In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass)
Abstract
1. Introduction
2. Results
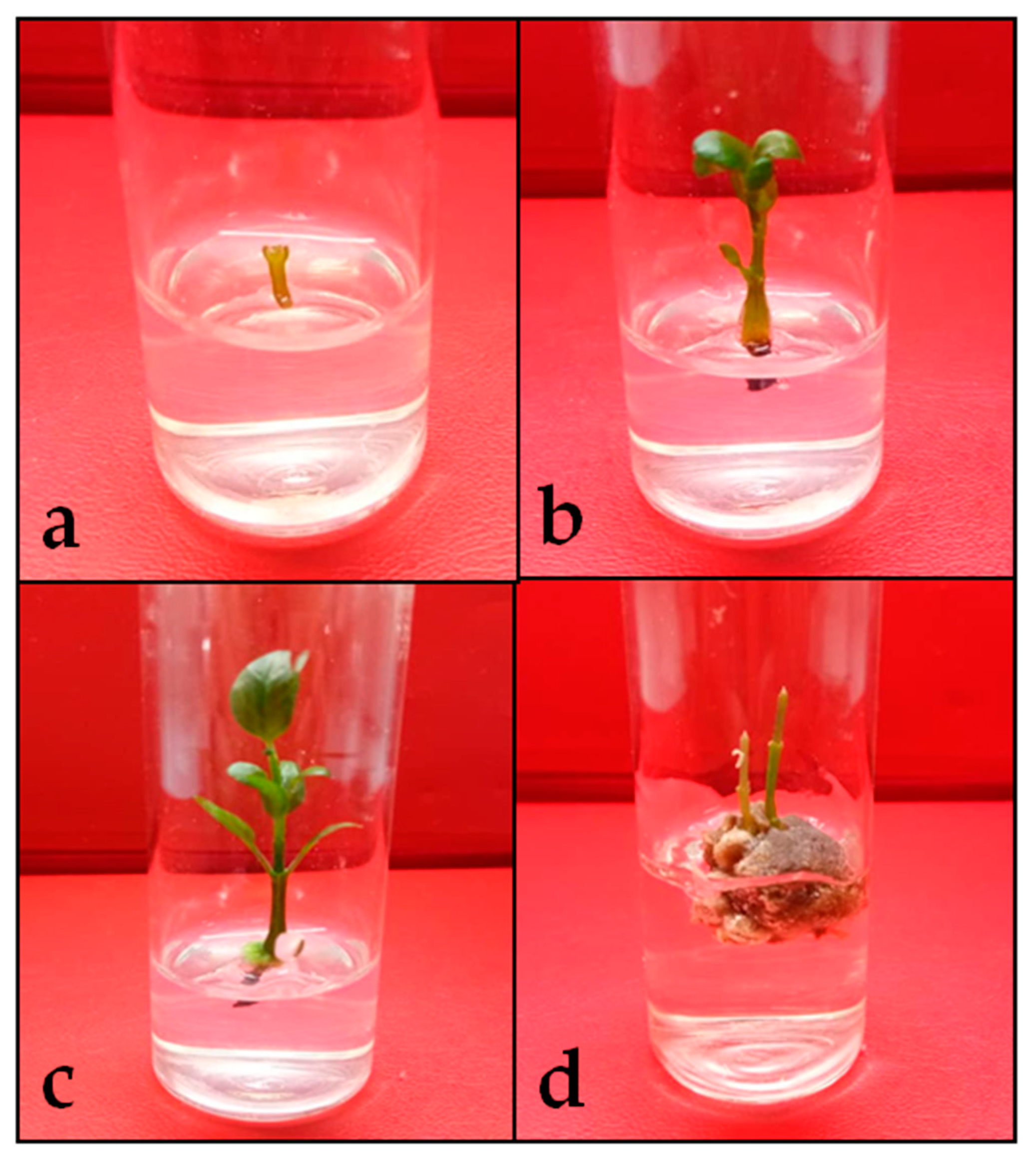
2.1. In Vitro Propagation of Clinacanthus nutans via Single Node Culture
2.1.1. Shoot Induction of C. nutans as Affected by Cytokinins
2.1.2. Synergistic Effect of Cytokinin and Auxins
2.1.3. Optimization of Basal Medium Strength
2.1.4. Effects of Sucrose Concentrations on the Growth of C. nutans
2.2. Quantification of Phenolics Contents and Antioxidant Activities of Clinacanthus nutans
2.2.1. Total Polyphenols, Phenolic Acids and Flavonoids Contents
2.2.2. Antioxidant Activities
2.2.3. Correlation Analysis between Variables
3. Discussion
4. Materials and Methods
4.1. In Vitro Propagation of Clinacanthus nutans via Single Node Culture
4.1.1. Planting Materials and Sterilization
4.1.2. Culture Medium and Conditions
4.1.3. Shoot Induction of C. nutans as Affected by Cytokinins
4.1.4. Synergistic Effect of Cytokinin and Auxins
4.1.5. Optimization of Basal Medium Strength
4.1.6. Effect of Sucrose Concentrations on the Growth of C. nutans
4.1.7. Experimental Unit and Culture Maintenance
4.2. Quantification of Phenolics Contents and Antioxidant Activities of Clinacanthus nutans
4.2.1. Planting Materials
4.2.2. Chemicals and Reagents
4.2.3. Preparation of Extract
4.2.4. Total Polyphenols Content
4.2.5. Total Phenolic Acids Content
4.2.6. Total Flavonoids Content
4.2.7. DPPH Free Radical Scavenging Activity
4.2.8. ABTS Scavenging Activity
4.2.9. Ferric Reducing Antioxidant Power (FRAP)
4.2.10. Superoxide Anion Radical Scavenging Activity
4.2.11. Iron (II) Chelating Activity
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marušk, A.; Proscevicius, J.; Bimbiraitė-Survilienė, K.; Kornyšova, O.; Ragažinskienė, O.; Ratautaitė, V. Comparison of phytochemical composition of medicinal plants by means of chromatographic and related techniques. Procedia Chem. 2010, 2, 83–91. [Google Scholar] [CrossRef]
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H. General overview of medicinal plants: A review. J. Phytopharm. 2017, 6, 349–351. [Google Scholar]
- MOA. Malaysia Department of Agriculture. Statistic of Crops Production Handbook. 2019. Available online: http://www.moa.gov.my (accessed on 27 September 2019).
- Fong, S.Y.; Piva, T.; Urban, S.; Huynh, T. Genetic homogeneity of vegetatively propagated Clinacanthus nutans (Acanthaceae). J. Med. Plants Res. 2014, 8, 903–914. [Google Scholar]
- Shim, S.Y.; Aziana, I.; Khoo, B.Y. Perspective and insight on Clinacanthus nutans Lindau in traditional medicine. Int. J. Integr. Biol. 2013, 14, 7–9. [Google Scholar]
- Tiew, W.P.; P’ng, X.W.; Chin, J.H.; Akowuah, G.A. Effect of methanol extract of Clinacanthus nutans on serum biochemical parameters in rats. J. Appl. Pharm. Sci. 2014, 6, 77–86. [Google Scholar]
- Teshima, K.I.; Kaneko, T.; Ohtani, K.; Kasai, R.; Lhieochaiphant, S.; Picheansoonthon, C.; Yamasaki, K. C-Glycosyl Flavones from Clinacanthus nutans (Natural Medicine Note). Nat. Med. 1997, 51, 557–561. [Google Scholar]
