Abstract
Synthetic insecticides are widely used against mosquitoes, but misuse has led to environmental and health concerns. Plant-derived alternatives, such as essential oils, seem to offer a safer option, minimizing these problems without compromising efficacy. In this study, we evaluated the essential oil from Siparuna gesnerioides (Kunth) A.DC., a Neotropical plant, for its effectiveness in controlling Aedes (Stegomyia) aegypti (Linnaeus) larvae, a major vector of human diseases. We first assessed the phytochemistry of the essential oil and used in silico approaches to predict potential physiological targets of its larvicidal activities. Selectivity assays were conducted with Belostoma anurum (Herrich-Schäffer), a non-target predatory water bug. The major constituents of S. gesnerioides essential oil were γ-elemene (45.8%) and germacrene D (43.8%). This essential oil effectively killed larvae from both susceptible and resistant mosquito strains (LC50 = 0.070 μg/mL). However, such concentrations killed more than 80% of B. anurum nymphs. Molecular modeling suggested that the essential oil major components (γ-elemene and germacrene D) interact stably with mosquito acetylcholinesterases (AChEs), indicating a potential mechanism of action. Our results reinforce the potential of Siparuna essential oils in mosquito control. Nevertheless, the non-selective impact on mosquito predators, as seen with S. gesnerioides, highlights the need for caution in field applications.
1. Introduction
Aedes aegypti (Diptera: Culicidae) is a relevant vector for diseases such as yellow fever, dengue, Zika, chikungunya, and Mayaro fever, found across the Americas, Africa, and Asia [1,2,3]. Conventional control strategies, including the elimination of breeding sites and the application of insecticides, have been pivotal in managing these vector-borne diseases [4,5]. However, despite their effectiveness, these methods have drawn criticism due to the rise in global prevalence of A. aegypti and the development of resistance in mosquito populations [6,7]. Furthermore, the widespread use of insecticides has raised concerns about their detrimental impacts on human health and the environment, especially on non-target fauna [8,9,10,11].
In response to these challenges, efforts have been made to explore alternative approaches to achieve more sustainable mosquito control. Among these approaches, biorational products (e.g., plant extracts and essential oils) derived from botanical species have gained attention for their potential to control insect vectors with reduced risks compared to synthetic chemicals [12,13]. For instance, A. aegypti can be effectively managed using plant-based essential oils, although the efficacy of these biorational insecticides can vary depending on genetic and environmental factors that affects their chemical composition [14,15,16,17,18].
The Neotropical region, rich in diverse flora, remains an underutilized source of biologically active substances. Plants of the genus Siparuna (Siparunaceae), commonly known as Negramina, are widespread in South America and have been traditionally used in folk medicine [19,20,21,22]. Despite this plant genus contains a diverse range of species [23,24], most of the insecticidal potential investigations has focused on the Siparuna guianensis Aubl., whose essential oils (alone or in nanocomposites) are shown to be effective in controlling pests such ticks [16], moths [25,26], aphids [27], and mosquitoes [17,28,29] with low toxicity to non-target organisms [27,28,29].
In this study, we assessed the chemical composition and toxicity of essential oils extracted from S. gesnerioides (Kunth) A.DC. and compared these parameters with the essential oil extracted from S. guianensis collected in the same region. As undesired effects of plant-based biorational products are not only related to synthetic insecticides [30,31,32], we further analyzed the potential undesired effects of these essential oils on the giant water bugs, Belostoma anurum (Hemiptera: Belostomatidae), whose nymphs are generalist predators, representing a natural control agent for mosquitoes [9,33,34,35], and have been shown to be indirectly affected by synthetic and plant-based insecticides [9,35,36]. Furthermore, by applying in silico predictions, we further investigate the molecular interaction between the major constituents of the Siparuna essential oils and the A. aegypti acetylcholinesterases (AChEs), a detoxifying enzyme whose disrupted activities can lead to neurotransmission malfunctions in mosquitoes and have been target by several essential oils.
2. Results
2.1. Yield and Chemical Composition of Essential Oils
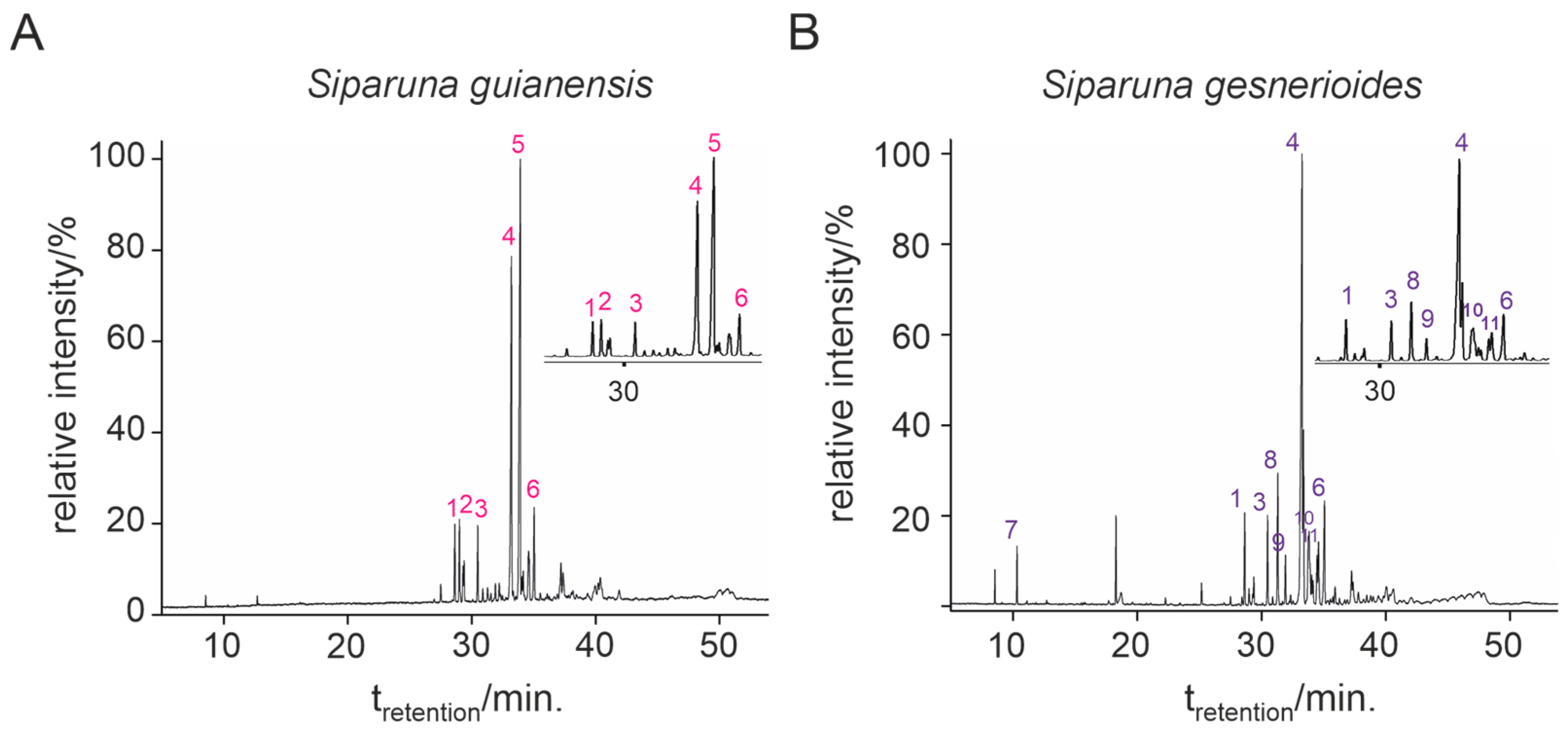
Siparuna gesnerioides presented the highest essential oil yield (0.384%) compared to S. guianensis (0.210%). Both essential oils presented a viscous liquid of bright yellow color, sui generis odor, and less dense than water. Qualitative and quantitative variations in chemical composition were observed between both essential oils. Six compounds were identified for S. guianensis (Table 1, Figure 1A), which represented 100% of the essential oil composition. For S. gesnerioides, nine compounds were identified (Table 1, Figure 1B), which represented 94.8% of the essential oil composition. All essential oils presented more than 94% of sesquiterpenes in their composition and very low contents of monoterpenes.

Table 1.
Chemical composition, concentrations (%), and terpene classification for the essential oil of Siparuna guianensis and Siparuna gesnerioides.
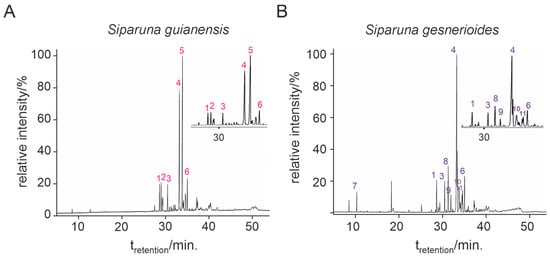
Figure 1.
