Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms
Abstract
1. Introduction
2. Results
2.1. Electrical Conductivity of the Substrate
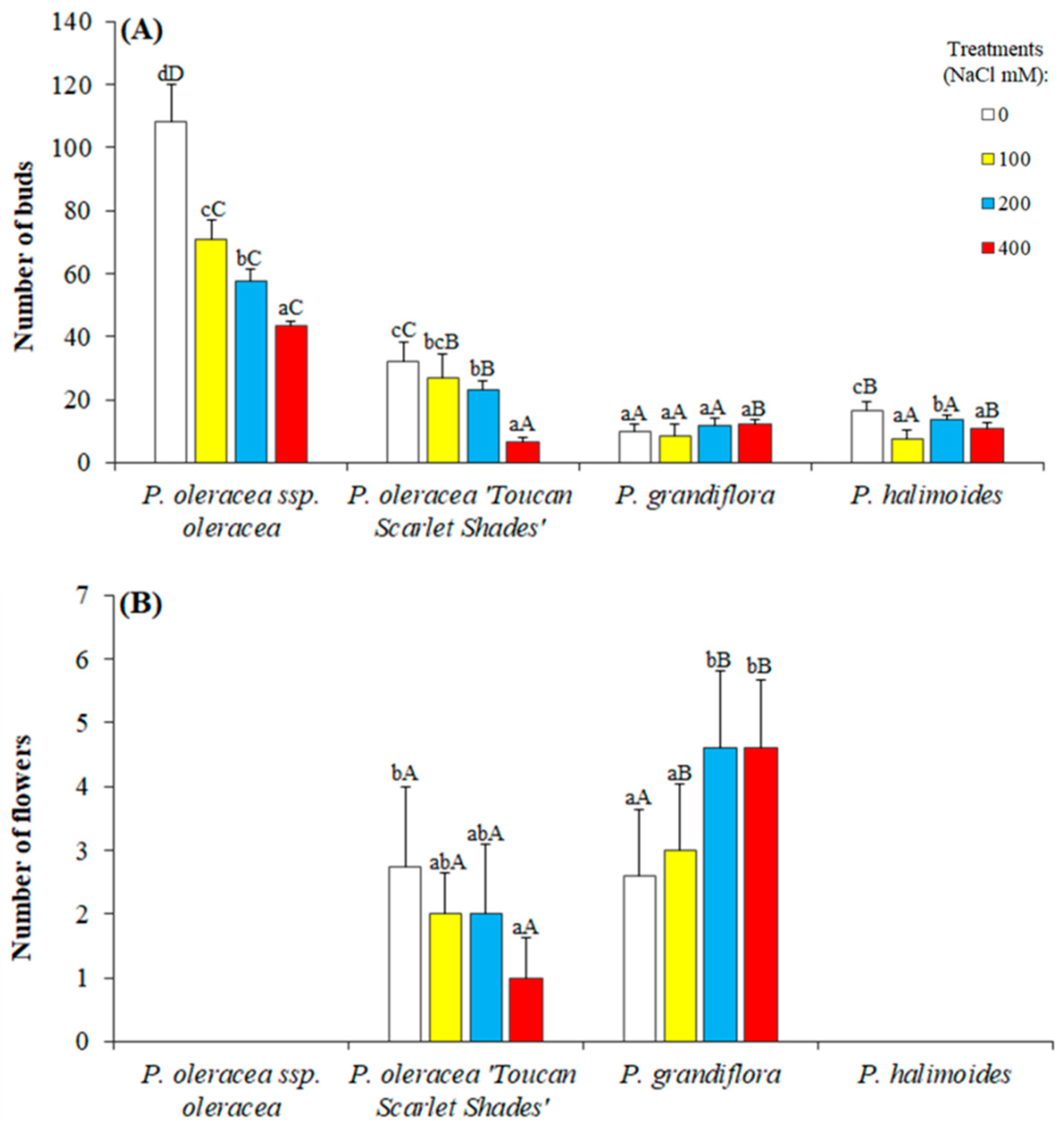
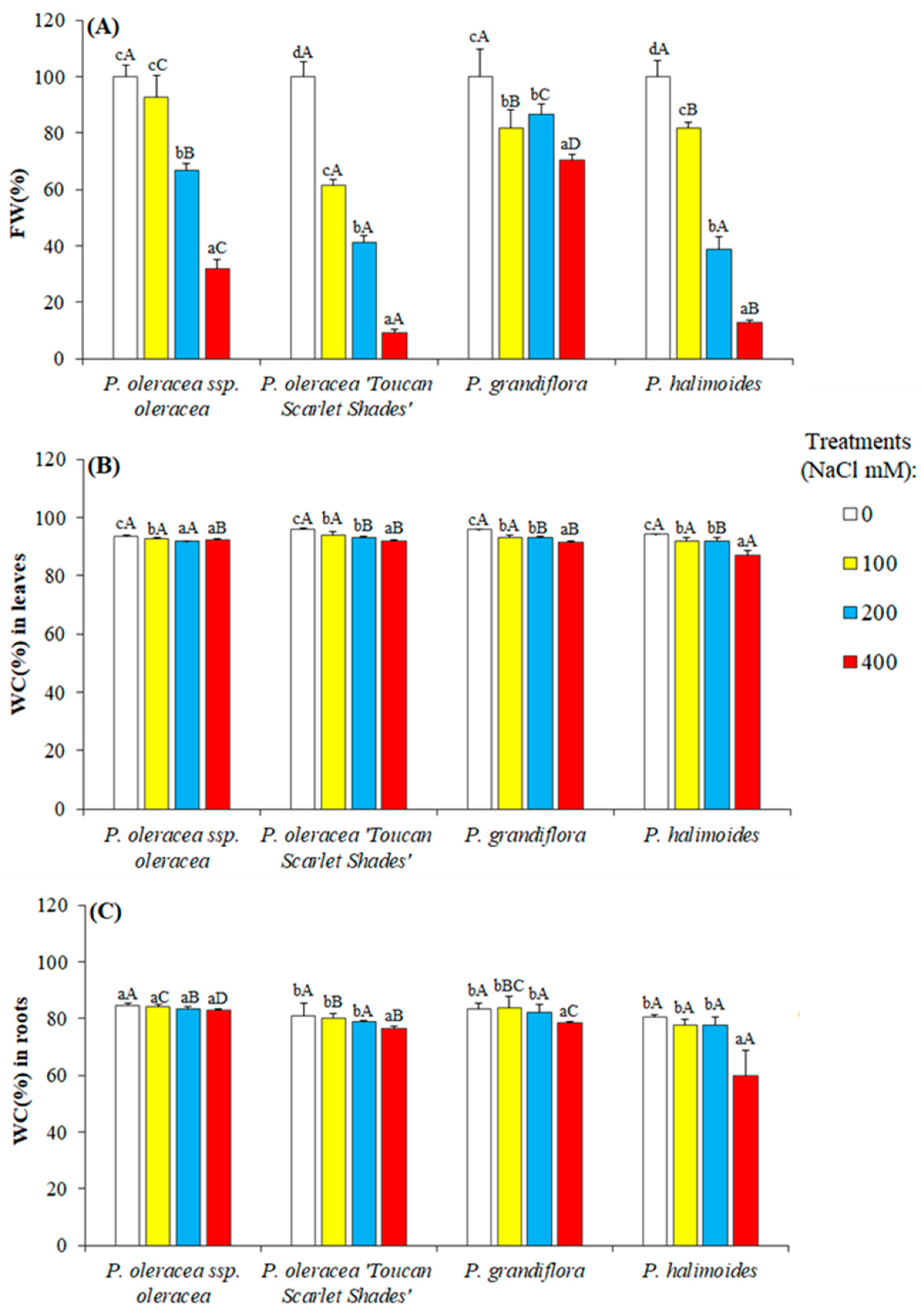
2.2. Growth Parameters
2.3. Photosynthetic Pigments
2.4. Osmolytes
2.5. Malodialdehyde and Non-Enzymatic Antioxidants
2.6. Ionic Content
2.7. Principal Component Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Stress Treatments
4.2. Plant Growth Parameters
4.3. Biochemical Plant Responses to Salt Stress
4.3.1. Photosynthetic Pigments
4.3.2. Osmolytes
4.3.3. Malondialdehyde
4.3.4. Total Phenolic and Flavonoid Content
4.3.5. Ion Content
4.3.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Van Breusegem, F.; Vranová, E.; Dat, J.F.; Inzé, D. The role of active oxygen species in plant signal transduction. Plant Sci. 2001, 161, 405–414. [Google Scholar] [CrossRef]
- Bartels, D.; Sunkar, R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005, 24, 23–58. [Google Scholar] [CrossRef]
- Katerji, N.; Van Hoorn, J.; Hamdy, A.; Mastrorilli, M. Salinity effect on crop development and yield, analysis of salt tolerance according to several classification methods. Agric. Water Manag. 2003, 62, 37–66. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Wild, A. Soils, Land and Food: Managing the Land During the Twenty-First Century; Cambridge University Press: Cambridge, UK, 2003; pp. 192–196. [Google Scholar]
- Owens, S. Salt of the Earth. EMBO Rep. 2001, 2, 877–879. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Meeting, World Health Organization. Evaluation of Certain Food Additives and Contaminants: Fifty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Cuevas, J.; Daliakopoulos, I.N.; Del Moral, F.; Hueso, J.J.; Tsanis, I.K. A Review of Soil-Improving Cropping Systems for Soil Salinization. Agronomy 2019, 9, 295. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.M.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Shoukat, A.; Hussan, M.U.; Sarwar, M.I. A review: Impact of salinity on plant growth. Nat. Sci. 2019, 17, 34–40. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B.; Mittra, B.N. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 2004, 18, 167–174. [Google Scholar] [CrossRef]
- He, M.; He, C.-Q.; Ding, N.-Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.; Hanson, A.D. Quaternary Ammonium and Tertiary Sulfonium Compounds in Higher Plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Rahnama, H.; Ebrahimzadeh, H. The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol. Plant. 2005, 49, 93–97. [Google Scholar] [CrossRef]
