Effects of Elevated Carbon Dioxide and Chronic Warming on Nitrogen (N)-Uptake Rate, -Assimilation, and -Concentration of Wheat
Abstract
1. Introduction
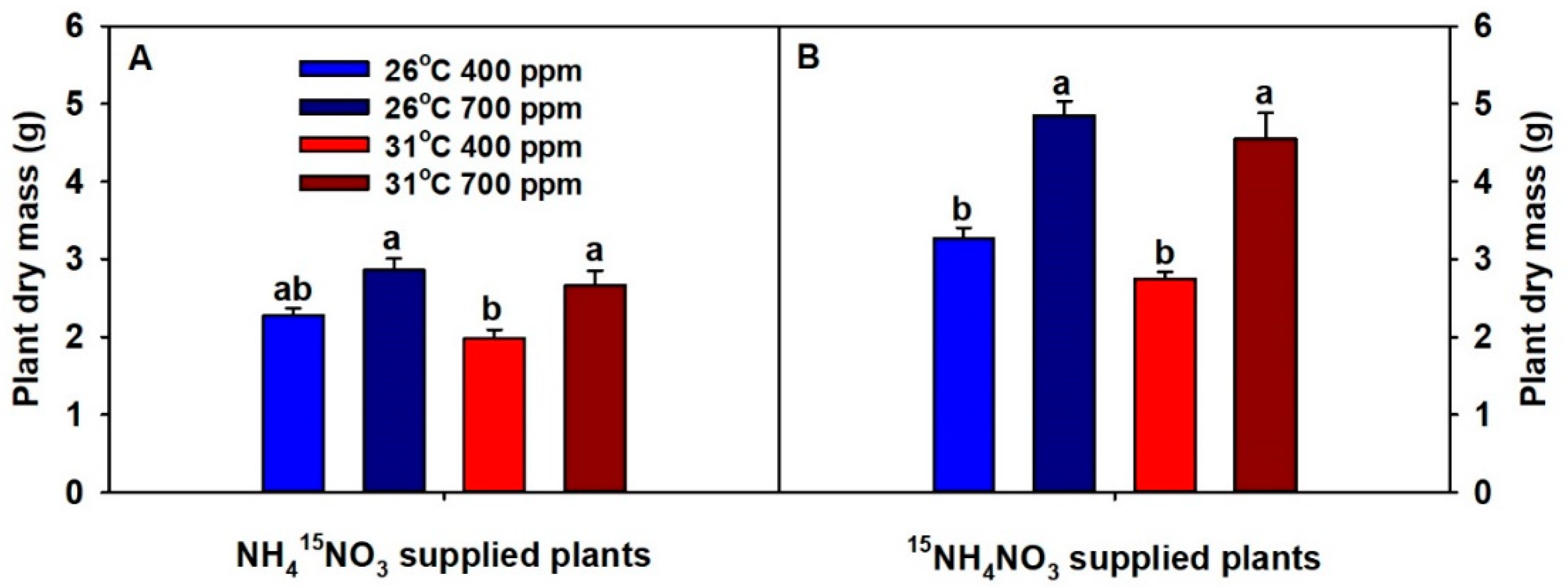