Chemical composition of each of the constituents of Siparuna guianensis (A) and Siparuna gesnerioides (B) essential oils by gas chromatography coupled to mass spectrometry.
2.2. Toxicity of Siparuna Essential Oils Against Aedes aegypti Larvae
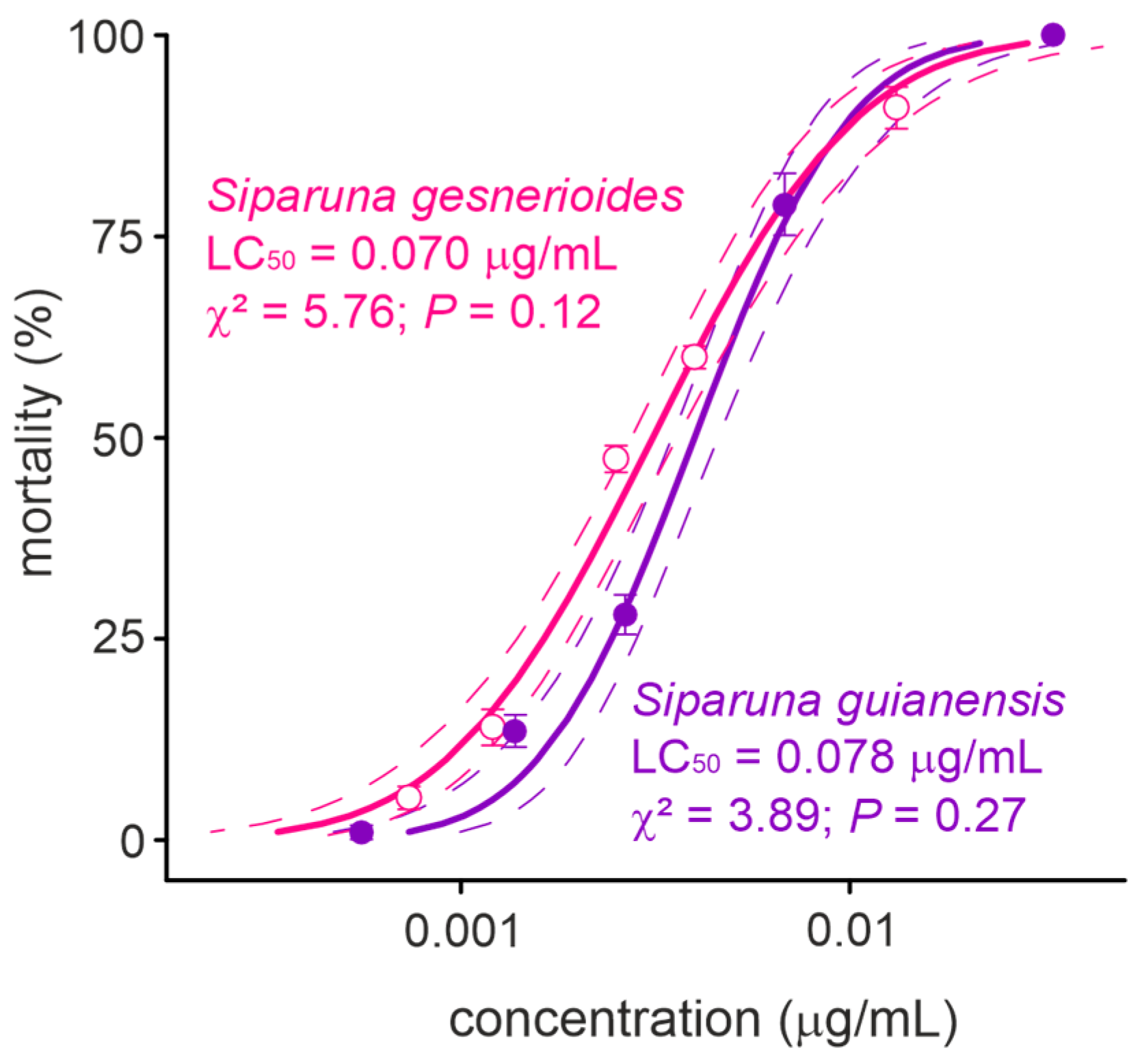
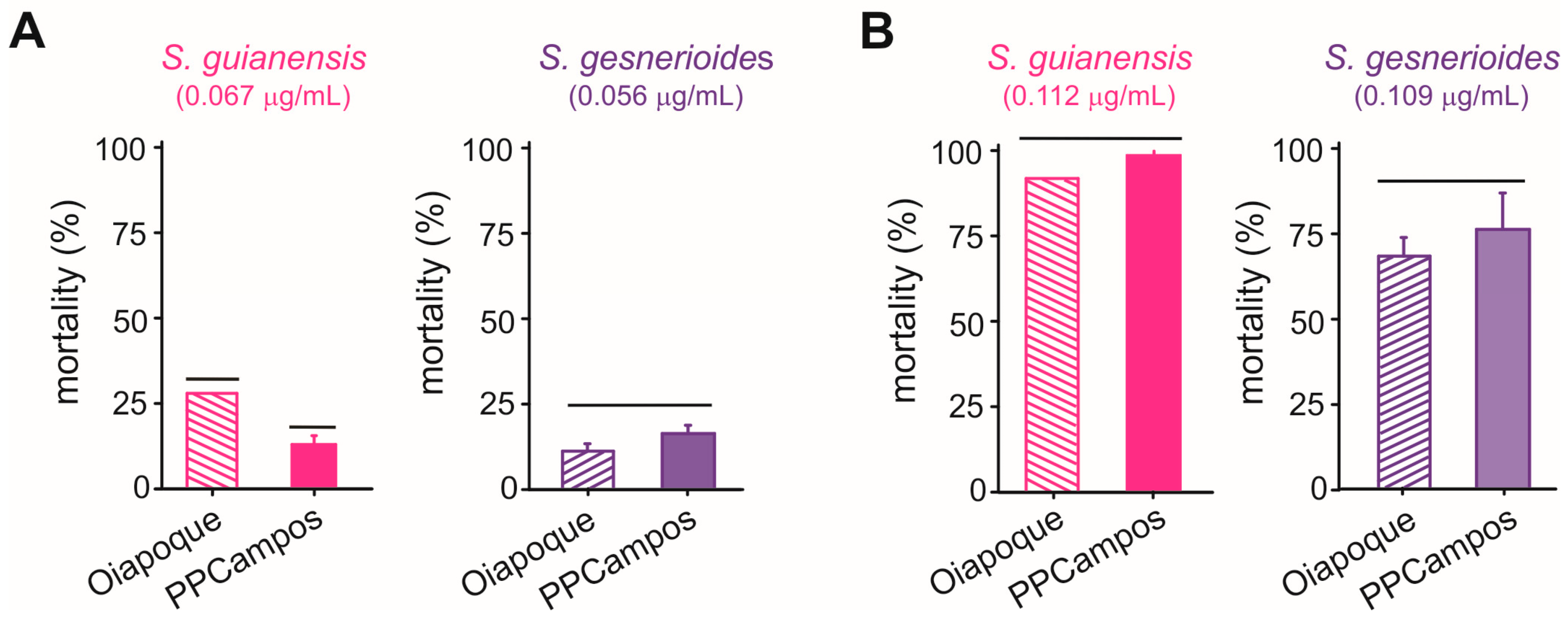
The concentration-mortality results obtained for S. guianensis (n = 700; χ2 = 3.89; p = 0.27) and S. gesnerioides (n = 700; χ2 = 5.76; p = 0.12) on A. aegypti larvae after a 24 h exposure were successfully fit to a Probit model (Figure 2), which allowed the estimation of the lethal concentrations for each of the Siparuna essential oils on the PPCampos strain. Aedes aegypti larvae were similarly killed by S. gesnerioides (LC50 = 0.070 [0.066–0.074] μg/mL) and S. guianensis (LC50 = 0.078 [0.073–0.082] μg/mL) (Table 2; Figure 2). Both Siparuna essential oils were similarly toxic to insecticide-susceptible and -resistant larvae of A. aegypti (Figure 3). Interestingly, the exposure to the LC25 (0.067 μg/mL) of S. guianensis essential oil killed significantly more insecticide-resistant larvae compared to susceptible mosquito larvae (Figure 3). Such differential activities were not presented to larvae exposed to the LC25 (0.056 μg/mL) of S. gesnerioides essential oil (Figure 3). At higher essential oil concentrations (i.e., LC80) there was no significant difference in terms of mortality of insecticide-susceptible and resistant larvae (Figure 3).