- Allen, J.A.; Chambers, J.L.; Stine, M. Prospects for increasing the salt tolerance of forest trees: A review. Tree Physiol. 1994, 14, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hajibagheri, M.A.; Clipson, N.J.W. Halophytes. Q. Rev. Biol. 1986, 61, 313–337. [Google Scholar] [CrossRef]
- Van De Wouw, M.; Kik, C.; Van Hintum, T.; Van Treuren, R.; Visser, B. Genetic erosion in crops: Concept, research results and challenges. Plant Genet. Resour. 2010, 8, 1–15. [Google Scholar] [CrossRef]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and Domesticating Crops Adapted to Drought and Salinity: A New Paradigm for Increasing Food Production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef]
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Zhang, T.; Su, R.; Zhang, Y.; Wang, L.; You, J.; Dossa, K. Depicting the Core Transcriptome Modulating Multiple Abiotic Stresses Responses in Sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2019, 20, 3930. [Google Scholar] [CrossRef]
- Al Hassan, M. Comparative Analyses of Plant Responses to Drought and Salt Stress in Related Taxa: A Useful Approach to Study Stress Tolerance Mechanisms. Ph.D. Thesis, Universitat Politecnica de Valencia, Valencia, Spain, 2016. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Misra, A.N.; Latowski, D.; Strzałka, K. The xanthophyll cycle activity in kidney bean and cabbage leaves under salinity stress. Russ. J. Plant Physiol. 2006, 53, 102–109. [Google Scholar] [CrossRef]
- Qados, A.M.A. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Rhodes, D.; Nadolska-Orczyk, A.; Rich, P. Salinity, osmolytes and compatible solutes. In Salinity: Environment-Plants-Molecules; Springer: Dordrecht, Germany, 2002; pp. 181–204. [Google Scholar] [CrossRef]
- Joshi, R.; Mangu, V.R.; Bedre, R.; Sanchez, L.; Pilcher, W.; Zandkarimi, H.; Baisakh, N. Salt adaptation mechanisms of halophytes: Improvement of salt tolerance in crop plants. In Elucidation of Abiotic Stress Signaling in Plants; Springer: New York, NY, USA, 2015; pp. 243–279. [Google Scholar]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Xu, J.; Tian, Y.-S.; Peng, R.-H.; Xiong, A.-S.; Zhu, B.; Jin, X.-F.; Gao, F.; Fu, X.-Y.; Hou, X.; Yao, Q.-H. AtCPK6, a functionally redundant and positive regulator involved in salt/drought stress tolerance in Arabidopsis. Planta 2010, 231, 1251–1260. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Fisher, D.G. Structural aspects of the leaves of seven species of Portulaca growing in Hawaii. Can. J. Bot. 1990, 68, 1803–1811. [Google Scholar] [CrossRef]
