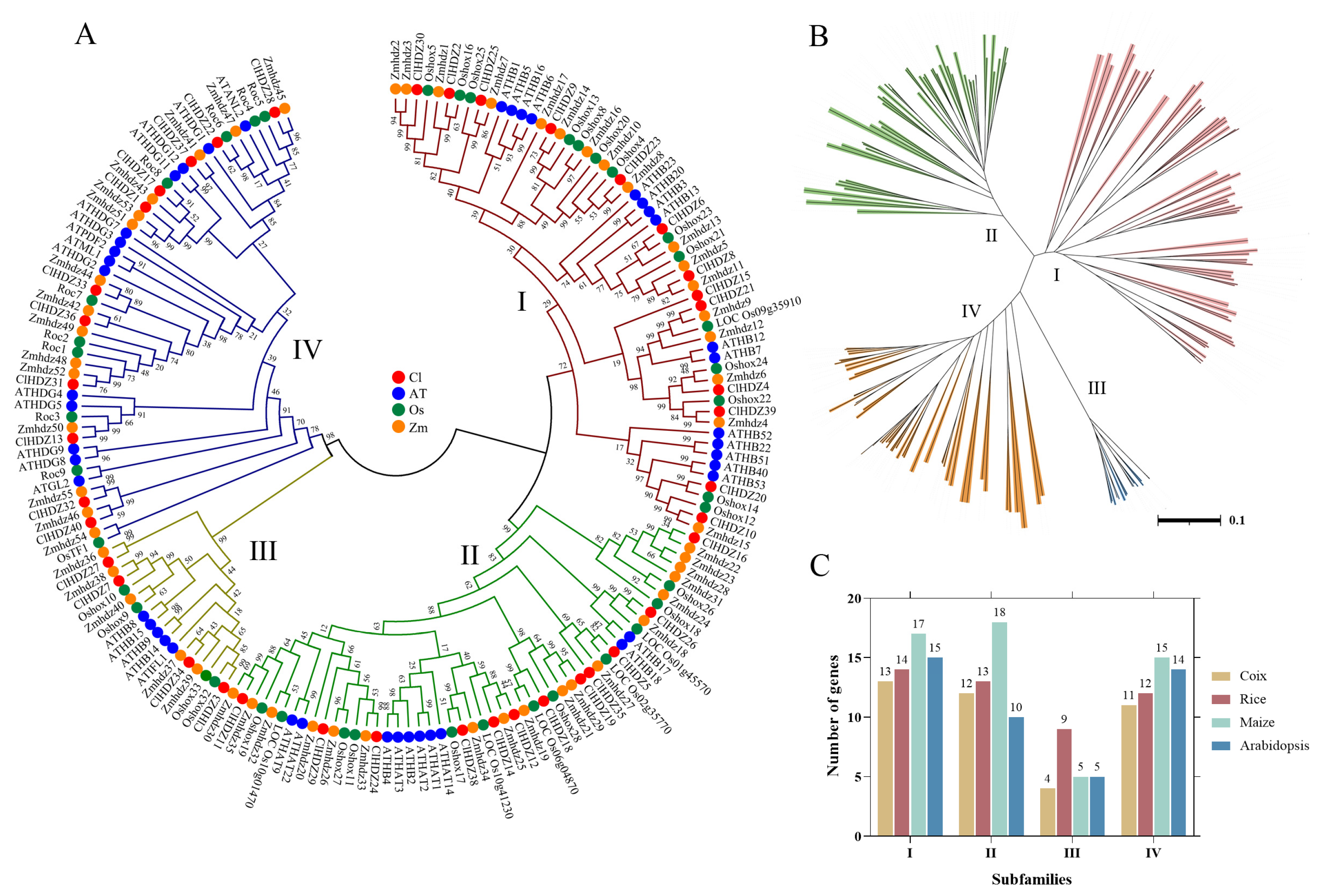
Figure 1.
Phylogenetic tree of HD-Zip genes from Coix, Arabidopsis, rice, and maize; 40 ClHDZ genes, 44 AtHDZ genes, 48 Oshox genes, and 55 Zmhdz genes are clustered into four subgroups (I–IV). HD-Zip genes from Coix, Arabidopsis, rice, and maize are denoted by red, blue, green, and HD-Zip transcription factors play an important role in shape. The tree was generated with the Clustal X 2.0 software using the neighbor-joining (N-J) method. (A) Phylogenetic trees of the HD-Zip genes from different species. (B) The distribution of the four subfamilies (I–IV) of the HD-Zip genes; (C) The number of genes in each HD-Zip subfamily in different species.
It is well known that HD-Zip gene family members are involved in the regulation of plant growth and development, such as light response, organ tissue development, shade avoidance response, pigment accumulation, hormone synthesis, and so on [8,9,10]. In maize, OCLl from the HD-Zip IV subfamily has been identified as a regulator of flowering time [11]. In tomatoes, SlHB8 from the HD-Zip III subfamily has been shown to act as a negative regulator of lignin formation and xylem formation, regulating leaf and stem development [12]. A recent study has identified an HD-Zip III TF named BjPHV as a negative regulator of non-glandular trichome initiation in Brassica L. [13]. Mounting evidence indicates that HD-Zip I TFs are widely involved in plant response to abiotic stress [14,15,16]. For instance, overexpression of MdHB-7 in apples can effectively reduce root damage caused by salt stress and also plays a positive role in ROS regulation. On the contrary, silencing MdHB-7 can improve the sensitivity of apples to salt stress [17]. Under drought stress, ZmHB53 overexpressing maize plants showed tolerance during germination, as well as improved seedling drought tolerance [18]. In addition, the HD-Zip I TF ZmHDZ9 enhances drought resistance of maize by regulating the accumulation of abscisic acid and lignin [19]. Further studies showed that lily LlHB16 promotes thermotolerance, whereas LlHOX6 interacts with LlHB16 to limit its transactivation, thereby impairing heat stress responses in lilies [20]. Screening of HD-Zip family genes in cucumber has shown that two members of the HD-Zip I subfamily, CsHDZ02 and CsHDZ33, are induced to be expressed by high-temperature stress [21].
Coix lacryma-jobi L. originated in Southeast Asia and has been cultivated in China for more than six thousand years [22]. Coix is an annual or perennial cash crop, its seeds contain rich nutrients and medicinal components. In addition, Coix has good adaptability to many biological and abiotic stresses, including drought, high temperature, pests, and diseases [23]. At present, the genome sequencing of Coix has been completed [24], which has provided important genome resources for the mining and utilization of genes related to resistance.
However, studies on the function of the HD-Zip gene family in Coix are very limited. The aim of this study was to investigate the gene structure, chromosome localization, collinearity, phylogenetic relationship, and TFBS of the Coix HD-Zip gene family, and to explore its expression pattern under high temperature, drought, and salt stress. In addition, ClHDZ4 was selected for further study based on bioinformatics analysis and expression patterns, and the subcellular localization of this protein was determined. Finally, the functional analysis of ClHDZ4 heterologous expression in yeast was investigated.
2. Results
2.1. Identification and Characteristic Analysis of HD-Zip Genes in Coix
In this study, 40 HD-Zip genes were identified in the Coix genome. According to the Latin abbreviation of Coix and the physical location of the gene, the Coix HD-Zip TFs were named ClHDZ1 to ClHDZ40 (Table S1). The predicted length of ClHDZ proteins varied from 223 to 883 amino acids (aa) in length, with an average of 481 aa, which is similar to that reported in soybean (462 aa) [25]. Moreover, the isoelectric point (pI) of ClHDZ proteins ranged from 4.58 to 11.44, and their molecular weights ranged from 24 to 96 kDa (Table S1). In addition, the predicted subcellular localization data revealed that most Coix HD-Zip proteins were located in the nucleus (Table S1). Table S1 provides information on other ClHDZ gene characteristics.