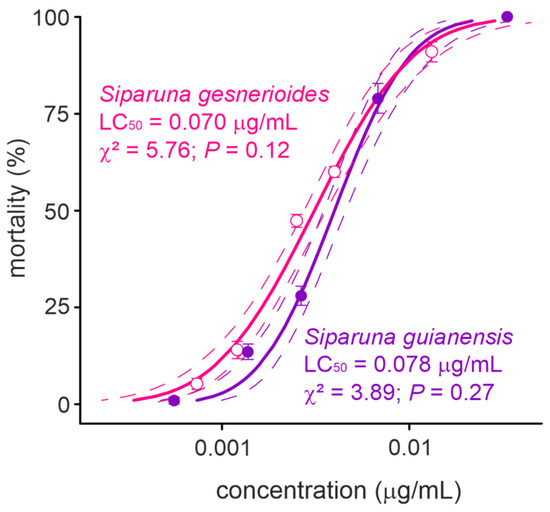
Figure 2.
Toxicity (24 h of exposure) of Siparuna gesnerioides and Siparuna guianensis to Aedes aegypti larvae. Solid lines represent the estimated mortality values by the PROBIT model. Dotted lines represent the 95% confidence intervals and symbols shows the mean (±SEM) obtained for four replicates.

Table 2.
Toxicity of essential oils of Siparuna guianensis and Siparuna gesnerioides to fourth instar larvae (L4) of Aedes aegypti over a 24 h exposure period. The lethal concentration (LC) values are expressed in μg/mL.
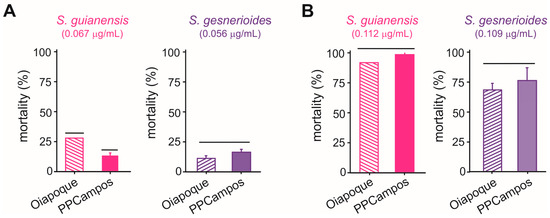
Figure 3.
Mortality (%) of Aedes aegypti larvae of insecticide-resistant (Oiapoque) and insecticide-susceptible (PPCampos) strains when exposed to Siparuna guianensis and Siparuna gesnerioides at their LC25 (A) and LC80 (B) values. Bars grouped at the same horizontal lines indicate the absence of significant differences according to Tukey’s HSD test (p < 0.05). Mortality was recorded over a 24 h period.
2.3. Molecular Interactions Between Essential Oil Major Constituents and Aedes aegypti Acetylcholinesterases
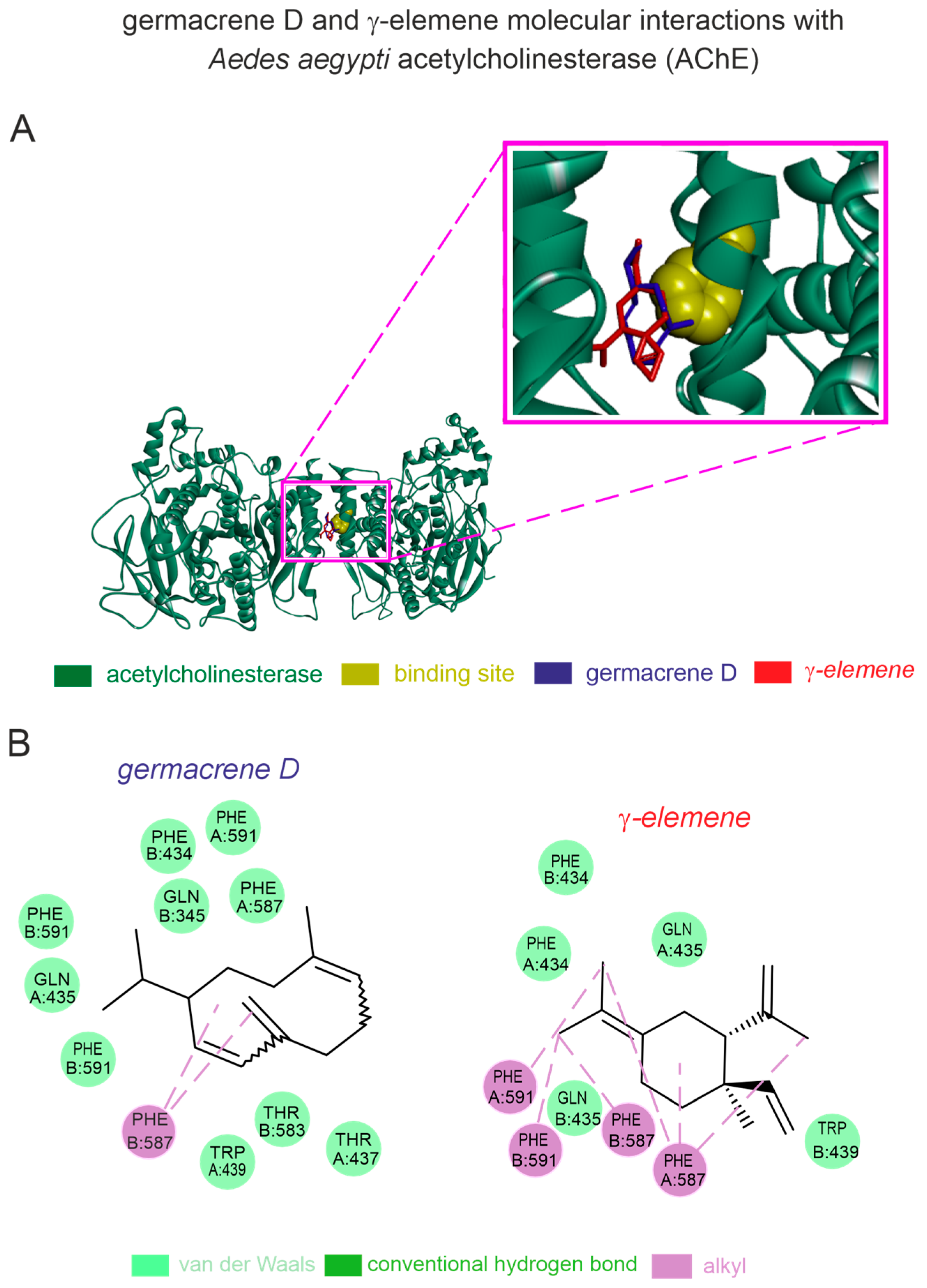
Based on our in silico computational predictions, the germacrene D and γ-elemene present in can interact with A. aegypti AChE through stable binding pockets. The homology modeling of the A. aegypti AChE showed a high-quality structure with 93% Ramachandran favored values and a QMEAN factor of −1.04. Both germacrene D (−6.8 kcal [−28.45 kJ]/mol) and γ-elemene (−6.7 kcal [−28.03 kJ]/mol) exhibited stronger binding affinities to the A. aegypti AChE. The germacrene D—A. aegypti AChE complex displayed van der Waals interactions with TRP439, THR583, THR437, PHE434, GLN435, PHE587, PHE434, and PHE591, as well as alkyl interactions with PHE587 (Figure 4). The γ-elemene complex showed van der Waals interactions with TRP439, GLN435, and PHE434, and pi-alkyl interactions with PHE587 and PHE591 (Figure 4).
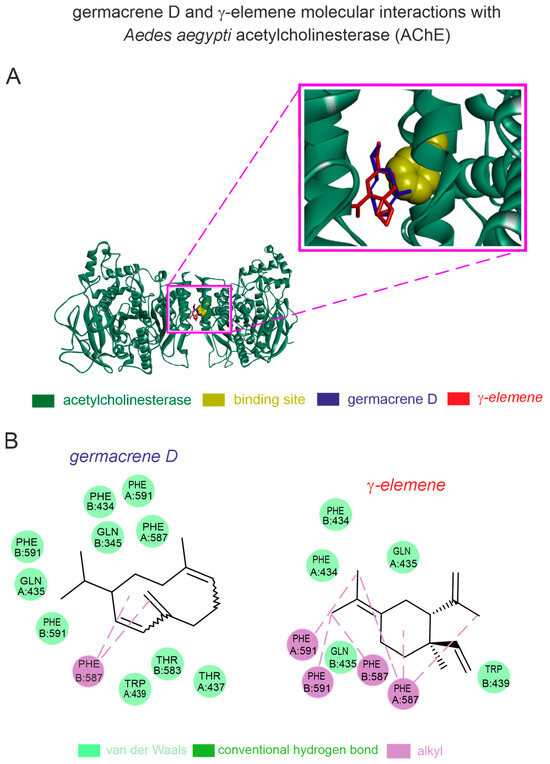
Figure 4.