- Yazici, I.; Türkan, I.; Sekmen, A.H.; Demiral, T. Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation. Environ. Exp. Bot. 2007, 61, 49–57. [Google Scholar] [CrossRef]
- Sdouga, D.; Ben Amor, F.; Ghribi, S.; Kabtni, S.; Tebini, M.; Branca, F.; Trifi-Farah, N.; Marghali, S. An insight from tolerance to salinity stress in halophyte Portulaca oleracea L.: Physio-morphological, biochemical and molecular responses. Ecotoxicol. Environ. Saf. 2019, 172, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Grieve, C.; Suarez, D. Purslane (Portulaca oleracea L.): A halophytic crop for drainage water reuse systems. Plant Soil 1997, 192, 277–283. [Google Scholar] [CrossRef]
- Kafi, M.; Rahimi, Z. Effect of salinity and silicon on root characteristics, growth, water status, proline content and ion accumulation of purslane (Portulaca oleracea L.). Soil Sci. Plant Nutr. 2011, 57, 341–347. [Google Scholar] [CrossRef]
- Uddin, K.; Juraimi, A.S.; Ali, E.; Ismail, M.R. Evaluation of Antioxidant Properties and Mineral Composition of Purslane (Portulaca oleracea L.) at Different Growth Stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F. Screening of Purslane (Portulaca oleracea L.) Accessions for High Salt Tolerance. Sci. World J. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Hamidov, A. Environmentally useful technique—Portulaca oleracea golden purslane as a salt removal species. WSEAS Trans. Environ. Dev. 2007, 2, 117–122. [Google Scholar]
- Karakaş, S.; Çullu, M.A.; Dikilitaş, M. Comparison of two halophyte species (Salsola soda and Portulaca oleracea) for salt removal potential under different soil salinity conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Osma, E.; Ozyigit, I.I.; Demir, G.; Yasar, U. Assessment of some heavy metals in wild type and cultivated purslane (Portulaca oleracea L.) and soils in Istanbul, Turkey. Fresen. Environ. Bull. 2014, 23, 2181–2189. [Google Scholar]
- Hammami, H.; Parsa, M.; Mohassel, M.H.R.; Rahimi, S.; Mijani, S. Weeds ability to phytoremediate cadmium-contaminated soil. Int. J. Phytoremediat. 2015, 18, 48–53. [Google Scholar] [CrossRef]
- Negi, S. Heavy metal accumulation in Portulaca oleracea Linn. J. Pharmacogn. Phytochem. 2018, 7, 2978–2982. [Google Scholar]
- Zaman, S.; Bilal, M.; Du, H.; Che, S. Morphophysiological and Comparative Metabolic Profiling of Purslane Genotypes (Portulaca oleracea L.) under Salt Stress. BioMed Res. Int. 2020, 2020, 1–17. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Hakim, A. Salinity-induced changes in the morphology and major mineral nutrient composition of purslane (Portulaca oleracea L.) accessions. Biol. Res. 2016, 49, 24. [Google Scholar] [CrossRef]