2.2. Phylogenetic Trees of HD-Zip Genes in Arabidopsis, Rice, Maize, and Coix
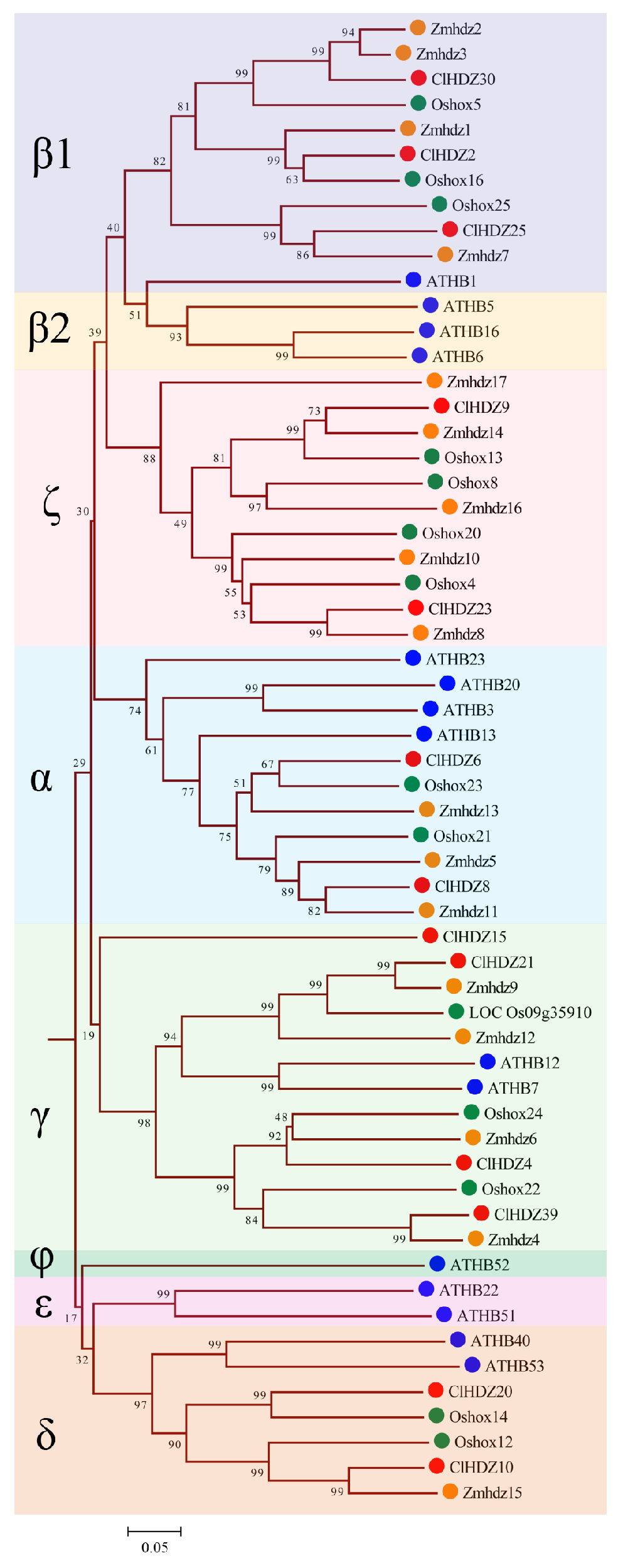
For the comparative evolutionary analysis of this gene family in rice, maize, Arabidopsis, and Coix, a phylogenetic tree was constructed using Neighbor-Joining (NJ) methods in MEGA 11.0 (Figure 1). Based on phylogenetic analysis, 187 HD-Zip proteins were clearly separated into four subfamilies (HD-Zip I to IV), which was in line with earlier discoveries in maize and other species. The results revealed that HD-Zip I and HD-Zip III subfamilies consisted of the largest and smallest members, respectively, except in maize. Furthermore, 40 Coix HD-Zip genes were divided into four subfamilies based on their phylogenetic relationships; subfamily I contained 13 ClHDZs, followed by subfamilies II and IV, which had 12 and 11 members, respectively. Furthermore, subfamily I was further subdivided into eight clades, designated α, β1, β2, γ, δ, ε, ζ, and φ, based on the classification of rice, Arabidopsis, and maize (Figure 2). And ζ does not contain the HD-Zip gene of Arabidopsis, whereas β2, ε, and φ exclude HD-Zip genes from maize, rice, and Coix. Given that Arabidopsis is a dicotyledon, while the other three species are gramineous and monocotyledons.
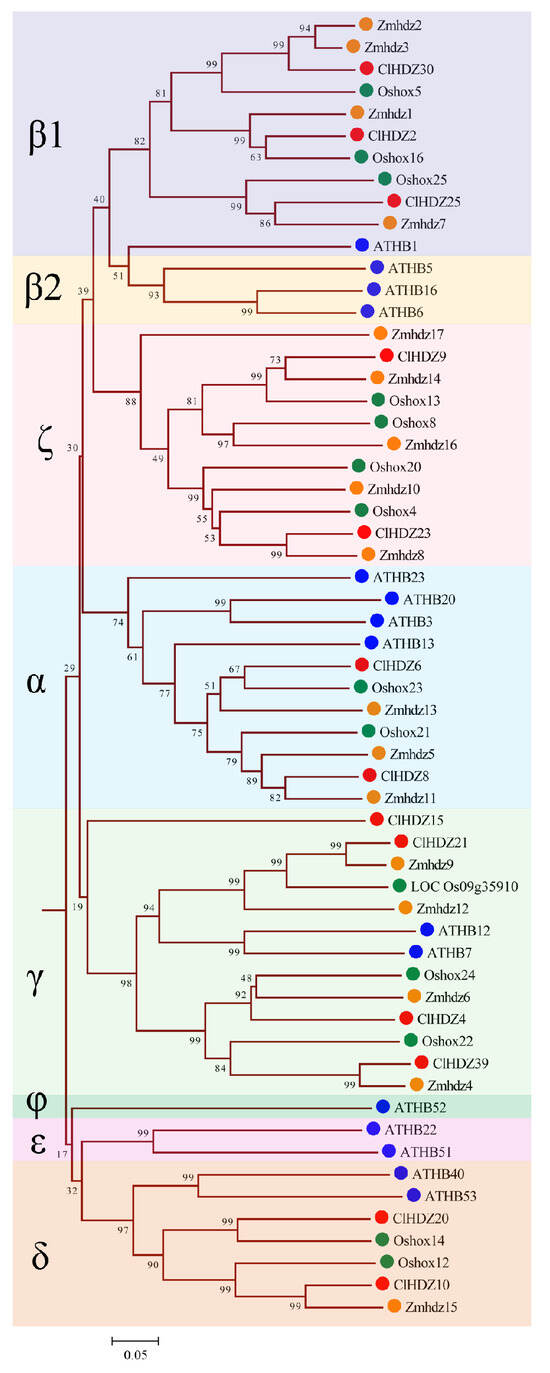
Figure 2.
Phylogenetic tree based on HD-Zip I protein sequences from Coix, rice, Arabidopsis, and maize. The tree was generated with the MEGA11.0 program using the NJ method. Coix, rice, Arabidopsis, maize, and sorghum HD-Zip I proteins are marked with different colored dots. HD-Zip I subfamily was further subdivided into eight clades, designated α, β1, β2, γ, δ, ε, ζ, and φ. The red circle represents ClHDZs, the blue circle represents ATHBs, the green circle represents Oshoxs, and the orange circle represents Zmhdzs.
2.3. Chromosomal Distribution and Collinear Analysis
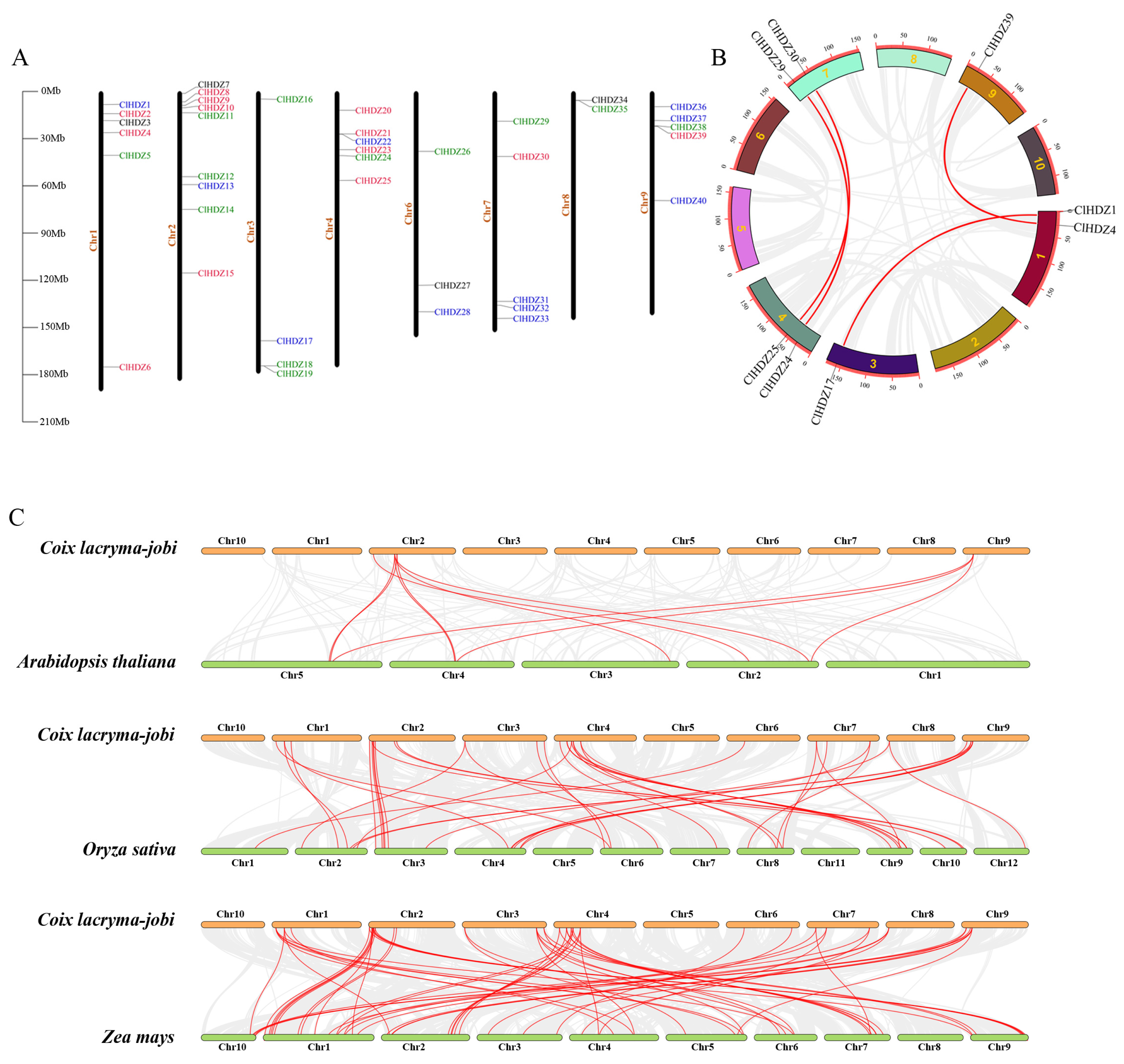
Based on the Coix annotation file, the locations of the 40 Coix HD-Zips on the chromosomes were mapped using TBtools v2.082. As shown in Figure 3A, the 40 ClHDZ genes were randomly and unevenly distributed on 8 of the 10 chromosomes, except for chromosomes 5 and 10. The result demonstrated that chromosome 2 had the highest quantity of genes, with 9 ClHDZ genes, followed by chromosomes 1 and 4, which contained 6 ClHDZ genes.