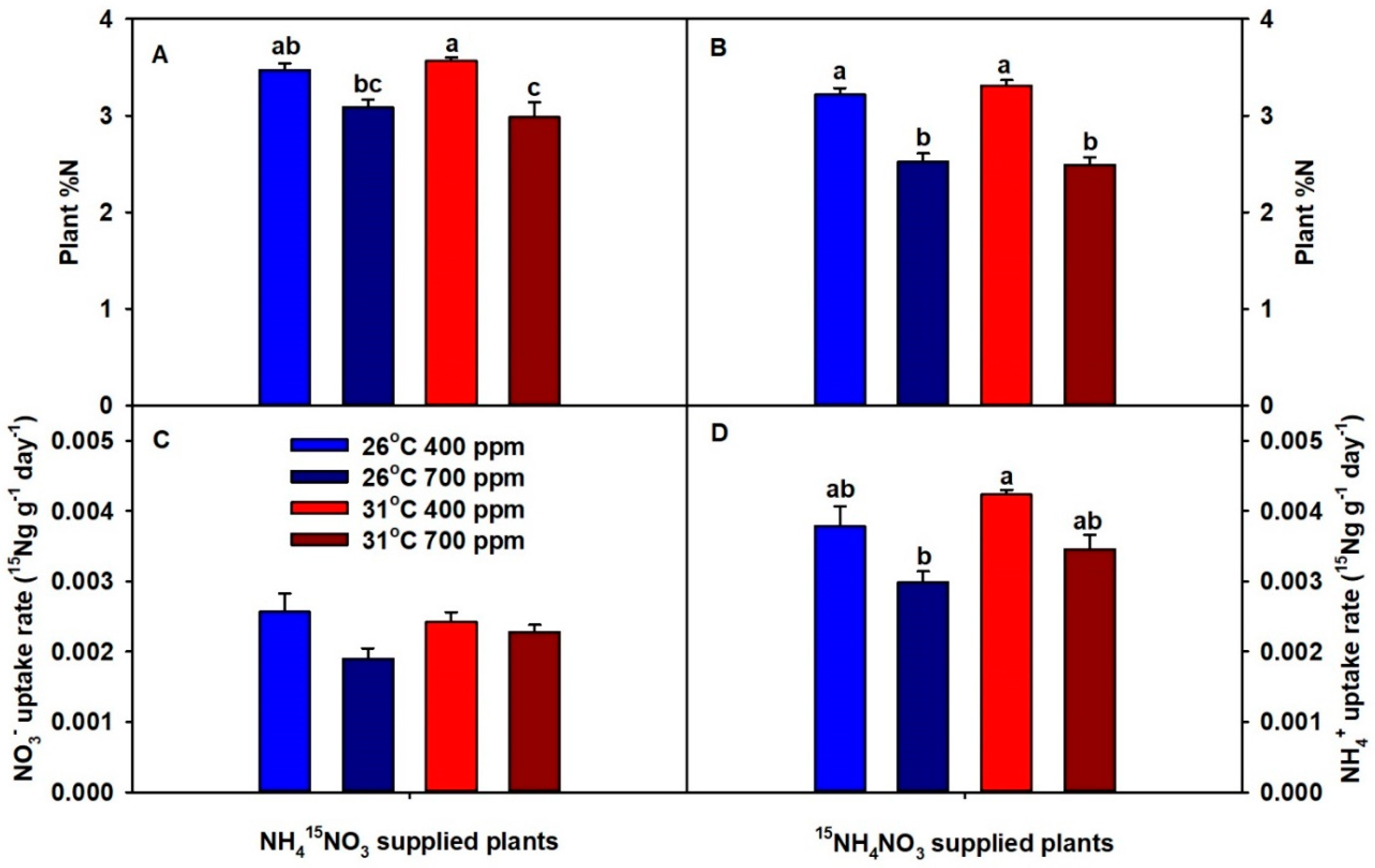
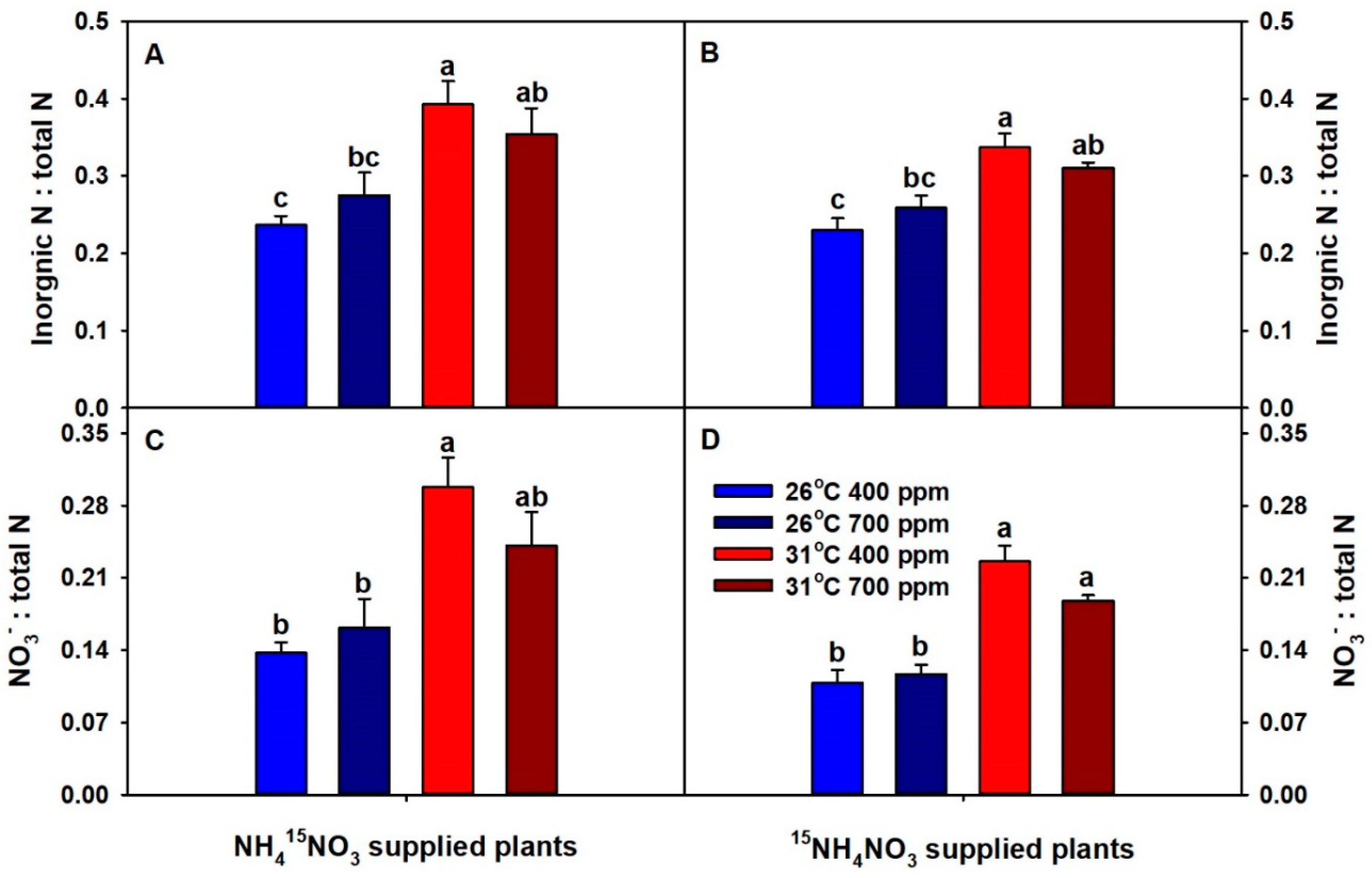
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material, Growth Conditions, and Treatments
4.2. Plant N and Protein Analysis
4.3. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wheat: Overview. Available online: https://www.ers.usda.gov/topics/crops/wheat/ (accessed on 22 January 2020).