- Ozturk, M.; Altay, V.; Güvensen, A. Portulaca oleracea: A vegetable from saline habitats. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.N., Ed.; Springer-Multimedia: Cham, Switzerland, 2020; pp. 1–14. [Google Scholar]
- Mulry, K.R.; Hanson, B.A.; Dudle, D.A. Alternative Strategies in Response to Saline Stress in Two Varieties of Portulaca oleracea (Purslane). PLoS ONE 2015, 10, e0138723. [Google Scholar] [CrossRef]
- Borsai, O.; Al Hassan, M.; Boscaiu, M.; Sestras, R.E.; Vicente, O. The genus Portulaca as a suitable model to study the mechanisms of plant tolerance to drought and salinity. EuroBiotech J. 2018, 2, 104–113. [Google Scholar] [CrossRef]
- Kichenaradjou, M.; Jawaharlal, M.; Arulmozhiyan, R.D.; Vijayaraghavan, H. Evaluation of ornamental groundcovers on physiological and quality parameters in salt affected soil condition. J. Pharmacogn. Phytochem. 2018, 7, 553–557. [Google Scholar]
- Guralnick, L.J.; Gilbert, K.E.; Denio, D.; Antico, N. The Development of Crassulacean Acid Metabolism (CAM) Photosynthesis in Cotyledons of the C4 Species, Portulaca grandiflora (Portulacaceae). Plants 2020, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Borsai, O.; Al Hassan, M.; Boscaiu, M.; Sestras, R.E.; Vicente, O. Effects of salt and drought stress on seed germination and seedling growth in Portulaca. Rom. Biotechnol. Lett. 2018, 23, 13340–13349. [Google Scholar]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709. [Google Scholar]
- Grigore, M.-N.; Toma, C. Succulence. In Anatomical Adaptations of Halophytes; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Grigore, M.N.; Toma, C. Kranz Anatomy. In Anatomical Adaptations of Halophytes; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Poljakoff-Mayber, A. Morphological and anatomical changes in plants as a response to salinity stress. In Plants in Saline Environments; Poljakoff-Mayber, A., Gale, J., Eds.; Springer: New York, NY, USA, 1975; pp. 97–117. [Google Scholar]
- Bernstein, L. osmotic adjustment of plants to saline media. II. dynamic phase. Am. J. Bot. 1963, 50, 360–370. [Google Scholar] [CrossRef]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Hand, M.J.; Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.; Tonfack, B.; Meguekam, T.L.; Youmbi, E. Effects of Salt Stress on Plant Growth, Nutrient Partitioning, Chlorophyll Content, Leaf Relative Water Content, Accumulation of Osmolytes and Antioxidant Compounds in Pepper (Capsicum annuum L.) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 481–490. [Google Scholar] [CrossRef]