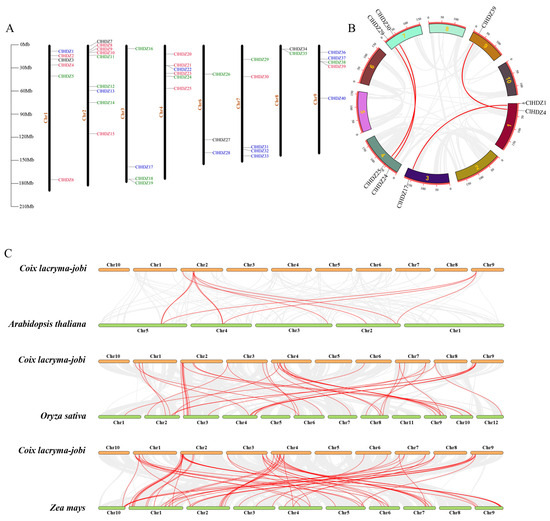
Figure 3.
Chromosome distribution and Collinearity analysis. (A) Chromosomal location of HD-Zip genes in Coix. Different colors represent different subfamilies. (B) Collinearity analysis of the HD-Zip gene in Coix. (C) HD-Zip gene collinearity between Coix and other species genomes. The collinear pairs were connected by red lines.
The potential evolutionary mechanism and replication events of the Coix HD-Zip gene family were analyzed (Figure 3B), and it was found that there were 4 homologous genes on the Coix chromosomes, namely ClHDZ1/ClHDZ17, ClHDZ4/ClHDZ39, ClHDZ24/ClHDZ29, and ClHDZ25/ClHDZ30. The results showed that some genes in the Coix HD-Zip gene family may have been formed by segmental duplication. Meanwhile, collinearity analysis was performed on the Coix and Arabidopsis genomes, and it was found that there were 10 collinear pairs, and none of the HD-Zip III members in Coix were in the collinear regions (Figure 3C). Moreover, a total of 52 collinearity pairs were found between Coix and maize, and the HD-Zip genes involved accounted for more than 70% of each genome. Also, 39 collinearity pairs were detected in the Coix and rice (Table S3). These results indicate that the HDZ gene families of Coix and maize have a closer homologous evolutionary relationship than that of rice.
2.4. Structural Feature Analysis of HD-Zip Genes in Coix
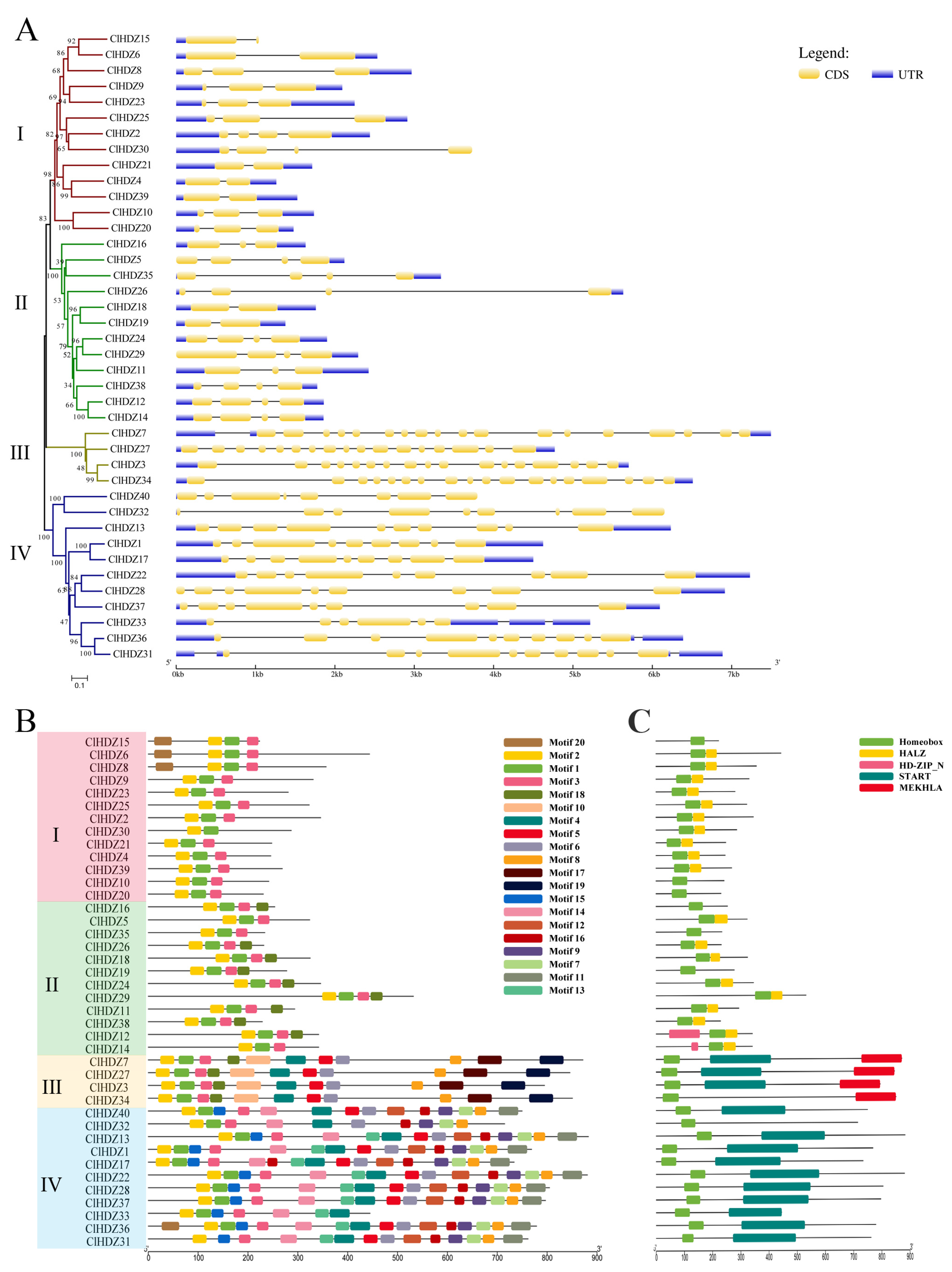
Exon/intron structure analysis using the coding sequences of each Coix HD-Zip gene was performed in order to gain more information about the structural variety of these genes. In Figure 4A, the exon numbers of the 40 ClHDZ genes ranged from 2 to 18. Further comparative analysis showed that genes with close clustering relationships in the evolutionary tree had similar gene structures, and members belonging to the same subfamily shared similar numbers of exons. In the HD-Zip I subfamily, most of the members had two or three exons, and the gene structure of the members of this family was simpler than that of the other families. Members of the HD-Zip II subfamily shared a similar gene structure with members of the HD-Zip I subfamily, and their exon counts are comparable to those of the HD-Zip I subfamily. The gene structure of HD-Zip III and HD-Zip IV subfamily members was relatively complex, and the number of exons in the HD-Zip III subfamily was the largest, with 17–18 exons. Members of the HD-Zip IV subfamily also had 6–10 exons.
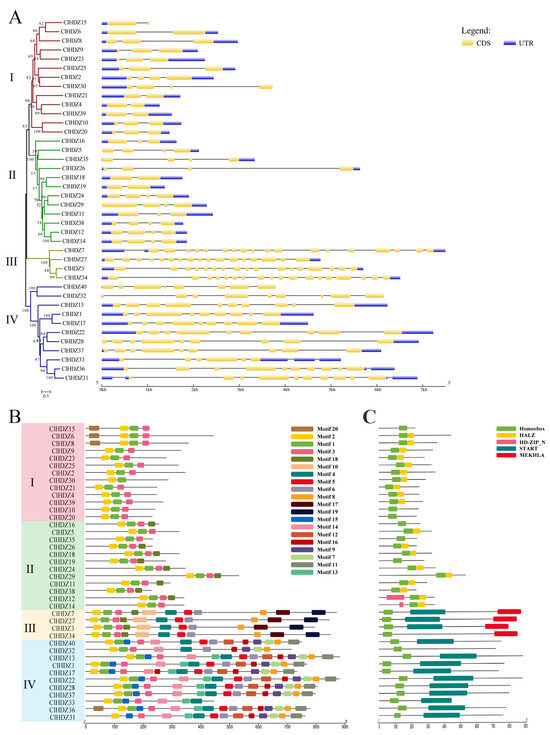
Figure 4.
Gene structure and Conserved motifs of HD-Zip genes in Coix. (A) Exons are indicated by yellow rectangles. Gray lines connecting two exons represent introns. (B) Distribution of the 20 conserved motifs in the ClHDZ genes following analysis by MEME tool. The different-colored boxes represent different motifs and their position in each protein sequence of ClHDZ. (C) Distribution of domains in ClHDZ genes. Different-colored rectangles indicate the location and distribution of different domains in each ClHDZ genes.