- Teshima, K.I.; Kaneko, T.; Ohtani, K.; Kasai, R.; Lhieochaiphant, S.; Picheansoonthon, C.; Yamasaki, K. Sulfur-containing glucosides from Clinacanthus nutans. Phytochemistry 1998, 48, 831–835. [Google Scholar] [CrossRef]
- Sriwanthana, B.; Chavalittumrong, P.; Chompuk, L. Effect of Clinacanthus nutans on human cell-mediated immune response in vitro. Thai J. Pharm. Sci. 1996, 20, 261–267. [Google Scholar]
- MOA. National Key Economic Area. 2012. Available online: http://www.moa.gov.my (accessed on 3 June 2019).
- Pratoomsoot, C.; Sruamsiri, R.; Dilokthornsakul, P.; Chaiyakunapruk, N. Quality of reporting of randomised controlled trials of herbal interventions in ASEAN plus six countries: A systematic review. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef]
- Tu, S.F.; Liu, R.H.; Cheng, Y.B.; Hsu, Y.M.; Du, Y.C.; El-Shazly, M.; Wu, Y.C.; Chang, F.R. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014, 19, 20382–20390. [Google Scholar] [CrossRef]
- Hamid, H.A.; Yahya, I.H.; Yusoff, M.M.; Zareen, S. Bioassay-guided isolation and antioxidant activity of sulfur-containing compounds from Clinacanthus nutans. J. Chin. Chem. Soc. 2016, 63, 1033–1037. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hill, K.; Schaller, G.E. Enhancing plant regeneration in tissue culture: A molecular approach through manipulation of cytokinin sensitivity. Plant. Signal. Behav. 2013, 8, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Gallavotti, A. The role of auxin in shaping shoot architecture. J. Exp. Bot. 2013, 64, 2593–2608. [Google Scholar] [CrossRef]
- Janarthanam, B.; Gayathri, B.; Sumath, E. A rapid, high frequency regeneration of Justicia gendarussa Burm. f. Bangla. J. Sci. Ind. Res. 2011, 46, 201–204. [Google Scholar] [CrossRef]
- Thomas, T.D.; Yoichiro, H. In vitro propagation for the conservation of a rare medicinal plant Justicia gendarussa Burm. f. by nodal explants and shoot regeneration from callus. Acta Physiol. Plant. 2010, 32, 943–950. [Google Scholar] [CrossRef]
- Purkayastha, J.; Sugla, T.; Paul, A.; Solleti, S.; Sahoo, L. Rapid In Vitro multiplication and plant regeneration from nodal explants of Andrographis paniculata: A valuable medicinal plant. In Vitro Cell Dev.-pl. 2008, 44, 442–447. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536–547. [Google Scholar] [CrossRef]
- Najar, R.A.; Fayaz, M.; Bhat, M.H.; Bashir, M.; Kumar, A.; Jain, A.K. An efficient micropropagation protocol for direct organogenesis from nodal explants of medicinal climber, Tylophora indica. Biosci. Biotech. Res. Comm. 2018, 11, 144–153. [Google Scholar] [CrossRef]
- Yadav, K.; Singh, N. Micropropagation of Spilanthes acmella Murr.—An important medicinal plant. Nat. Sci. 2010, 8, 5–11. [Google Scholar]
- Chen, B.; Zhang, J.; Zhang, W.; Zhang, C.; Xiao, Y. The rapid propagation technique of the medicinal plant Clinacanthus nutans by tissue culture. N. Y. Sci. J. 2015, 8, 23–27. [Google Scholar]