- Ayala-Astorga, G.I.; Lilia, A.-M. Salinity effects on protein content, lipid peroxidation, pigments, and proline in Paulownia imperialis (Siebold & Zuccarini) and Paulownia fortunei (Seemann & Hemsley) grown In Vitro. Electron. J. Biotechnol. 2010, 13, 13–14. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Jasso-Plata, N.; Ochoa, I. Growth and Physiological Responses of Phaseolus Species to Salinity Stress. Int. J. Agron. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Siddiqi, E.H.; Ashraf, M. Can leaf water relation parameters be used as selection criteria for salt tolerance in safflower (Carthamus tinctorius L.). Pak. J. Bot. 2008, 40, 221–228. [Google Scholar]
- Akcin, A.; Yalcin, E. Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall. and Suaeda prostrata Pall. subsp. prostrata (Amaranthaceae). Braz. J. Bot. 2016, 39, 101–106. [Google Scholar] [CrossRef]
- Al Hassan, M.; Chaura, J.; López-Gresa, M.P.; Borsai, O.; Daniso, E.; Donat-Torres, M.P.; Mayoral, O.; Vicente, O.; Boscaiu, M. Native-Invasive Plants vs. Halophytes in Mediterranean Salt Marshes: Stress Tolerance Mechanisms in Two Related Species. Front. Plant Sci. 2016, 7, 473. [Google Scholar] [CrossRef]
- Rabhi, M.; Castagna, A.; Remorini, D.; Scattino, C.; Smaoui, A.; Ranieri, A.; Abdelly, C. Photosynthetic responses to salinity in two obligate halophytes: Sesuvium portulacastrum and Tecticornia indica. S. Afr. J. Bot. 2012, 79, 39–47. [Google Scholar] [CrossRef]
- Rangani, J.; Parida, A.K.; Panda, A.; Kumari, A. Coordinated Changes in Antioxidative Enzymes Protect the Photosynthetic Machinery from Salinity Induced Oxidative Damage and Confer Salt Tolerance in an Extreme Halophyte Salvadora persica L. Front. Plant Sci. 2016, 7, 50. [Google Scholar] [CrossRef]
- Redondo-Gómez, S.; Wharmby, C.; Castillo, J.M.; Mateos-Naranjo, E.; Luque, C.J.; De Cires, A.; Enrique Figueroa, M. Growth and photosynthetic responses to salinity in an extreme halophyte, Sarcocornia fruticosa. Physiol. Plant. 2006, 128, 116–124. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total. Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Hasanuzzaman, M.; Lao, M.T. Oxidative stress and antioxidant defense in plants under salinity. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 291–309. [Google Scholar] [CrossRef]
- Sun, H.; Sun, X.; Wang, H.; Ma, X. Advances in salt tolerance molecular mechanism in tobacco plants. Hereditas 2020, 157, 1–6. [Google Scholar] [CrossRef]