Moreover, we identified the conserved motif of the protein using the Motif Elicitation tool to gain a better understanding of the structural features of the Coix HD-Zip genes. As shown in Figure 4B, Motifs 1 and 2 were present in all HDZ proteins, while the ClHDZ31 protein lacked Motif 3. For further functional annotation (Table S4), Motif 1 and Motif 2 were homeobox domains (HD), and Motif 3 was homeobox-associated leucine zipper (HALZ). These three motifs collectively formed conserved motifs of the features of the HD-Zip gene family, which was consistent with the structural basis of the classification of the HD-Zip family by Ariel et al. (2007) [2]. With the exception of the normal HD domain and LZ domain, the results demonstrated that the HD-Zip I and HD-Zip II subfamily members shared similar structures and comprised only a small number of simple motifs. HD-Zip III and HD-Zip IV subfamily members have more motifs and more complex domains. The START domain, which was unique to HD-Zip III and HD-Zip IV subfamily members, was made up of Motifs 4, 5, 6, 10, 13, and 14. Only the HD-Zip III subfamily had the MEKHLA domain, which was formed by motif 19. The significant differences in gene structure and domain across the four subfamilies may be related to their different functions in plant growth and development and response to environmental stress.
2.5. Prediction and Enrichment Analysis of Transcription Factor Binding Sites in Coix HD-Zip
The transcription factor binding sites (TFBS) of ClHDZ were analyzed using the JASPAR database and MEME FIMO (Table S5). A total of 702 TFBS were identified in the promoter sequence of the ClHDZ. Enrichment analysis of these TFBS (Tables S1 and S6) revealed that 501 motifs in the promoter sequence had high statistical significance (p-value < 0.05), belonging to 32 transcription factor families. Among them, the number of ERF transcription factor family is the largest, with 60. It is noteworthy that BAD1 (MA2408.1), which belongs to the TCP transcription factor family, had the highest odds_ratio (189.86). This motif was significantly enriched in the promoter sequence of ClHDZ.
The analysis of the significantly enriched motifs in the ClHDZ promoter sequence (Table S7 and Figure S1) revealed that Zm00001d020267 (MA1817.2), which belonged to the ERF transcription factor family, was the most abundant in the ClHDZ promoter sequence. Notably, some transcription factors (such as ERF, MYB, and ARF) were significantly enriched in the promoter sequence of the ClHDZ, suggesting that these TFs might have played important roles in the regulatory network of ClHDZ.
2.6. The Expression Files of Coix HD-Zip Genes in Response to Drought and Heat Stress
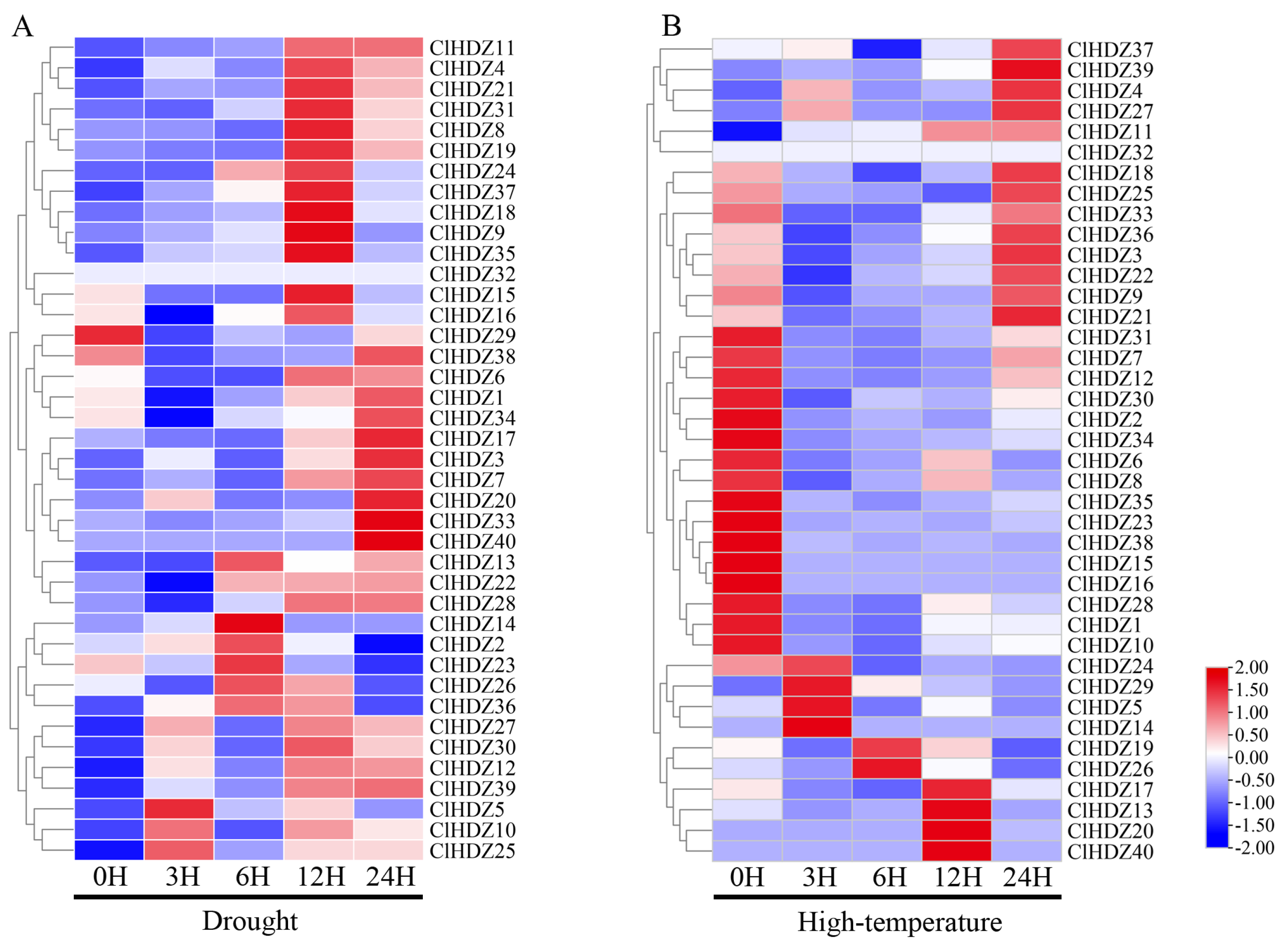
To investigate the potential roles of Coix HD-Zip genes in drought and high temperature, we collected two transcriptome datasets for all 40 ClHDZ genes from the NCBI database and generated a heat map. During drought stress (Figure 5A), the expression profiles of 11 ClHDZ genes were significantly up-regulated at 12 h; whereas 9 ClHDZ genes were significantly up-regulated at 24 h of treatment; additionally, one gene (ClHDZ32) was found to have no expression information. As for high-temperature stress (Figure 5B), the expression of nine ClHDZ genes was apparently down-regulated at any time. The expression of most ClHDZs was increased by high-temperature induction, and there were three main expression modes: six ClHDZs were rapidly induced and up-regulated at 1 h or 3 h; ClHDZ17, ClHDZ13, ClHDZ20, and ClHDZ40 were up-regulated at 12 h; and the expression level of 12 ClHDZs was significantly up-regulated at 24 h.
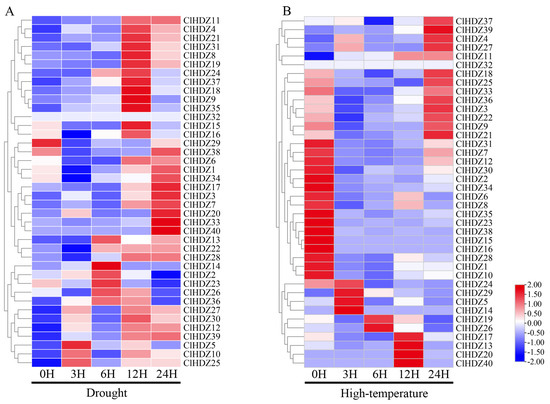
Figure 5.
Heat map of HD-Zip gene expression pattern in Coix. (A) The expression levels of ClHDZ under drought stress. (B) The expression levels of ClHDZ at different periods of high-temperature stress.