In silico prediction of interactions of germacrene-D and γ-elemene with acetylcholinesterase (AChE) from the dengue-transmitting mosquito. (A) Protein 3D structure of the mosquito-related AChE with germacrene-D and γ-elemene molecules; (B) 2D interaction maps of AChE interaction sites with germacrene-D and γ-elemene.
2.4. Selectivity of Siparuna Essential Oils to Belostoma anurum Nymphs
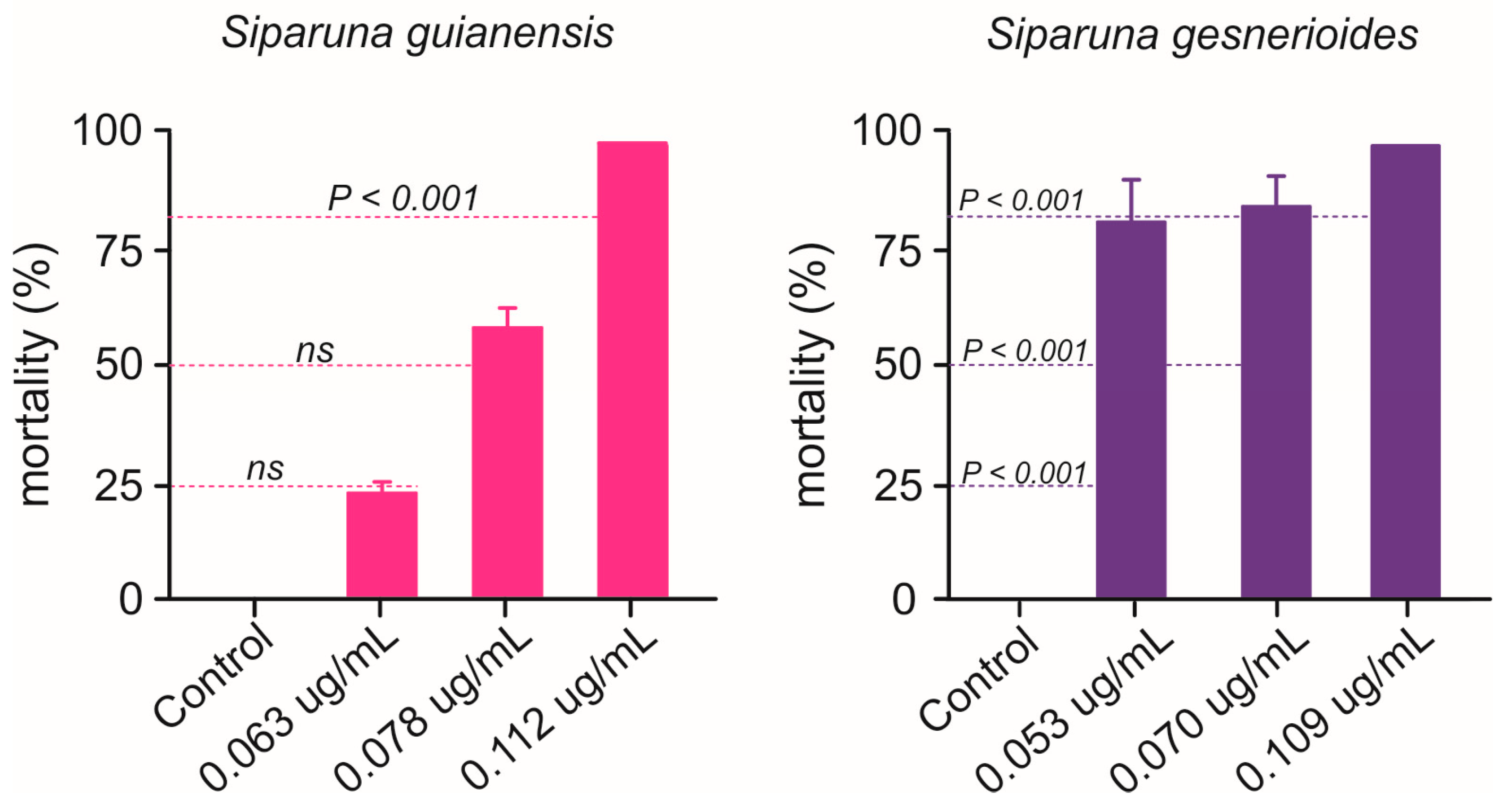
Nymphs of B. anurum were adversely affected when exposed to Siparuna essential oils (Figure 5). Siparuna gesnerioides essential oil exhibited higher toxicity by killing over 83% of B. anurum nymphs at concentration as lower as 0.053 μg/mL, the estimated LC25 for A. aegypti larvae (Figure 5). Nymphs exposed to S. gesnerioides essential oil LC50 (0.070 μg/mL) exhibited a mortality rate of 86.7%, while 100% of nymphs were killed at this essential oil LC80 (0.109 μg/mL) (Figure 5). At low (LC25 = 0.063 μg/mL) and intermediary (LC50 = 0.078 μg/mL) concentrations, the S. guianensis essential oil toxicity to B. anurum nymphs did not differ to that recorded for mosquito larvae (Figure 5). However, when the exposure was at highest concentration LC80 (0.109 μg/mL), the mortality rate of 100.0% of B. anurum nymphs, which is significantly higher than the mortality estimated for the mosquito larvae (Figure 5).
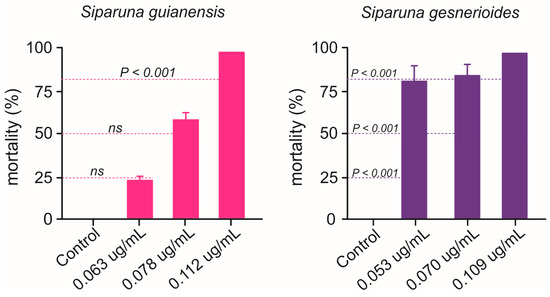
Figure 5.
Mortality (%) of Belostoma anurum exposed to concentrations (LC25, LC50, and LC80) of essential oils of Siparuna guianensis and Siparuna gesnerioides obtained with Aedes aegypti. Dashed lines represent the expected mortality based on the estimation for A. aegypti larvae. Significance levels (Z test, p < 0.05) are shown for each of concentration (i.e., LC25, LC50, and LC80). ns means the absence of statistically relevant differences. Mortality was recorded over a 24 h period.
3. Discussion
In this study, we demonstrated that while the essential oils of Siparuna gesnerioides and S. guianensis are sesquiterpene-rich, with germacrene D (in both essential oil types) and γ-elemene (only in S. guianensis essential oil) as major constituents. S. gesnerioides yielded a notably higher oil content (0.38%) compared to S. guianensis (0.21%). Both essential oils showed similar larvicidal activity against A. aegypti, though S. guianensis oil at LC25 exhibited greater lethality toward insecticide-resistant larvae. In contrast, S. gesnerioides essential oil was less selective to B. anurum nymphs, exhibiting higher lethality at lower concentrations (LC25), emphasizing the distinct selectivity of both essential oils. We further demonstrated, by means of an in silico analysis, that germacrene D and γ-elemene bind stably to A. aegypti AChE, suggesting a potential mode of action for these essential oils.
Plant-derived insecticides have re-emerged as viable alternatives in pest management, with essential oil of several Siparuna species showing toxic activity against agricultural and medically significant arthropods [16,17,25,26,27,29]. Our findings support the use Siparuna essential oils as biorational insecticides, particularly for controlling A. aegypti larvae, demonstrating that S. gesnerioides essential oil exhibited an LC50 values below 0.08 μg/mL. This larvicidal potency is within the range described for other Siparuna essential oils [17,28,38] and aligns with findings described elsewhere [39] noting that only 6% of essential oils tested (337 essential oil types from 225 plant species) as larvicides achieved LC50 values below 10 μg/mL. The essential oil yield (0.38%) for S. gesnerioides is within the range (i.e., between 0.10 and 0.61%) reported for other Siparuna essential oils [25,40,41,42].
Differences in essential oil composition between species, despite similar environmental conditions, may stem from genetic or physiological factors, as has been observed in other plants [16,43]. Our efforts represent the first phytochemical description of the essential oil of S. gesnerioides. The concentrations of the main constituents in our S. guianensis essential oils differ from those reported in previous studies [16,17,28,44], which also found variations in the amounts of the major compounds. Furthermore, while S. guianensis and S. gesnerioides essential oils share four compounds, including the major constituent germacrene D, some distinctions (e.g., the absence of γ-elemene in S. guianensis essential oil) may help to explain the recorded differences in mortality rates for A. aegypti and the non-target water bug B. anurum. These changes in the composition and concentration of the S. guianensis and S. gesnerioides essential oils may be due to plant physiological and genetic aspects since the two species were collected in the same area.