- Lutts, S.; Majerus, V.; Kinet, J.-M. NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol. Plant. 1999, 105, 450–458. [Google Scholar] [CrossRef]
- De Lacerda, C.F.; Cambraia, J.; Oliva, M.A.; Ruiz, H.A. Osmotic adjustment in roots and leaves of two sorghum genotypes under NaCl stress. Braz. J. Plant Physiol. 2003, 15, 113–118. [Google Scholar] [CrossRef]
- Al Hassan, M.; Morosan, M.; López-Gresa, M.P.; Prohens, J.; Vicente, O.; Boscaiu, M. Salinity-Induced Variation in Biochemical Markers Provides Insight into the Mechanisms of Salt Tolerance in Common (Phaseolus vulgaris) and Runner (P. coccineus) Beans. Int. J. Mol. Sci. 2016, 17, 1582. [Google Scholar] [CrossRef]
- Cha-Um, S.; Kirdmanee, C. Effect of salt stress on proline accumulation, photosynthetic ability and growth characters in two maize cultivars. Pak. J. Bot. 2009, 41, 87–98. [Google Scholar]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 53, 247–257. [Google Scholar] [CrossRef]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lidon, A.L.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Suprasanna, P. Prospects of Halophytes in Understanding and Managing Abiotic Stress Tolerance. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 29–56. [Google Scholar]
- Kumar, D.; Al Hassan, M.; Naranjo, M.A.; Agrawal, V.; Boscaiu, M.; Vicente, O. Effects of salinity and drought on growth, ionic relations, compatible solutes and activation of antioxidant systems in oleander (Nerium oleander L.). PLoS ONE 2017, 12, e0185017. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. In California Agricultural Experiment Station Publications Series; College of Agriculture, University of California: Davis, CA, USA, 1950. [Google Scholar]
- Rahdari, P.; Tavakoli, S.; Hosseini, S.M. Studying of salinity stress effect on germination, proline, sugar, protein, lipid and chlorophyll content in purslane (Portulaca oleracea L.) leaves. J. Stress. Physiol. Biochem. 2011, 8, 182–193. [Google Scholar]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Lei, Z.; Huang, Q.; Li, T.; Shen, F.; Cheng, Q. A Salt Tolerance Evaluation Method for Sunflower (Helianthus annuus L.) at the Seed Germination Stage. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]





| Photosynthetic Pigment (mg g−1 DW) | Treatment (mM NaCl) | P. oleracea L. subsp. oleracea | P. oleracea “Toucan Scarlet Shades” | P. grandiflora | P. halimoides |
|---|---|---|---|---|---|
| Chl a | 0 | 7.82 ± 0.83 bC | 3.18 ± 0.21 bA | 4.08 ± 0.69 cB | 3.25 ± 0.17 aA |
| 100 | 9.90 ± 0.67 cC | 2.57 ± 0.42 aA | 3.27 ± 0.34 bAB | 3.76 ± 0.79 bB | |