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Barneix, A.J. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J. Plant Physiol. 2007, 164, 581–590. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Holm, P.B.; Krupinska, K. Leaf senescence and nutrient remobilisation in barley and wheat. Plant Biol. 2008, 10, 37–49. [Google Scholar] [CrossRef]
- Bloom, A.J.; Burger, M.; Asensio, J.S.R.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef]
- Taub, D.R.; Wang, X. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integr. Plant Biol. 2008, 50, 1365–1374. [Google Scholar] [CrossRef]
- Jauregui, I.; Aroca, R.; Garnica, M.; Zamarreño, Á.M.; García-Mina, J.M.; Serret, M.D.; Parry, M.; Irigoyen, J.J.; Aranjuelo, I. Nitrogen assimilation and transpiration: Key processes conditioning responsiveness of wheat to elevated [CO2] and temperature. Physiol. Plant. 2015, 155, 338–354. [Google Scholar] [CrossRef]
- Cai, C.; Yin, X.; He, S.; Jiang, W.; Si, C.; Struik, P.C.; Luo, W.; Li, G.; Xie, Y.; Xion, Y.; et al. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob. Chang. Biol. 2016, 22, 856–874. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Blumenthal, D.; Morgan, J.A.; Pendall, E.; Carrillo, Y.; Follett, R.F. Contrasting effects of elevated CO2 and warming on nitrogen cycling in a semiarid grassland. New Phytol. 2010, 187, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, D.M.; Heckathorn, S.A.; Bista, D.R.; Mishra, S.; Boldt, J.K.; Krause, C.R. Elevated CO2 plus chronic warming reduce nitrogen uptake and levels or activities of nitrogen-uptake and -assimilatory proteins in tomato roots. Physiol. Plant. 2017, 159, 354–365. [Google Scholar] [CrossRef] [PubMed]
- King, J.S.; Thomas, R.B.; Strain, B.R. Morphology and tissue quality of seedling root systems of Pinus taeda and Pinus ponderosa as affected by varying CO2, temperature, and nitrogen. Plant Soil 1997, 195, 107–119. [Google Scholar] [CrossRef]
- Li, C.; Zhu, J.G.; Sha, L.N.; Zhang, J.S.; Zeng, Q.; Liu, G. Rice (Oryza sativa L.) growth and nitrogen distribution under elevated CO2 concentration and air temperature. Ecol. Res. 2017, 32, 405–411. [Google Scholar] [CrossRef]
- Nelson, L.; Blumenthal, D.M.; Williams, D.G.; Pendall, E. Digging into the roots of belowground carbon cycling following seven years of Prairie Heating and CO2 Enrichment (PHACE), Wyoming USA. Soil Biol. Biochem. 2017, 115, 169–177. [Google Scholar] [CrossRef]
- Wan, S.; Norby, R.J.; Pregitzer, K.S.; Ledford, J.; O’Neill, E.G. CO2 enrichment and warming of the atmosphere enhance both productivity and mortality of maple tree fine roots. New Phytol. 2004, 162, 437–446. [Google Scholar] [CrossRef]
- Lilley, J.M.; Bolger, T.P.; Peoples, M.B.; Gifford, R.M. Nutritive value and the nitrogen dynamics of Trifolium subterraneum and Phalaris aquatica under warmer, high CO2 conditions. New Phytol. 2001, 150, 385–395. [Google Scholar] [CrossRef]
- Rosenthal, D.M.; Ruiz-Vera, U.M.; Siebers, M.H.; Gray, S.B.; Bernacchi, C.J.; Ort, D.R. Biochemical acclimation, stomatal limitation and precipitation patterns underlie decreases in photosynthetic stimulation of soybean (Glycine max) at elevated [CO2] and temperatures under fully open air field conditions. Plant Sci. 2014, 226, 136–146. [Google Scholar] [CrossRef]
- Salazar-Parra, C.; Aranjuelo, I.; Pascual, I.; Erice, G.; Sanz-Sáez, Á.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Araus, J.L.; Morales, F. Carbon balance, partitioning and photosynthetic acclimation in fruit-bearing grapevine (Vitis vinifera L. cv. Tempranillo) grown under simulated climate change (elevated CO2, elevated temperature and moderate drought) scenarios in temperature gradient greenhouses. J. Plant Physiol. 2015, 174, 97–109. [Google Scholar] [CrossRef]
- Johnson, S.N.; Hartley, S.E. Elevated carbon dioxide and warming impact silicon and phenolic-based defenses differently in native and exotic grasses. Glob. Chang. Biol. 2018, 24, 3886–3896. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Pais, I.P.; Leitão, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.J.C.; et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci. 2018, 9, 287. [Google Scholar] [CrossRef]