Although the main modes of action are not fully understood, comprehensive reviews have described that terpene-rich essential oils can disrupt the functions of ligand (e.g., octopamine, tyramine, and GABA)-activated receptors [45,46,47,48,49,50], non-selective ion (transient receptor potential—TRP) channels [27,51,52,53], and inhibiting the activities of acetylcholinesterase [54,55]. In this study, our molecular predictions indicated that both major constituents of S. guianensis and S. gesnerioides essential oils, germacrene D and γ-elemene, stably bind to A. aegypti AChE, suggesting that these molecules may disrupt the functions of these enzymes, adding insight into the mode of action of these Siparuna essential oils. Indeed, germacrene D, a common sesquiterpene in Siparuna as in other plant species, has shown to mediate plant–insect interactions and a broad insecticidal activity [56,57,58], disrupting the AChE and TRP channel functions in target and non-target organism [51,59]. Regarding γ-elemene, several studies have shown its insecticidal/repellent activities [60,61,62,63], resulting in its inclusion on patented formulations [64]. However, our work provides the first direct evidence of potential molecular targets for γ-elemene in insects. It is worth noting that our efforts do not exclude the potential involvement of other constituents presented in minor concentrations, as recent investigations have shown that insecticidal/repellent activities often depend not on the most abundant compound but on specific interactions among oil constituents [65,66].
Despite the beneficial potential of essential oils from S. gesnerioides and S. guianensis for controlling mosquito larvae, and contrasting with previous investigations that have shown the low toxicity of Siparuna essential oils to non-target organisms [25,26,27,29], our findings indicate these plant-based biorational tools can also be toxic to non-target aquatic arthropods such as the nymphs of B. anurum. Such findings reinforce the necessity for the careful assessment of the unintended impacts associated with the use of plant-based biorational pesticides used for controlling mosquito larvae [12,13]. In contrast to our findings, previous investigations on larvae [35] have reported no lethality in B. anurum when exposed to extracts from Chiococca alba, even at concentrations capable of killing 80% of A. aegypti larvae, indicating that those extracts exhibited higher selectivity towards the predatory insects when compared to the essential oils tested here.
4. Materials and Methods
4.1. Collection of Plant Material and Extraction of Essential Oil
Leaves of S. guianensis and S. gesnerioides were collected in the municipality of Norcasia (5°34′27″ N 74°53′20″ O; altitude 700 m), Caldas State, Colombia. Taxonomic identification was confirmed by experts at the herbarium (Universidad de Caldas, Manizales, Caldas State, Colombia), where samples were deposited with reference numbers JAO 957 (S. guianensis) and JASG 1522 (S. guianensis). For each species, we collected young and old leaves from different parts of the plants, ensuring the randomness of the sample. The material was placed in paper bags, identified, and transported to the Zoology Laboratory of the Universidad de Caldas (Manizales, Colombia). The leaves were dried in the shade at room temperature. After drying, the leaves were placed in plastic bags and stored until the extraction of the essential oil. The essential oils were obtained in the Kupay laboratory (Bogota–Cundinamarca, Colombia) by steam distillation. Briefly, the essential oils were extracted using a steam distillation unit (10 Kg) that resembles those used by commercial distillers. In the extractions, about 1600 g of dried leaves were used and the distillation time was four hours.
4.2. Chemical Composition of the Essential Oil
The identification and quantification of essential oil constituents were performed using gas chromatography with a flame ionization detector (GC-FID, QP2010SE, Shimadzu, Japan) and gas chromatography coupled to mass spectrometry (GC-MS, QP2010SE, Shimadzu, Japan) according to the methodology of [67]. For these characterizations, the following conditions were adopted: the carrier gas used was helium (He) for both detectors with flow rate and linear velocity of 2.80 mL min−1 and 50.80 cm s−1 (GC-FID) and 1.98 mL min−1 and 50.90 cm s−1 (GC-MS), respectively. The injector temperature was 220 °C at a split ratio of 1:30; fused silica capillary column (30 m × 0.25 mm); Rtx®-5MS stationary phase (0.25 μm film thickness). The oven temperature had the following schedule: initial temperature of 40 °C, which remained for 3 min, and then the temperature was gradually increased at a rate of 3 °C min−1 until it reached 180 °C, where it remained for 10 min, having a total analysis time of 59.67 min. The temperatures that were used in the flame ionization (FID) and MS detectors were 240 and 200 °C, respectively. The samples used were drawn from the vial in a volume of 1 μL of a 1% solution of EO in 95% hexane.
Analyses using gas chromatography coupled to mass spectrometry (GC-MS) were performed in an electron impact device with an energy of 70 eV; scan rate of 1000; scan interval of 0.50 fragments s−1; and detected fragments from 29 to 400 (m/z). Analyses using gas chromatography with a flame ionization detector (GC-FID) were performed with a flame formed by H2 and atmospheric air with a temperature of 300 °C. Flow rates of 40 mL min−1 and 400 mL min−1 were used for H2 and air, respectively. The identification of the components of the essential oils was performed by comparing the mass spectra obtained with those available in the spectrophotometer database (Wiley 7, NIST 05, and NIST 05s) and by the retention index (IR). For the IR calculation, a mixture of saturated C7-C40 alkanes (Supelco Inc., Bellefonte, PA, USA) submitted under the same chromatographic conditions as the essential oil was used, and the adjusted retention time of each compound was obtained using GC-FID. Then, the calculated values for each compound were compared with those in the literature [68,69,70]. The relative percentage of each compound in the EO was calculated by the ratio of the integral area of the peaks to the total area of all constituents in the sample with a relative area above 0.5%.
4.3. Aedes aegypti and Belostoma Anurum Rearing Conditions
We used A. aegypti larvae (fourth instar—L4) of the insecticide-susceptible (PPCampos) and insecticide-resistant (Oiapoque) strains that have been reared under controlled conditions in an insecticide-free environment over eight years. The larvae were kept in dechlorinated water, being fed daily with turtle food at a controlled temperature (temperature: 25 ± 2 °C; relative humidity: 60 ± 2%; photoperiod of 12 h of light) until the L4 stage, which is ideal for conducting larvicidal activity assays, following the methodology previously described in [7].
Nymphs of B. anurum were obtained from a laboratory strain that has been kept in an insecticide-free environment for over a decade. The initial strain consisted of individuals collected from fish farming facilities at the Federal University of Viçosa (UFV, Viçosa, MG, Brazil, 20°45′ S, 42°52′ W). Field-collected adult insects were maintained in a plastic pot (2L) in pairs (1 male and 1 female) with 1L of dechlorinated water under controlled conditions of a temperature of 25 ± 2 °C and a photoperiod of 12:12 L:D. Each aquarium had the presence of water hyacinth plants (Eichhornia crassipes (Mart.)), which were used as a resting and mating shelter. After mating, males with egg pads on their backs were kept in the plastic pot until hatching. After hatching, the first instar nymphs were individualized in glass containers (15 mL) with 10 mL of dechlorinated water to avoid cannibalism. The specimens were fed daily with A. aegypti larvae (L4) until the nymphs reached the second instar, which was used in bioassays.
4.4. Larvicidal Activity of Essential Oils Against Aedes Aegypti Larvae
We evaluated the larvicidal activity of S. gesnerioides essential oil in A. aegypti larvae (L4) of PPCampos (insecticide susceptibility pattern) strain, comparing its potential with the larvicidal activity recorded for the S. guianensis essential oil. For each essential oil type, we used at least five concentrations ranging from 0.033 μg/mL up to 0.196 μg/mL for S. guianensis essential oil, and from 0.037 μg/mL up to 0.131 μg/mL for S. gesnerioides essential oil. These essential oil concentrations were chosen based on a preliminary test to discover the smallest concentration capable of killing 100% of larvae and the highest concentration that did not kill any larvae tested. We always used dimethylsulfoxide (DMSO) at a concentration of 3.33 μg/mL to dilute the essential oils. Groups of 25 larvae were separated using a Pasteur pipette and subsequently distributed into glass vials (250 mL of volumetric capacity) containing 50 mL of the essential oil-containing solutions, which consisted in our experimental unit. Our control treatment consists of groups of 25 larvae subjected to solutions (50 mL) containing only DMSO (3.33 μg/mL). For each essential oil concentration and control treatment, we used four replicates, totalizing 100 larvae per treatment. Mortality was recorded after a 24 h exposure period. Larvae that did not show movement or response to stimulation with a Pasteur pipette were considered dead.