| 200 | 7.87 ± 1.13 bC | 3.60 ± 0.19 cAB | 3.07 ± 0.36 aA | 4.28 ± 0.51 cB | |
| 400 | 5.45 ± 0.29 aB | 3.21 ± 0.61 bcA | 3.12 ± 0.46 aA | 5.57 ± 0.31 dB | |
| Chl b | 0 | 5.83 ± 0.97 bC | 1.20 ± 0.19 aA | 3.13 ± 0.96 bB | 1.29 ± 0.24 aA |
| 100 | 8.79 ± 1.20 cB | 0.95 ± 0.14 aA | 1.69 ± 0.41 aA | 1.21 ± 0.26 aA | |
| 200 | 6.09 ± 1.76 bB | 1.23 ± 0.08 aA | 1.48 ± 0.29 aA | 1.23 ± 0.07 aA | |
| 400 | 4.32 ± 0.42 aB | 1.27 ± 0.13 aA | 1.41 ± 0.19 aA | 1.79 ± 0.14 bA | |
| Caro | 0 | 0.49 ± 0.32 aA | 0.62 ± 0.04 aB | 0.50 ± 0.06 aA | 0.66 ± 0.06 aB |
| 100 | 0.23 ± 0.29 aA | 0.68 ± 0.12 aB | 0.52 ± 0.20 aB | 0.97 ± 0.25 bC | |
| 200 | 0.32 ± 0.32 aA | 0.91 ± 0.13 bB | 0.45 ± 0.15 aA | 1.20 ± 0.14 cC | |
| 400 | 0.42 ± 0.10 aA | 0.84 ± 0.23 bB | 0.66 ± 0.03 bA | 1.78 ± 0.15 dC |
| Chloride Ions (µmol g−1 DW) | Treatment (mM NaCl) | P. oleracea L. ssp. oleracea | P. oleracea “Toucan Scarlet Shades” | P. grandiflora | P. halimoides |
|---|---|---|---|---|---|
| Leaves | 0 | 136.35 ± 25.41aA | 244.18 ± 45.78aB | 256.34 ± 19.01aB | 217.45 ± 46.27aB |
| 100 | 1001.88 ± 132.14bC | 449.37± 99.91bA | 542.94 ± 133.24bA | 738.21 ± 20.06bB | |
| 200 | 1977.47 ± 164.27cC | 537.64 ± 108.71bA | 691.44 ± 116.61bA | 1105.85 ± 50.17cB | |
| 400 | 2436.03 ± 182.24dC | 908.43 ± 88.77cB | 618.84 ± 26.94bA | 1027.84 ± 248.03cB | |
| Roots | 0 | 357.26 ± 105.75aB | 125.47 ± 17.96aA | 337.74 ± 64.09aB | N.A. |
| 100 | 794.84 ± 181.52bA | 710.152 ± 44.68bA | 901.01 ± 108.31bA | N.A. | |
| 200 | 846.95 ± 236.21bA | 1444.22 ± 230.83cB | 1121.48 ± 211.66bcAB | N.A. | |
| 400 | 1489.29 ± 119.33cA | 2328.77 ± 173.74dB | 1336.98 ± 179.31cA | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsai, O.; Hassan, M.A.; Negrușier, C.; Raigón, M.D.; Boscaiu, M.; Sestraș, R.E.; Vicente, O. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants 2020, 9, 1660. https://doi.org/10.3390/plants9121660
Borsai O, Hassan MA, Negrușier C, Raigón MD, Boscaiu M, Sestraș RE, Vicente O. Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants. 2020; 9(12):1660. https://doi.org/10.3390/plants9121660
Chicago/Turabian StyleBorsai, Orsolya, Mohamad Al Hassan, Cornel Negrușier, M. Dolores Raigón, Monica Boscaiu, Radu E. Sestraș, and Oscar Vicente. 2020. "Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms" Plants 9, no. 12: 1660. https://doi.org/10.3390/plants9121660
APA StyleBorsai, O., Hassan, M. A., Negrușier, C., Raigón, M. D., Boscaiu, M., Sestraș, R. E., & Vicente, O. (2020). Responses to Salt Stress in Portulaca: Insight into Its Tolerance Mechanisms. Plants, 9(12), 1660. https://doi.org/10.3390/plants9121660