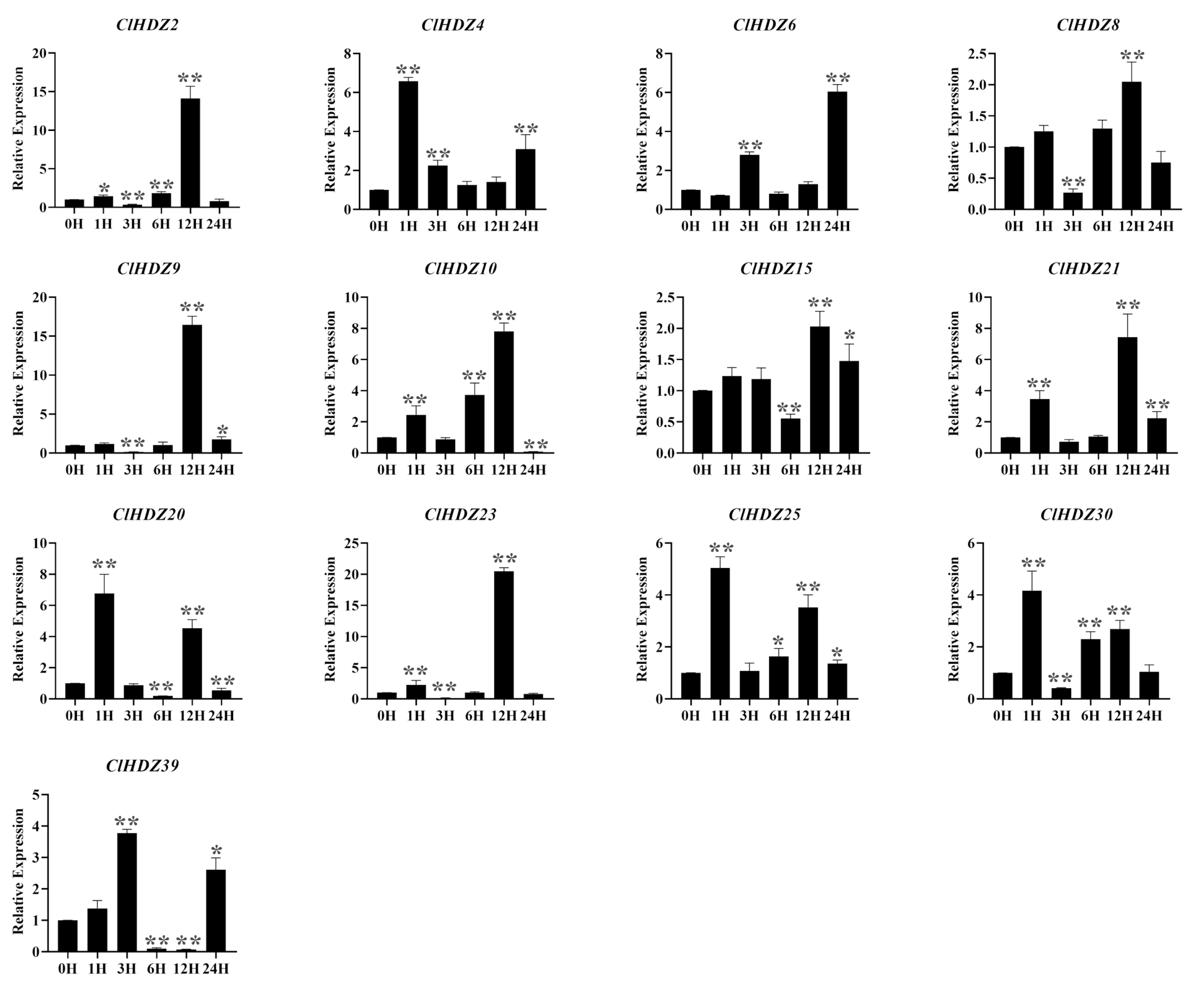
2.7. Expression Pattern of the HD-Zip I Genes in Coix Under Salt Stress
According to reports, HD-Zip I genes played an important role in responses and tolerance to various abiotic stresses, especially drought and salt stress. The expression pattern of HD-Zip I genes in NaCl-treated Coix was detected by qRT-PCR (Figure 6). As shown in the figure, the expression levels of four genes rapidly responded and peaked at 1 h, among which the expression levels of ClHDZ4 and ClHDZ20 reached a maximum value of more than 6-fold. Furthermore, ClHDZ25/ClHDZ30 exhibited a similar expression pattern after NaCl treatment. For example, the expression of ClHDZ25 was up-regulated and reached a maximum at 12 h, but then decreased and gradually up-regulated threefold at 12 h. Additionally, the expression level of 7 ClHDZ genes exhibited a rapid and strong up-regulation at 12 h.
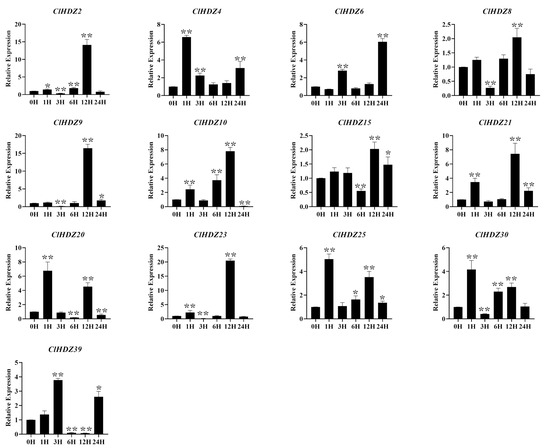
Figure 6.
Expression analysis of 13 HD-Zip I genes in Coix following salt treatments by qRT-PCR. Y-axis and X-axis indicate relative expression levels and the time courses of stress treatments, respectively. Mean values and standard deviations (SDs) were obtained from three biological and three technical replicates. The error bars indicate standard deviation. Significance levels: * p < 0.05; ** p < 0.01.
2.8. Subcellular Localization of ClHDZ4 and Its Role in Enhancing Yeast Stress Tolerance Through Ectopic Expression
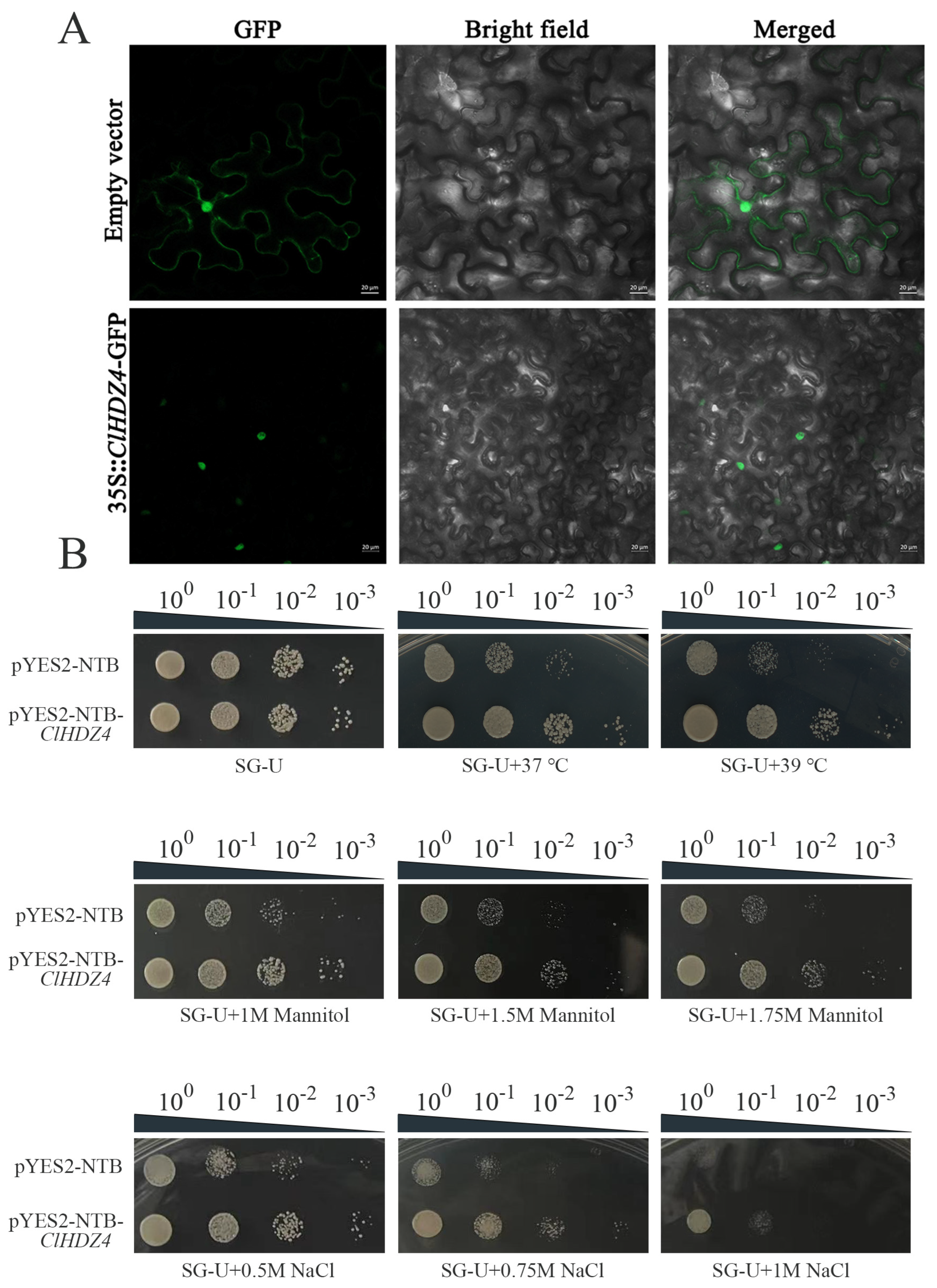
The subcellular localization of ClHDZ4 was identified by constructing a recombinant plasmid p1305-CaMV35S-ClHDZ4-GFP and expressing it in the lower epidermis of tobacco leaves. As shown in Figure 7A, fusion fluorescence signals on the nucleus were observed, whereas the control group (p1305-CaMV35S-GFP) exhibited in both the cytoplasm and the nucleus.
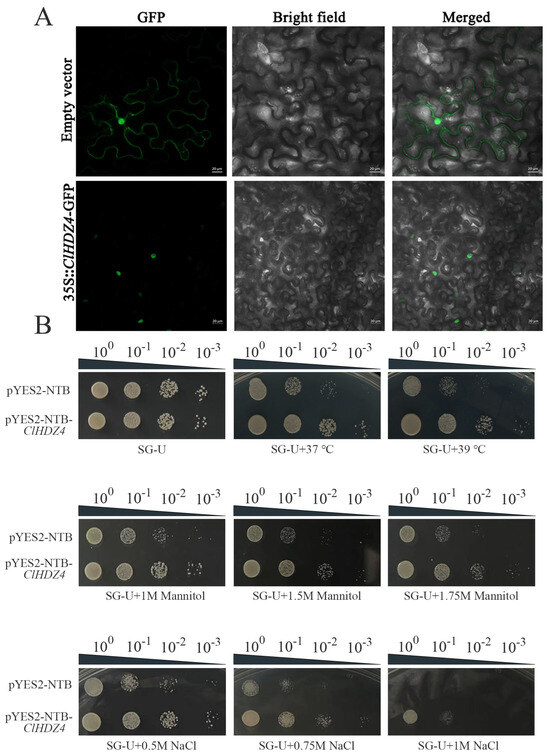
Figure 7.