We further evaluated the potential of both S. guianensis and S. gesnerioides essential oils to kill larvae of insecticide-resistant (Oiapoque) strain. We subjected these insecticide-resistant larvae to the estimated LC25 (S. guianensis: 0.067 μg/mL; S. gesnerioides: 0.056 μg/mL) and LC80 (S. guianensis: 0.112 μg/mL; S. gesnerioides: 0.109 μg/mL) of the essential oils for the individuals of the PPCampos strain. The exposure to the LC25 evaluates the lethality at lower concentrations, while the LC80 represents the minimum insecticide concentration required by regulatory agencies for toxicological tests aimed at characterizing insecticide efficacy. The exposure procedures were identical to those described above. For each treatment, we used 100 fourth instar larvae (i.e., four groups of 25 larvae as replicates). Mortality was assessed at 24 h after the start of the experiment. All toxicity bioassays were conducted at controlled temperature (25 ± 2 °C), humidity (60 ± 2%), and photoperiod (12 h light phase).
4.5. Molecular Interactions Between the Essential Oil Major Constituents (Germacrene D and Γ-Elemene) and Acetylcholinesterases (AChEs) of Aedes Aegypti
We used in silico approaches to predict potential molecular interactions of the essential oil major constituents (germacrene D and γ-elemene) and the acetylcholinesterases (AChEs) of A. aegypti. Amino acid sequences of AChE of A. aegypti were retrieved from the National Center for Biotechnology Information (NCBI) database. The 3D structures of proteins were constructed by modeling approach using Swiss Model Workspace “https://swissmodel.expasy.org (accessed on 24 January 2025)” with the template of X-ray crystal structure of PDB code 6XYY for the A. aegypti AChE. The clashes in crystallographic structures and amino acid positioning in the active site were checked using Swiss model [71]. The validation of the generated models was performed by analyzing the Ramachandran plot [72,73], and the QMEAN factor was also analyzed [74].
To predict docking calculations, germacrene D and γ-elemene ligands were downloaded from PubChem [75] at National Institutes of Health (NIH) and saved in sdf format. Additionally, the ligands and AChE protein were prepared using Autodock Tools [76] adding charges, polar hydrogens, selecting grid to the protein and saving all molecules in pdbqt format [77]. Docking positions were generated for the germacrene D and γ-elemene interacting with AChE of A. aegypti, returning affinity energy values (kcal [4.184 kJ]/mol) using the AutoDock Vina [78]. The best position for each ligand–protein complex was used for generating 2D interaction maps with Discovery Studio [79].
4.6. Essential Oil Toxicity on Nymphs of Belostoma anurum
We exposed the 2nd instar nymphs of B. anurum to essential oils concentrations estimated to the PPCampos larvae. We used the S. guianensis concentrations of 0.063 μg/mL; 0.078 μg/mL; 0.112 μg/mL, which corresponds to the LC25, LC50, and LC80 values. Similar concentrations (LC25 = 0.053 μg/mL; LC50 = 0.070 μg/mL; LC80 = 0.109 μg/mL) were used for the S. gesnerioides essential oil. In order to avoid cannibalism, nymphs were individually exposed to the essential oils, where each of them was placed into a glass vial (20 mL) having 15 mL of the essential oil-containing solutions. Control individuals were exposed to DMSO (3.33 μg/mL)-containing solutions. For each treatment, we used 30 nymphs (i.e., three groups of 10 individualized nymphs as replicates). Mortality was assessed at 24 h after the start of the experiment. All toxicity bioassays were carried out under controlled conditions of temperature (25 ± 2 °C), humidity (60 ± 2%), and photoperiod (12 h light phase).
4.7. Statistical Analysis
The concentration-mortality results obtained in the toxicological bioassays with A. aegypti larvae were subjected to a Probit analysis using the PROBIT procedure in the SAS 9.2 statistical software platform (SAS Institute, Cary, NC, USA, 2008). The mortality of the insecticide-resistant larvae and B. anurum nymphs were subjected to analysis of variance (ANOVA) and compared by Tukey’s HSD test (p < 0.05) using SigmaPlot 14.0 (Systat Software, San Jose, CA, USA).
5. Conclusions
Our findings demonstrate that S. gesnerioides and S. guianensis possess comparable larvicidal activities, highlighting the potential of Siparuna essential oils as effective, environmentally friendly alternatives for integrated pest management. Notably, the increased toxicity of S. guianensis against A. aegypti larvae of a strain shown to exhibit resistance to insecticide larvae reinforces its promise as a strategy to combat resistance in mosquito populations. Furthermore, the molecular insights into the interaction of γ-elemene with AChE enhance our understanding of the mechanisms underlying the insecticidal effects of these essential oils, contributing valuable information for the development of natural pest control agents. However, the recorded lower selectivity of both Siparuna essential oils against B. anurum nymphs emphasizes the necessity of conducting thorough risk assessments for plant-based biorational insecticides. This challenge calls for the development of optimized formulations that can maximize the efficacy of Siparuna essential oils while minimizing their toxicity to non-target organisms.
Author Contributions
M.L.M.-C.: investigation, formal analysis, writing—original draft preparation; E.E.O.: conceptualization, formal analysis, supervision, funding acquisition, writing—original draft preparation, writing—review and editing; B.T.-R.: conceptualization, supervision, funding acquisition, writing—review and editing; T.B.: conceptualization, formal analysis, writing—review and editing; C.F.-H.: conceptualization, writing—review and editing; J.G.M.A.: investigation, formal analysis and writing—original draft preparation; R.P.L.M.: conceptualization, formal analysis, writing—review and editing; L.A.M.: investigation, formal analysis and writing—review and editing; R.W.S.A.: conceptualization, formal analysis, writing—review and editing; L.G.D.: conceptualization, supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science, Technology, and Innovation of Colombia (MinCiencias), the University of Caldas (project code: 0319320 for MLMC), and the Centro de Bioinformática y Biología Computacional de Colombia (BIOS), the BIOS research group and the convocatory 934-2023 of MinCiencias. It was also supported by the Coordination for the Improvement of Higher Education Personnel (CAPES, funding code 001). Additional support came from the National Council for Scientific and Technological Development of Brazil (CNPq, 408598/2023-9 and 309890/2022-5 for EEO; 152709/2022-3 for MLM-C).
Data Availability Statement
The data presented are available on request from the corresponding authors.
Acknowledgments
The authors thank the funding agencies from Colombia and Brazil for providing the funds needed to the conduction of the research.
Conflicts of Interest
The authors declare no competing interests.
References
- Jones, R.; Kulkarni, M.A.; Davidson, T.M.V.; Talbot, B.; Sander, B.; González, C. Arbovirus vectors of epidemiological concern in the Americas: A scoping review of entomological studies on Zika, dengue and chikungunya virus vectors. PLoS ONE 2020, 15, e0220753. [Google Scholar] [CrossRef] [PubMed]
- Pless, E.; Gloria-Soria, A.; Evans, B.R.; Kramer, V.; Bolling, B.G.; Tabachnick, W.J. Multiple introductions of the dengue vector, Aedes aegypti, into California. PLoS Negl. Trop. Dis. 2017, 11, e0005718. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.B.; Campbell, C.L.; Juneja, P.; Jiggins, F.M.; Sylla, M.; Black, W.C. Exon-enriched libraries reveal large genic differences between Aedes aegypti from Senegal, West Africa, and populations outside Africa. G3 Genes Genomes Genet. 2017, 7, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Roiz, D.; Wilson, A.L.; Scott, T.W.; Fonseca, D.M.; Jourdain, F.; Müller, P.; Velayudhan, R.; Corbel, V. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl. Trop. Dis. 2018, 12, 45–68. [Google Scholar] [CrossRef]
- World Health Organization. Larval Source Management: A Supplementary Malaria Vector Control Measure: An Operational Manual. 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/85379/9789241505604_eng.pdf (accessed on 22 April 2025).