- Wang, J.; Hasegawa, T.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in grain protein and amino acids composition of wheat and rice under short-term increased [CO2] and temperature of canopy air in a paddy from East China. New Phytol. 2019, 222, 726–734. [Google Scholar] [CrossRef]
- Coleman, J.S.; Bazzaz, F.A. Effects of CO2 and temperature on growth and resource use of co-occurring C3 and C4 annuals. Ecology 1992, 73, 1244–1259. [Google Scholar] [CrossRef]
- Arndal, M.F.; Schmidt, I.K.; Kongstad, J.; Beier, C.; Michelsen, A. Root growth and N dynamics in response to multi-year experimental warming, summer drought and elevated CO2 in a mixed heathland-grass ecosystem. Funct. Plant Biol. 2014, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Pérez, P.; Martínez-Carrasco, R.; Usadel, B.; Kostadinova, S.; Morcuende, R. Quantitative RT-PCR platform to measure transcript levels of C and N metabolism-related genes in durum wheat: Transcript profiles in elevated [CO2] and high temperature at different levels of N supply. Plant Cell Physiol. 2015, 56, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, D.M.; Heckathorn, S.A.; Bista, D.R.; Boldt, J.K. Elevated carbon dioxide plus chronic warming causes dramatic increases in leaf angle in tomato, which correlates with reduced plant growth. Plant Cell Environ. 2019, 42, 1247–1256. [Google Scholar] [CrossRef]
- Bassirirad, H. Kinetics of nutrient uptake by roots: Responses to global change. New Phytol. 2000, 147, 155–169. [Google Scholar] [CrossRef]
- Mitchell, R.A.C.; Mitchell, V.J.; Driscoll, S.P.; Franklin, J.; Lawlor, D.W. Effects of increased CO2 concentration and temperature on growth and yield of winter wheat at two levels of nitrogen application. Plant Cell Environ. 1993, 16, 521–529. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, Z.; Jiang, D.; Högy, P.; Fangmeier, A. Independent and combined effects of elevated CO2 and post-anthesis heat stress on protein quantity and quality in spring wheat grains. Food Chem. 2019, 277, 524–530. [Google Scholar] [CrossRef]
- Wetselaar, R.; Farquhar, G.D. Nitrogen losses from tops of plants. Adv. Agron. 1980, 33, 263–302. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Reisdorf-Cren, M.; Orsel, M. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol. 2008, 10, 23–36. [Google Scholar] [CrossRef]
- Glenn Hard Red Spring Wheat. Available online: http://ndsuresearchfoundation.org/glenn (accessed on 6 November 2018).
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Smart, D.R.; Ferro, A.; Ritchie, K.; Bugbee, B.G. On the use of antibiotics to reduce rhizoplane microbial populations in root physiology and ecology investigations. Physiol. Plant. 1995, 95, 533–540. [Google Scholar] [CrossRef]
- Mishra, S.; Heckathorn, S.; Frantz, J.; Yu, F.; Gray, J. Effects of boron deficiency on geranium grown under different nonphotoinhibitory light levels. J. Am. Soc. Hortic. Sci. 2009, 134, 183–193. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid1. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayawardena, D.M.; Heckathorn, S.A.; Boldt, J.K. Effects of Elevated Carbon Dioxide and Chronic Warming on Nitrogen (N)-Uptake Rate, -Assimilation, and -Concentration of Wheat. Plants 2020, 9, 1689. https://doi.org/10.3390/plants9121689
Jayawardena DM, Heckathorn SA, Boldt JK. Effects of Elevated Carbon Dioxide and Chronic Warming on Nitrogen (N)-Uptake Rate, -Assimilation, and -Concentration of Wheat. Plants. 2020; 9(12):1689. https://doi.org/10.3390/plants9121689
Chicago/Turabian StyleJayawardena, Dileepa M., Scott A. Heckathorn, and Jennifer K. Boldt. 2020. "Effects of Elevated Carbon Dioxide and Chronic Warming on Nitrogen (N)-Uptake Rate, -Assimilation, and -Concentration of Wheat" Plants 9, no. 12: 1689. https://doi.org/10.3390/plants9121689
APA StyleJayawardena, D. M., Heckathorn, S. A., & Boldt, J. K. (2020). Effects of Elevated Carbon Dioxide and Chronic Warming on Nitrogen (N)-Uptake Rate, -Assimilation, and -Concentration of Wheat. Plants, 9(12), 1689. https://doi.org/10.3390/plants9121689