Subcellular localization and detection of drought, NaCl, and high-temperature tolerance of the yeasts transformed with ClHDZ4. (A) Subcellular localization of ClHDZ4 in tobacco leaves. (B) Growth of the INVSc1 yeast strain transformed with ClHDZ4.
In order to investigate whether ClHDZ4 responds to drought, salt, and high-temperature stress, the effects of ClHDZ4 on yeast growth and stress resistance were analyzed in yeast containing the pYES2::ClHDZ4 vector (Figure 7B). According to the comparison with a control yeast strain, we determined whether ClHDZ4 can enhance the drought, salt, and high-temperature resistance of the yeast strain. Under normal conditions, there was no significant difference in strain growth size between yeast overexpressing ClHDZ4 and empty vector. However, with the increase in PEG and NaCl concentrations in the medium, the growth of control yeast cells was significantly inhibited. Under 1M NaCl treatment, the yeast strain containing ClHDZ4 grew better than the control, and the control yeast did not grow at 10-1-fold dilution. Similar results were observed under drought treatment; the growth and survival rate of the transgenic yeast cells harboring the ClHDZ4 gene was improved. With regard to heat tolerance function, the growth of yeast cells harboring the ClHDZ4 gene was improved under 37 °C treatment compared to the control. When the temperature was increased to 39 °C, the growth of continuously diluted control yeast cells on SD/-Ura medium was significantly inhibited. These results indicate that the ClHDZ4 gene enhanced the resistance of yeast cells to drought, NaCl, and high temperature.
3. Discussion
Plants are regulated by a variety of TFs in the process of growth and development, and HD-Zip TFs are a plant-specific gene family whose functions have been widely reported in plants such as Arabidopsis [26], rice (Oryza sativa L.) [27] and maize (Zea mays L.) [28]. Hence, 40 Coix HD-Zip genes were genome-wide identified and characterized in this study.
Coix HD-Zip genes could be categorized into four subfamilies according to their structure, and the number of members of each subfamily shows variability in different species. For example, the number of members corresponding to the four subfamilies HD-Zip I to IV in Coix was 13, 12, 4, and 11, respectively, while the number of members of the four subfamilies in maize was 17, 18, 5, and 15, respectively (Figure 1). It is well known that HD-Zip I subfamily genes can be further divided into 8 branches (α, β1, β2, γ, ε, δ, ζ, and φ) [29]. In the present study, phylogenetic analysis showed that the 13 HD-Zip I proteins of Coix were divided into five clades without the φ, ε, and β2 clades, indicating that Coix had lost members of these three clades during evolution. Similarly, in monocotyledons such as rice [27], maize [28], and perennial ryegrass (Lolium perenne L.) [30], it had been reported that φ, ε, and β2 evolutionary branches of HD-Zip I proteins were absent. Comparative evolutionary analysis showed that the phylogenetic relationship of the HD-Zip genes was highly consistent with the relative relationship between these species.
Analysis of gene structure and conserved motifs in Coix showed that members of the same subfamily shared similar motif distribution and exon number, which was consistent with observations made with respect to other species. In Figure 4, ClHDZs of subfamily III had the largest number of exons and domains. Moreover, the HD-Zip III subfamilies identified in cucumber, watermelon, and kiwifruit [21,31,32] contained the lowest number of genes, but they played important roles in plant growth and development, such as the formation of apical meristematic tissue and vascular system [2]. The similarity of structures indicates that they may perform similar functions. The ClHDZ4 gene screened in this study had a similar structure and the same number of exons as Oshox22/24 in rice [27] and Zmhdz4/6 in maize [28], and all five genes belonged to the γ branch of the HD-Zip I subfamily, which was hypothesized to have similar functions.
Plants adapt to environmental stresses by inducing the expression of stress-related genes, such as drought, and the expression of HD-Zip I genes in many species responds to drought treatment and plays an important role in drought stress [14,33]. Analysis of transcription factor binding sites in the promoter region of the ClHDZ genes showed that the promoter region contains multiple motifs that bind to ERF, MYB, and ARF transcription factors. According to the literature, ERF, MYB, and ARF are widely involved in regulating plant responses to abiotic stresses [34,35,36]. The TFBS enrichment analysis results show that the BAD1 (MA2408.1) motif, which belongs to the TCP transcription factor family, has the highest odds ratio in the ClHDZ promoter. This suggests that the TCP transcription factor BAD1 was likely to be an important regulator of ClHDZ. It has been reported that the TCP transcription factor family plays an important role in the plant response to drought stress [37,38]. Therefore, the expression level of the ClHDZ genes under drought stress was investigated. The results showed that members of the HD-Zip I subfamily in Coix responded positively to drought stress, which was the same as that in Arabidopsis, wheat, and apple [17,39,40]. It was found that AtHB12, a member of the γ branch of HD-Zip I subfamily, was induced by drought stress, and overexpression of AtHB12 enhanced the tolerance of transgenic Arabidopsis to drought stress [41]. Similarly, Md HB-7 and Md HB7-like, which were members of the γ branch of the HD-Zip I subfamily, enhanced the drought adaptation and water-use efficiency of transgenic apple by regulating the stomatal density [17,42]. Given this, members of this branch might play an important role in drought stress response. The accumulated studies revealed that HD-Zip I TFs were also involved in the regulation of salt and high-temperature stresses [43]. For example, CaHDZ15 (HD-Zip I gene) in pepper [44] directly targeted and activated heat shock factor A6a (HSFA6a), which further activated CaHSFA2, and its overexpression significantly improved the tolerance of transgenic tobacco to high-temperature stress. In Sophora alopecuroides, overexpression of SaHDZ22 [45] increased Arabidopsis tolerance to salt stress. In this study, the expression level of HD-Zip I genes was also induced under high-temperature stress and salt stress. Among them, ClHDZ4 has a collinear relationship with Zmhdz4 and Zmhdz6. Moreover, overexpression of Zmhdz4 has been reported to enhance drought resistance of transgenic maize plants [46]. In addition, AtHB7, AtHB12, and ClHDZ4 belong to the same clade, and the expression levels of ATHB7 and ATHB12 were also significantly up-regulated under NaCl and drought stress [39]. The expression level of ClHDZ4 was up-regulated under drought, salt, and high-temperature stress, and its overexpression could enhance the stress resistance of yeast strains.
4. Materials and Methods
4.1. Retrieving and Identifying HD-Zip Family Genes in Coix
The genome annotations of the Coix were fetched from Coge (https://genomevolution.org/coge/, accessed on 21 February 2025). The accession numbers for the HD-domain profile (PF00046) and LZ domain profile (PF02183) were downloaded from InterPro (https://www.ebi.ac.uk/interpro/entry/pfam/, accessed on 21 February 2025). Subsequently, their Hidden Markov Model (HMM) profiles were used to search the Coix protein database via the HMMER program with an E-value less than 1 × 10−5 as the threshold [47]. The candidate HD-Zip protein sequences underwent verification through the SMART (http://smart.embl.de, accessed on 21 February 2025) and CDD database on the NCBI website (https://www.ncbi.nlm.nih.gov/cdd, accessed on 21 February 2025). Protein properties were obtained via EXPASY (https://web.expasy.org/protparam/, accessed on 21 February 2025), including theoretical isoelectric points (pI) and molecular weights (Mw). The online Cell-PLoc server and CELLO v.2.5 (Plant-PLoc, http://www.csbio.sjtu.edu.cn/bioinf/plant/ (accessed on 21 February 2025) and http://cello.life.nctu.edu.tw/ (accessed on 21 February 2025)) were used to predict the subcellular localization of HD-Zip proteins in Coix [48].
4.2. Phylogenetic Analysis, Chromosome Localization, and Collinear Analysis
The multiple alignment of 40 Coix HD-Zip proteins, 44 Arabidopsis HD-Zip proteins, and 48 rice HD-Zip proteins was performed using the ClustalW program in MEGA7.0 with default parameters. Next, MEGA11.0 was used to create a phylogenetic tree using the neighbor-joining method (NJ) with 1000 bootstrap replicates and default parameters [49].
The GFF annotation file from the Coge database provided the chromosomal location data for the Coix HD-Zip genes. The Gene Location Visualize feature in the Tbtools software was used to visualize the physical maps of the Coix HD-Zip genes. To conduct inter-species collinearity analysis, genome sequences and annotation files for Arabidopsis, maize, and rice were obtained from the Phytozome v13 website. The collinearity relationships between Coix and three other species were analyzed and visualized using TBtool [50].
4.3. Gene Structure, Conserved Motifs, Transcription Factor Binding Site (TFBS) Analysis, and Enrichment Analysis
Based on the exon and intron positions of HD-Zip genes in the GFF annotation file of the Coix genome, the intron-exon structure was analyzed and mapped using TBtools software. The Multiple Expectation Maximization for Motif Elucidation (MEME) tool in TBtools v2.082 software was used to predict and analyze the conserved protein motif of Coix seed HD-Zip protein, with the maximum number of motifs set to 20, and other parameters set to default.
The promoter sequences of all protein-coding genes of Arabidopsis thaliana were downloaded from the EPD database (https://epd.expasy.org/epd/, accessed on 22 February 2025) as the control set, while the 2000 bp sequence upstream of the ClHDZ genes promoter was used as the test set [51].