- Reiter, P. Control of Urban Zika Vectors: Should We Return to the Successful PAHO/WHO Strategy? PLoS Negl. Trop. Dis. 2016, 10, e0004769. [Google Scholar] [CrossRef]
- Haddi, K.; Tomé, H.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 2017, 7, srep46549. [Google Scholar] [CrossRef]
- Valbon, W.; Araújo, S.H.C.; Nery, R.S.; Barbosa, J.F.; Newland, P.L.; Oliveira, E.E. Sublethal exposure to pyriproxyfen does not impair the abilities of the backswimmer Buenoa amnigenus to prey upon Aedes aegypti larvae. Ecotoxicology 2022, 31, 998–1008. [Google Scholar] [CrossRef]
- Valbon, W.R.; Hatano, E.; Oliveira, N.R.X.; Ataíde, Á.D.; Corrêa, M.J.M.; Gomes, S.F.; Martins, G.F.; Haddi , K.; Alvarenga, E.S.; Oliveira , E.E. Detrimental effects of pyriproxyfen on the detoxification and abilities of Belostoma anurum to prey upon Aedes aegypti larvae. Environ. Pollut. 2021, 284, 117130. [Google Scholar] [CrossRef]
- Devillers, J. Fate and ecotoxicological effects of pyriproxyfen in aquatic ecosystems. Environ. Sci. Pollut. Res. 2020, 27, 16052–16068. [Google Scholar] [CrossRef]
- Moura, J.A.S.; Souza-Santos, L.P. Environmental risk assessment (ERA) of pyriproxyfen in non-target aquatic organisms. Aquat. Toxicol. 2020, 222, 105448. [Google Scholar] [CrossRef]
- Costa, L.T.M.; Smagghe, G.; Viteri Jumbo, L.O.; Santos, G.R.; Aguiar, R.W.S.; Oliveira, E.E. Selective actions of plant-based biorational insecticides: Molecular mechanisms and reduced risks to non-target organisms. Curr. Opin. Environ. Sci. Health 2025, 44, 100601. [Google Scholar] [CrossRef]
- Botina, L.L.; Martins, G.F. Biological mosquiticidal agents: Potential and effects on non-target organisms. Curr. Opin. Environ. Sci. Health 2024, 41, 100567. [Google Scholar] [CrossRef]
- Aungtikun, J.; Soonwera, M. Improved adulticidal activity against Aedes aegypti (L.) and Aedes albopictus (Skuse) from synergy between Cinnamomum spp. essential oils. Sci. Rep. 2021, 11, 4685. [Google Scholar] [CrossRef]
- França, L.P.; Amaral, A.C.F.; Ramos, A.d.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.; Branches, A.D.S.; Silva, J.N.; Silva, N.G.; et al. Piper capitarianum essential oil: A promising insecticidal agent for the management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. 2021, 28, 9760–9776. [Google Scholar] [CrossRef]
- Diniz, J.A.; Marchesini, P.; Zeringóta, V.; Matos, R.d.S.; Novato, T.P.L.; Melo, D.; Vale, L.; Lopes, W.D.Z.; Gomes, G.A.; Monteiro, C. Chemical composition of essential oils of different Siparuna guianensis chemotypes and their acaricidal activity against Rhipicephalus microplus (Acari: Ixodidae): Influence of α-bisabolol. Int. J. Acarol. 2022, 48, 36–42. [Google Scholar] [CrossRef]
- Aguiar, R.W.S.; Dos Santos, S.F.; Da Silva Morgado, F.; Ascencio, S.D.; De Mendonça Lopes, M.; Viana, K.F.; Didonet, J.; Ribeiro, B.M. Insecticidal and repellent activity of Siparuna guianensis Aubl. (Negramina) against Aedes aegypti and Culex quinquefasciatus. PLoS ONE 2015, 10, e0116765. [Google Scholar] [CrossRef]
- Jannuzzi, H.; Mattos, J.K.D.A.; Silva, D.B.D.; Gracindo, L.A.M.; Vieira, R.F. Avaliação agronômica e química de dezessete acessos de erva-cidreira [Lippia alba (Mill.) NE Brown] -quimiotipo citral, cultivados no Distrito Federal. Rev. Bras. Plantas Med. 2011, 13, 258–264. [Google Scholar] [CrossRef]
- Ferraz, V.; Poletto, K.Q.; Júnior, A.F.C.; Alves, A. Antimicrobial activity and medicinal biomass of Siparuna guianensis in Brazilian Cerrado forest, a global hotspot. J. Med. Plant Res. 2015, 9, 968–980. [Google Scholar]
- Vásquez, J.; Alarcón, J.C.; Jiménez, S.L.; Jaramillo, G.I.; Gómez-Betancur, I.C.; Rey-Suárez, J.P.; Jaramillo, K.M.; Muñoz, D.C.; Marín, D.M.; Romero, J.O. Main plants used in traditional medicine for the treatment of snake bites n the regions of the department of Antioquia, Colombia. J. Ethnopharmacol. 2015, 170, 158–166. [Google Scholar] [CrossRef]
- Félix-Silva, J.; Antônio Silva-Junior, A.; Zucolotto, S.M.; De M Fernandes-Pedrosa, F. Medicinal plants for the treatment of local tissue damage induced by snake venoms: An overview from traditional use to pharmacological evidence. Evid. -Based Complement. Altern. Med. 2017, 2017, 5748256. [Google Scholar] [CrossRef]
- Fernandes, D.A.; Gomes, B.A.; Mendonça, S.C.; Pinheiro, C.d.C.; Sanchez, E.O.F.; Leitão, S.G.; Fully, A.L.; Leitao, G. Alkaloids from Siparuna (Siparunaceae) are Predicted as the Inhibitors of Proteolysis and Plasma Coagulation Caused by Bothrops Jararaca Snake Venom. 2024, SSRN 4755935. Available online: https://papers.ssrn.com/abstract=4755935 (accessed on 22 April 2025).
- Leitão, G.G.; Simas, N.K.; Soares, S.S.V.; De Brito, A.P.P.; Claros, B.M.G.; Brito, T.B.M.; Monache, F.D. Chemistry and pharmacology of Monimiaceae: A special focus on Siparuna and Mollinedia. J. Ethnopharmacol. 1999, 65, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.; Novon, G.H. New species of Siparuna (Siparunaceae) IV. A new subcanopy tree from white-sand areas in Brazil and Venezuela. Novon 2005, 15, 202–206. [Google Scholar]
- Ferreira, T.P.; Oliveira, E.E.; Tschoeke, P.H.; Pinheiro, R.G.; Maia, A.M.S.; Aguiar, R.W.S. Potential use of Negramina (Siparuna guianensis Aubl.) essential oil to control wax moths and its selectivity in relation to honey bees. Ind. Crops Prod. 2017, 109, 151–157. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Haddi, K.; Ribeiro, B.M.; Corrêia, R.F.T.; Tomé, H.V.V.; Santos-Amaya, O.; Pereira, E.J.G.; Guedes, R.N.C.; Santos, G.R.; Oliveira, E.E.; et al. Essential oil of Siparuna guianensis as an alternative tool for improved lepidopteran control and resistance management practices. Sci. Rep. 2018, 8, 7215. [Google Scholar] [CrossRef] [PubMed]
- Toledo, P.F.S.; Ferreira, T.P.; Bastos, I.M.A.S.; Rezende, S.M.; Viteri Jumbo, L.O.; Didonet, J.; Andrade, B.S.; Melo, T.S.; Smagghe, G.; Oliveira, E.E.; et al. Essential oil from Negramina (Siparuna guianensis) plants controls aphids without impairing survival and predatory abilities of non-target ladybeetles. Environ. Pollut. 2019, 255, 113153. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.D.A.; D’Haveloose, N.P.; Cruz, R.A.S.; Araújo, R.S.; Carvalho, J.C.T.; Rocha, L.; Fernandes, L.P.; Da Costa, T.S.; Souto, R.N.P. Nano-emulsification Enhances the Larvicidal Potential of the Essential Oil of Siparuna guianensis (Laurales: Siparunaceae) Against Aedes (Stegomyia) aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 788–796. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.-M.G.; Oliveira, E.E.; Jumbo, L.O.V.; Ribeiro, B.M.; et al. Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624. [Google Scholar] [CrossRef]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.C.; Pereira, E.J.G.; Aguiar, R.W.S.; Oliveira, E. Rethinking biorational insecticides for pest management: Unintended effects and consequences. Pest. Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century-fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Turchen, L.M.; Wang, R.; Agathokleous, E. Bioinsecticides and non-target pest species. Curr. Opin. Environ. Sci. Health 2024, 41, 100570. [Google Scholar] [CrossRef]
- Valbon, W.R.; Haddi, K.; Gutiérrez, Y.; Cruz, F.M.; Azevedo, K.E.X.; Perez Campos, J.S.; Salaro, A.L.; Oliveira, E. Life History Traits and Predatory Performance of Belostoma anurum (Hemiptera: Belostomatidae), a Biological Control Agent of Disease Vector Mosquitoes. Neotrop. Entomol. 2019, 48, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Shaalan, E.; Canyon, D. Aquatic insect predators and mosquito control. Trop. Biomed. 2009, 26, 223–261. [Google Scholar]
- Borges, J.C.M.; Haddi, K.; Valbon, W.R.; Costa, L.T.M.; Ascêncio, S.D.; Santos, G.R.; Soares, I.M.; Barbosa, R.S.; Viana, K.F.; Silva, E.A.; et al. Methanolic Extracts of Chiococca alba in Aedes aegypti Biorational Management: Larvicidal and Repellent Potential, and Selectivity against Non-Target Organisms. Plants 2022, 11, 3298. [Google Scholar] [CrossRef] [PubMed]
- Valbon, W.R.; Cruz, F.M.; Ramos, G.S.; Tomé, H.V.V.; Oliveira, E.E. Sublethal exposure to deltamethrin reduces the abilities of giant water bugs to prey upon Aedes aegypti larvae. Chemosphere 2018, 191, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.L.; Jones, M.M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–194. [Google Scholar] [CrossRef]
- Moura, W.S.; Oliveira, E.E.; Haddi, K.; Corrêa, R.F.T.; Piau, T.B.; Moura, D.S.; Santos, S.F.; Grisolia, C.K.; Ribeiro, B.M.; Aguiar, R.W.S. Cassava starch-based essential oil microparticles preparations: Functionalities in mosquito control and selectivity against non-target organisms. Ind. Crops Prod. 2021, 162, 113289. [Google Scholar] [CrossRef]
- Luz, T.R.S.A.; de Mesquita, L.S.S.; Amaral, F.M.M.; do Coutinho, D.F. Essential oils and their chemical constituents against Aedes aegypti L. (Diptera: Culicidae) larvae. Acta Trop. 2020, 212, 105705. [Google Scholar] [CrossRef]
- García, J.; Gilardoni, G.; Cumbicus, N.; Morocho, V. Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin, A. Plants 2020, 9, 187. [Google Scholar] [CrossRef]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical Composition and Biological Activity of Five Essential Oils from the Ecuadorian Amazon Rain Forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Morocho, V.; Hidalgo-Tapia, M.; Delgado-Loyola, I.; Cartuche, L.; Cumbicus, N.; Valarezo, E. Chemical Composition and Biological Activity of Essential Oil from Leaves and Fruits of Limoncillo (Siparuna muricata (Ruiz & Pav.) A. DC.). Antibiotics 2023, 12, 82. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Kuckelhaus, G.P.; Tschoeke, P.H.; Cangussu, A.S.; Cibene, J.; Borges, M.; de Souza Moura, W.; de Souza Aguiar, R.W. Influence of seasonality on the yield and composition of the essential oil of Siparuna guianensis Aublet. Afr. J. Biotechnol. 2017, 16, 1611–1618. [Google Scholar]
- Andrade, M.A.; Cardoso, M.D.G.; De Andrade, J.; Silva, L.F.; Teixeira, M.L.; Resende, J.M.V.; Figueiredo, A.C.D.S.; Barroso, J.G. Chemical Composition and Antioxidant Activity of Essential Oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants 2013, 2, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, J.R.; Boina, D.R.; Chow, E.; Carlier, P.R.; Reina, M.; Gonzalez-Coloma, A. Mode of action of the plant-derived silphinenes on insect and mammalian GABAA receptor/chloride channel complex. Pestic. Biochem. Physiol. 2008, 91, 17–23. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant essential oils and formamidines as insecticides/acaricides: What are the molecular targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from american cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem. Mol. Biol. 2005, 35, 309–321. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Quantitative structure–activity relationships of monoterpenoid binding activities to the housefly GABA receptor. Pest. Manag. Sci. 2012, 68, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest. Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Jumbo, L.O.V.; Corrêa, M.J.M.; Gomes, J.M.; Armijos, M.J.G.; Valarezo, E.; Mantilla-Afanador, J.G.; Machado, F.P.; Rocha, L.; Aguiar, R.W.; Oliveira, E.E. Potential of Bursera graveolens essential oil for controlling bean weevil infestations: Toxicity, repellence, and action targets. Ind. Crops Prod. 2022, 178, 114611. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.; Aguiar, R.W.; Smagghe, G.; Oliveira, E.E. Disentangling the ecotoxicological selectivity of clove essential oil against aphids and non-target ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; da Cruz Araujo, S.H.; Mantilla Afanador, J.G.; Silva, A.C.F.; Machado, F.P.; Rocha, L.M.; Oliveira, E.E. Potential of Ocotea indecora Essential Oil for Controlling Drosophila suzukii: Molecular Predictions for Toxicity and Selectivity to Beneficial Arthropods. Neotrop. Entomol. 2024, 53, 189–199. [Google Scholar] [CrossRef]
- Miyazawa, M.; Nakahashi, H.; Usami, A.; Matsuda, N. Chemical composition, aroma evaluation, and inhibitory activity towards acetylcholinesterase of essential oils from Gynura bicolor DC. J. Nat. Med. 2016, 70, 282–289. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef]
- Stranden, M.; Borg-Karlson, A.K.; Mustaparta, H. Receptor Neuron Discrimination of the Germacrene D Enantiomers in the Moth Helicoverpa armigera. Chem. Senses 2002, 27, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Stranden, M.; Røstelien, T.; Liblikas, I.; Almaas, T.J.; Borg-Karlson, A.K.; Mustaparta, H. Receptor neurones in three heliothine moths responding to floral and inducible plant volatiles. Chemoecology 2003, 13, 143–154. [Google Scholar] [CrossRef]
- Martins, R.M.G.; Xavier-Júnior, F.H.; Barros, M.R.; Menezes, T.M.; de Assis, C.R.D.; de Melo, A.C.G.R.; Veras, B.O.; Ferraz, V.P.; Filho, A.A.; Yogui, G.T.; et al. Impact on cholinesterase-inhibition and in silico investigations of sesquiterpenoids from Amazonian Siparuna guianensis Aubl. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 252, 119511. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Senthilmurugan, S.; Vijayan, P.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Curzerene, trans-β-elemenone, and γ-elemene as effective larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus: Toxicity on non-target aquatic predators. Environ. Sci. Pollut. Res. 2018, 25, 10272–10282. [Google Scholar] [CrossRef]
- Benelli, G.; Govindarajan, M.; AlSalhi, M.S.; Devanesan, S.; Maggi, F. High toxicity of camphene and γ-elemene from Wedelia prostrata essential oil against larvae of Spodoptera litura (Lepidoptera: Noctuidae). Environ. Sci. Pollut. Res. 2018, 25, 10383–10391. [Google Scholar] [CrossRef]
- Lacruz, A.; Barrera-Cortés, J.; Lina-García, L.; Ramos-Valdivia, A.C.; Santillán, R. Nanoemulsified Formulation of Cedrela odorata Essential Oil and Its Larvicidal Effect against Spodoptera frugiperda (J.E. Smith). Molecules 2022, 27, 2975. [Google Scholar] [CrossRef]
- Enan, E. Compositions and Methods for Controlling Insects—Google Patents. US20080075796A1, 27 March 2008. Available online: https://patents.google.com/patent/US20080075796A1/en (accessed on 22 April 2025).
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.B.; et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, P.; Sanchez, S.; Duran, P.; Andreazza, F.; Isaacs, R.; Dong, K. Behavioral and physiological responses of Drosophila melanogaster and D. suzukii to volatiles from plant essential oils. Pest. Manag. Sci. 2021, 77, 3698–3705. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.A.; Silva, R.R.A.; Soares, E.E.d.O.; Corrêa, M.J.M.; Marques, C.S.; Ferreira, M.F.d.S. Optimization of inclusion complex’s preparation of Psidium cattleyanum S. essential oil and 2-hydroxypropyl-β-cyclodextrin by central composite design for application as larvicide in Aedes aegypti L. Ind. Crops Prod. 2023, 194, 116333. [Google Scholar] [CrossRef]
- El-Sayed, N.A.E.; Farag, A.E.S.; Ezzat, M.A.F.; Akincioglu, H.; Gülçin, İ.; Abou-Seri, S.M. Design, synthesis, in vitro and in vivo evaluation of novel pyrrolizine-based compounds with potential activity as cholinesterase inhibitors and anti-Alzheimer’s agents. Bioorg. Chem. 2019, 93, 103312. [Google Scholar] [CrossRef] [PubMed]
- Mallard, I.; Bourgeois, D.; Fourmentin, S. A friendly environmental approach for the controlled release of Eucalyptus essential oil. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 130–137. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 2017. Available online: http://www.juniperus.org/uploads/2/2/6/3/22639912/bk4frontisbnpreface-contents5thedonline2017.pdf (accessed on 22 April 2025).
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
- Ramachandran, G.N.; Sasisekharan, V. Conformation of Polypeptides and Proteins. Adv. Protein Chem. 1968, 23, 283–437. [Google Scholar] [CrossRef]
- Haas, J.; Barbato, A.; Behringer, D.; Studer, G.; Roth, S.; Bertoni, M. Continuous Automated Model EvaluatiOn (CAMEO) complementing the critical assessment of structure prediction in CASP12. Proteins Struct. Funct. Bioinform. 2018, 86, 387–398. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S. Update: Improved access to chemical data. Nucleic Acids Res. 2019, 47, 1102–1109. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar] [PubMed]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S.; Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Richmond, T.J. Dassault systèmes BIOVIA, discovery studio visualizer, 17.2, San Diego: Dassault Systèmes, 2016. J. Chem. Phys. 2000, 10, 21–9991. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).