The TFBS in both the test and control sets were predicted using the JASPAR database (https://jaspar.elixir.no/, accessed on 22 February 2025) and the FIMO tool in the MEME Suite (https://meme-suite.org/meme/tools/fimo, accessed on 22 February 2025), with a Match p-value threshold of <1 × 10−4 [52]. Subsequently, Fisher’s exact test was applied to the results for enrichment analysis, retaining only the motifs with a p-value < 0.05.
4.4. Expression Profiles of HD-Zip Genes in Coix
The expression profile of the HD-Zip gene in Coix under high-temperature and drought stress was analyzed using Coixtranscriptome data (NCBI BioProject: PRJNA812268), the mean TPM values of three replicates were calculated, and the TBtools software was used to draw a heatmap of gene expression.
4.5. Plant Material Acquisition, RNA Extraction, and qRT-PCR
The Wanyi 2 variety of Coix was cultured in the growth chambers at a temperature of 26 °C with a light/dark cycle of 16/8 h. Before the salt treatment, seedlings were pre-cultivated in 1/2 Hoagland nutrient solution for one month. Subsequently, it was treated with 200 mM NaCl, and samples were taken at day 0, 1, 3, 6, 12, and 24. Additionally, the samples were kept at −80 °C and frozen with liquid nitrogen. The RNA of the samples was extracted using the Aidlab plant RNA kit (Aidlab Biotech, Beijing, China), and the first-strand of cDNA was synthesized using the Prime ScriptTMRT reagent Kit (TaKaRa, Dalian, China). Real-time PCR was performed by CFX96TM Real-Time System and using TB Green Premix Ex Taq II qRT-PCR with a sample volume of 10 μL. The relative expression levels of each gene were determined using the standard 2−ΔΔCT method [53]. VQ30 was utilized as a reference gene, and specific primers for Coix HD-Zip genes were designed by Primer Premier 5.0 software.
4.6. Subcellular Localization Analysis of ClHDZ4
The coding sequence of ClHDZ4 (without the stop codon) was amplified with the primer pairs (Supplementary Table S2) and then cloned into a pCambia1305-35S::GFP vector fused with the N-terminus of the green fluorescent protein (GFP) gene. The GFP-only control vector and ClHDZ4-GFP vector were transformed into the Agrobacterium strain GV3101 and injected into the tobacco leaves for transient expression. Tobacco was grown in a greenhouse with a 16/8 h light and dark photoperiod at 24 °C for 4 weeks. After 48 h, the GFP fluorescence was detected by a laser scanning confocal microscope (Leica TCS SP8, Wetzlar, Germany).
4.7. The Overexpression of ClHDZ4 in Yeast
The coding sequence of ClHDZ4 was cloned into the pYES2/NTB (pYES2) vector and then transformed into the yeast strain INVSc1. Yeast monoclonal colonies were selected and confirmed through PCR analysis. Transformed yeast cells were incubated in SD-Ura liquid medium at 29 °C overnight until the optical density (OD)600 reached 1.2, then transformed yeast cells were shake-cultured at 250 rpm, at 29 °C, for 10 h in liquid SG-U medium. The yeast (containing pYES2 empty liquid or pYES2::ClHDZ4 recombinant vector) was diluted to 1:10, 100, 1000, and 10,000. Then, 4 μL of the original yeast liquid and the diluted yeast liquid were successively dropped on the SG-U solid medium. For drought and salt treatments, we added different concentrations of PEG or NaCl to the SG-U medium, while for high-temperature treatments, the SG-U solid medium was incubated for 7 days at 37 °C and 40 °C.
5. Conclusions
In this study, 40 HD-Zip genes of Coix were identified, which were classified into four subfamilies according to phylogenetic analysis and conserved domains. The Coix HD-Zip genes were randomly and unevenly distributed on 8 of the 10 chromosomes, all of which were predicted to be located in the nucleus. Moreover, ClHDZ genes showed different expression patterns under different stress treatments (drought, salt, and high temperature). In addition, ectopic expression of ClHDZ4 enhanced the growth of the yeast strain under drought, salt, or high-temperature treatment. Overall, the results of this study are helpful to further elucidate the regulatory mechanism of the HD-Zip gene in response to abiotic stress and provide reference information for genetic breeding.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14091318/s1. Table S1. Details of the identified Coix HD-Zip genes. Table S2. List of primer sequences used for experiments in this study; Table S3. Collinearity analysis of HD-Zip gene between Coix and other species; Table S4. Detailed information for the 30 motifs in the HD-Zip proteins of Coix; Table S5. Prediction of Transcription Factor Binding Sites in ClHDZ. Table S6. Enrichment analysis of transcription factor binding sites of ClHDZ. Table S7. Number of binding sites for each transcription factor in ClHDZ. Figure S1. Heat map analysis of the number of binding sites for each transcription factor in ClHDZ.
Author Contributions
Conceptualization, Y.W. (Yujiao Wang) and J.Z.; formal analysis, H.W.; funding acquisition, Y.W. (Yujiao Wang); methodology, Y.W. (Yujiao Wang); software, Y.W. (Yujiao Wang) and X.L.; supervision, C.Y. and B.J.; writing—original draft, Y.W. (Yongle Wang); writing—review and editing, Y.W. (Yujiao Wang), H.W., and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (3240151950) and the Anhui Academy of Agricultural Sciences talent introduction project (XXBS-202309).
Data Availability Statement
All data in this study can be found in the manuscript or the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.A.-O.; Zaheer, U.; Gao, S.J. Transcription Factors in Plant Stress Responses: Challenges and Potential for Sugarcane Improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L.; Chan, R.L. The True Story of The HD-Zip Family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Katsumata, H.; Abe, M.; Yabe, N.; Komeda, Y.; Yamamoto, K.T.; Takahashi, T. Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis. Plant Physiol. 2006, 141, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Steindler, C.; Morelli, G.; Ruberti, I. The Arabidopsis Athb-8, -9 and -14 genes are members of a small gene family coding for highly related HD-Zip proteins. Plant Mol. Biol. 1998, 38, 609–622. [Google Scholar] [CrossRef]
- Côté, C.L.; Boileau, F.; Roy, V.; Ouellet, M.; Levasseur, C.; Morency, M.-J.; Cooke, J.E.; Séguin, A.; MacKay, J.J. Gene family structure, expression and functional analysis of HD-Zip III genes in angiosperm and gymnosperm forest trees. BMC Plant Biol. 2010, 10, 273. [Google Scholar] [CrossRef]
- Viola, I.L.; Gonzalez, D.H. Structure and Evolution of Plant Homeobox Genes. In Plant Transcription Factors; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, X.; Zhang, B.; Xiao, Y.; Guo, J.; Liu, J.; Chen, Q.; Peng, F. Genome-wide identification, bioinformatics and expression analysis of HD-Zip gene family in peach. BMC Plant Biol. 2023, 23, 122. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhang, S.; Liu, X.; Zhou, Z.; Liu, Z.; Wang, K.; Tian, Y.; Wang, H.; Zhang, Y.; et al. Two zinc-finger proteins control the initiation and elongation of long stalk trichomes in tomato. J. Genet. Genom. 2021, 48, 1057–1069. [Google Scholar] [CrossRef]
- He, G.; Liu, P.; Zhao, H.; Sun, J. The HD-Zip II Transcription Factors Regulate Plant Architecture through the Auxin Pathway. Int. J. Mol. 2020, 21, 3250–3255. [Google Scholar] [CrossRef]
- Xie, Q.; Gao, Y.; Li, J.; Yang, Q.; Qu, X.; Li, H.; Zhang, J.; Wang, T.; Ye, Z.; Yang, C. The HD-Zip IV transcription factor SlHDZIV8 controls multicellular trichome morphology by regulating the expression of Hairless-2. J. Exp. Bot. 2020, 71, 7132–7145. [Google Scholar] [CrossRef]
- Depège-Fargeix, N.; Javelle, M.; Chambrier, P.; Frangne, N.; Gerentes, D.; Perez, P.; Rogowsky, P.M.; Vernoud, V. Functional characterization of the HD-Zip IV transcription factor OCL1 from maize. J. Exp. Bot. 2011, 62, 293–305. [Google Scholar] [CrossRef]
- Liu, X.; Wu, C.; Su, D.; Yang, Y.; Xian, Z.; Yu, C.; Li, Z.; Hao, Y.A.-O.; Chen, R. The SlHB8 Acts as a negative regulator in stem development and lignin biosynthesis. Int. J. Mol. Sci. 2021, 22, 13343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Liu, Y.; Li, Z.; Hu, Z.A.-O.; Zhang, M.; Yang, J.A.-O. An HD-Zip III transcription factor, BjPHVa, negatively regulates non-glandular trichome formation in Brassica juncea. Physiol. Plant. 2024, 176, e14553. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.C.; Hrmova, M.; Lopato, S.; Langridge, P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 2011, 190, 823–837. [Google Scholar] [CrossRef]
- Li, S.; Chen, N.; Li, F.; Mei, F.; Wang, Z.; Cheng, X.; Kang, Z.; Mao, H.A.-O. Characterization of wheat homeodomain-leucine zipper family genes and functional analysis of TaHDZ5-6A in drought tolerance in transgenic Arabidopsis. BMC Plant Biol. 2020, 20, 50. [Google Scholar] [CrossRef]
- Wang, K.; Xu, L.; Wang, Y.; Ying, J.; Li, J.; Dong, J.; Li, C.; Zhang, X.; Liu, L.A.-O. Genome-wide characterization of homeodomain-leucine zipper genes reveals RsHDZ17 enhances the heat tolerance in radish (Raphanus sativus L.). Physiol. Plant. 2022, 174, e13789. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Jia, X.; Gao, H.; Mao, K.; Ma, F.A.-O. The HD-Zip I transcription factor MdHB7-like confers tolerance to salinity in transgenic apple (Malus domestica). Physiol. Plant. 2021, 173, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yan, X.; Wang, N.; Zenda, T.; Dong, A.; Zhai, X.; Yang, Q.; Duan, H.A.-O. ZmHB53, a maize Homeodomain-Leucine Zipper I transcription factor family gene, contributes to abscisic acid sensitivity and confers seedling drought tolerance by promoting the activity of ZmPYL4. Plant Cell Environ. 2025. [Google Scholar] [CrossRef]
- Jiao, P.; Jiang, Z.; Miao, M.; Wei, X.; Wang, C.; Liu, S.; Guan, S.; Ma, Y. Zmhdz9, an HD-Zip transcription factor, promotes drought stress resistance in maize by modulating ABA and lignin accumulation. Int. J. Biol. Macromol. 2024, 258, 128849. [Google Scholar] [CrossRef]
- Wu, Z.A.-O.; Li, T.; Zhang, Y.; Zhang, D.; Teng, N.A.-O. HD-Zip I protein LlHOX6 antagonizes homeobox protein LlHB16 to attenuate basal thermotolerance in lily. Plant Physiol. 2024, 194, 1870–1888. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Wang, J.; Cao, Z.; Zhang, H.; Chen, P.; Yuhong, L. Genome wide identification, characterization and expression analysis of HD-Zip gene family in Cucumis sativus L. under biotic and various abiotic stresses. Int. J. Biol. Macromol. 2020, 158, 502–520. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Ball, T.; Yu, L.; Li, Y.; Xing, F. Revealing a 5,000-y-old beer recipe in China. Proc. Natl. Acad. Sci. USA 2016, 113, 6444–6448. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Yang, A.; He, J.; Xiao, C.; Lv, S.; Han, F.; Yuan, Y.; Yuan, Y.; Dong, X.; et al. The Coix genome provides insights into panicoideae evolution and papery hull domestication. Mol. Plant 2020, 13, 309–320. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Cai, Z.; Huang, Y.; Jin, W. Evolution and domestication footprints uncovered from the genomes of Coix. Mol. Plant 2019, 13, 295–308. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Zhao, H.; Zhao, Y.; Cheng, B.; Xiang, Y. Genome-wide analysis of soybean HD-Zip gene family and expression profiling under salinity and drought treatments. PLoS ONE 2014, 9, e87156. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Brocchieri, L.; Bürglin, T.R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol. Biol. Evol. 2009, 26, 2775–2794. [Google Scholar] [CrossRef]
- Agalou, A.; Purwantomo, S.; Overnäs, E.; Johannesson, H.; Zhu, X.; Estiati, A.; de Kam, R.J.; Engström, P.; Slamet-Loedin, I.H.; Zhu, Z.; et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol. Biol. 2008, 66, 87–103. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, Y.; Jiang, H.; Li, X.; Gan, D.; Peng, X.; Zhu, S.; Cheng, B. Systematic analysis of sequences and expression patterns of drought-responsive members of the HD-Zip gene family in maize. PLoS ONE 2011, 6, e28488. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, E.; Olsson, A.S.; Johannesson, H.; Hanson, J.; Engström, P.; Söderman, E. Homeodomain Leucine Zipper Class I genes in Arabidopsis. Expression Patterns and Phylogenetic Relationships. Plant Physiol. 2005, 139, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, L.; Zhang, J.; Yu, J.; Yang, Z.; Huang, B. Identification and characterization of novel homeodomain leucine zipper (HD-Zip) transcription factors associated with heat tolerance in Perennial ryegrass. Environ. Exp. Bot. 2019, 160, 1–11. [Google Scholar] [CrossRef]
- Yan, X.; Yue, Z.; Pan, X.; Si, F.; Li, J.; Chen, X.; Li, X.; Luan, F.; Yang, J.; Zhang, X.; et al. The HD-Zip gene family in Watermelon: Genome-wide identification and expression analysis under abiotic stresses. Genes 2022, 13, 2242. [Google Scholar] [CrossRef]
- Ye, K.Y.; Li, J.W.; Wang, F.M.; Gao, J.Y.; Liu, C.X.; Gong, H.J.; Qi, B.B.; Liu, P.P.; Jiang, Q.S.; Tang, J.M.; et al. Genome-wide analysis and expression profiling of the HD-Zip gene family in kiwifruit. BMC Genom. 2024, 25, 354. [Google Scholar] [CrossRef] [PubMed]
- Ré, D.A.; Dezar, C.A.; Chan, R.L.; Baldwin, I.T.; Bonaventure, G. Nicotiana attenuata NaHD20 plays a role in leaf ABA accumulation during water stress, benzylacetone emission from flowers, and the timing of bolting and flower transitions. J. Exp. Bot. 2011, 62, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Fan, J.D.; Liu, C.Y.; Zhao, Y.-Q.; Xu, Z.-S.; Lu, X.-J.; Ge, J.; Zhang, B.-W.; Li, M.-Q.; Yang, Y.; et al. Physiological and transcriptome analysis of changes in endogenous hormone contents and related synthesis and signaling genes during the heat stress in garlic (Allium sativum L.). BMC Plant Biol. 2025, 25, 464. [Google Scholar] [CrossRef]
- Balhara, R.; Verma, D.; Kaur, R.; Singh, K. MYB transcription factors, their regulation and interactions with non-coding RNAs during drought stress in Brassica juncea. BMC Plant Biol. 2024, 24, 999. [Google Scholar] [CrossRef]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Ding, S.; Cai, Z.; Du, H.; Wang, H. Genome-Wide Analysis of TCP Family Genes in Zea mays L. Identified a Role for ZmTCP42 in Drought Tolerance. Int. J. Mol. Sci. 2019, 20, 2762. [Google Scholar] [CrossRef]
- Liu, Y.J.; An, J.P.; Gao, N.; Wang, X.; Chen, X.; Wang, X.; Zhang, S.; You, C. MdTCP46 interacts with MdABI5 to negatively regulate ABA signalling and drought response in apple. Plant Cell Environ. 2022, 45, 3233–3248. [Google Scholar] [CrossRef]
- Ré, D.A.; Capella, M.; Bonaventure, G.; Chan, R.L. Arabidopsis AtHB7 and AtHB12 evolved divergently to fine tune processes associated with growth and responses to water stress. BMC Plant Biol. 2014, 14, 150. [Google Scholar] [CrossRef]
- Harris, J.C.; Sornaraj, P.; Taylor, M.; Bazanova, N.; Baumann, U.; Lovell, B.; Langridge, P.; Lopato, S.; Hrmova, M. Molecular interactions of the γ-clade homeodomain-leucine zipper class I transcription factors during the wheat response to water deficit. Plant Mol. Biol. 2016, 90, 435–452. [Google Scholar] [CrossRef]
- Romani, F.; Ribone, P.A.; Capella, M.; Miguel, V.N.; Chan, R.L. A matter of quantity: Common features in the drought response of transgenic plants overexpressing HD-Zip I transcription factors. Plant Sci. 2016, 251, 139–154. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, H.; Jia, X.; Zhou, K.; Wang, H.; Mao, K.; Ma, F. MdHB7-like confers drought tolerance and improves water-use efficiency through modulating stomatal density in apple (Malus domestica). Sci. Hortic. 2022, 294, 110758. [Google Scholar] [CrossRef]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-Zip gene family: Potential roles in improving plant growth and regulating stress-responsive mechanisms in plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef]
- Mou, S.A.-O.; He, W.; Jiang, H.A.-O.; Meng, Q.A.-O.; Zhang, T.A.-O.; Liu, Z.A.-O.X.; Qiu, A.A.-O.; He, S.A.-O. Transcription factor CaHDZ15 promotes pepper basal thermotolerance by activating HEAT SHOCK FACTORA6a. Plant Physiol. 2024, 195, 812–831. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Yan, F.; Wang, L.; Wang, Y.; Li, J.; Yang, X.; Gao, Z.; Liu, X.; Liu, Y.; et al. Genome-wide analysis of HD-Zip genes in Sophora alopecuroides and their role in salt stress response. Plant Genome 2024, 17, e20504. [Google Scholar] [CrossRef]
- Xie, X.; Ren, Z.; Su, H.; Abou-Elwafa, S.F.; Shao, J.; Ku, L.; Jia, L.; Tian, Z.; Wei, L. Functional study of ZmHDZ4 in maize (Zea mays) seedlings under drought stress. BMC Plant Biol. 2024, 24, 1209. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Yu, C.S.; Lin, C.J.; Hwang, J.K. Predicting subcellular localization of proteins for Gram-negative bacteria by support vector machines based on n-peptide compositions. Protein Sci. 2004, 13, 1402. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Dreos, R.; Ambrosini, G.; Périer, R.C.; Bucher, P. The Eukaryotic Promoter Database: Expansion of EPDnew and new promoter analysis tools. Nucleic Acids Res. 2015, 43, 92–96. [Google Scholar] [CrossRef]
- Rauluseviciute, I.; Riudavets-Puig, R.; Blanc-Mathieu, R.; Castro-Mondragon, J.A.; Ferenc, K.; Kumar, V.; Lemma, R.B.; Lucas, J.; Chèneby, J.; Baranasic, D.; et al. JASPAR 2024: 20th anniversary of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2024, 52, D174–D182. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).