- Monfort, L.E.F.; Bertolucci, S.K.V.; Lima, A.F.; de Carvalho, A.A.; Mohammed, A.; Blank, A.F.; Pinto, J.E.B.P. Effects of plant growth regulators, different culture media and strength MS on production of volatile fraction composition in shoot cultures of Ocimum basilicum. Ind. Crop. Prod. 2018, 116, 231–239. [Google Scholar] [CrossRef]
- Naik, P.M.; Godbole, M.; Nagella, P.; Murthy, H.N. Influence of different media, medium strength and carbon sources on adventitious shoot cultures and production of bacoside A in Bacopa monnieri (L.). Ceylon J. Sci. 2017, 746, 97–104. [Google Scholar] [CrossRef]
- Nordine, A.; Bousta, D.; El Khanchoufi, A.; El Meskaoui, A. An efficient and rapid In Vitro propagation system of Thymus hyemalis Lange, a wild medicinal and aromatic plant of Mediterranean region. Int. J. Pharma. Bio. Sci. 2013, 1, 118–129. [Google Scholar]
- Naz, R.; Anis, M.; Aref, I.M. Management of cytokinin–auxin interactions for in vitro shoot proliferation of Althaea officinalis L.: A valuable medicinal plant. Rend. Lincei. 2015, 26, 323–334. [Google Scholar] [CrossRef]
- Reddy, M.C.; Bramhachari, P.V.; Murthy, K.S.R. Optimized plant tissue culture protocol for in vitro morphogenesis of an endangered medicinal herb Ceropegia ensifolia Bedd. Trop. Subtrop. Agroecosystems 2015, 18, 95–101. [Google Scholar]
- Javed, F.; Ikram, S. Effect of sucrose induced osmotic stress on callus growth and biochemical aspects of two wheat genotypes. Pak. J. Bot. Bot. 2008, 40, 1487–1495. [Google Scholar]
- Lipavská, H.; Konrádová, H. Somatic embryogenesis in conifers: The role of carbohydrate metabolism. Vitr. Cell Dev.-Pl. 2004, 40, 23–30. [Google Scholar]
- Ebrahim, M.K. Comparison, determination and optimizing the conditions required for rhizome and shoot formation, and flowering of In Vitro cultured calla explants. Sci. Hortic. 2004, 101, 305–313. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Tamta, S.; Bhatt, I.D.; Rawal, R.S. Phenolic compounds, antioxidant capacity and antimutagenic activity in different growth stages of in vitro raised plants of Origanum vulgare L. Mol. Biol. Rep. 2019, 46, 2231–2241. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated In Vitro. Folia Hortic. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- Bose, B.; Kumaria, S.; Choudhury, H.; Tandon, P. Assessment of genetic homogeneity and analysis of phytomedicinal potential in micropropagated plants of Nardostachys jatamansi, a critically endangered, medicinal plant of alpine Himalayas. Plant. Cell Tiss. Org. 2016, 124, 331–349. [Google Scholar] [CrossRef]
- Umebese, C.E.; Falana, F.D. Growth, phytochemicals and antifungal activity of Bryophyllum pinnatum L. subjected to water deficit stress. Afr. J. Biotechnol. 2013, 12, 6599–6604. [Google Scholar]
- Amoo, S.O.; Aremu, A.O.; van Staden, J. Shoot proliferation and rooting treatments influence secondary metabolite production and antioxidant activity in tissue culture-derived Aloe arborescens grown ex vitro. Plant. Growth Regul. 2013, 70, 115–122. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumaria, S.; Job, N.; Tandon, P. Phyto-molecular profiling and assessment of antioxidant activity within micropropagated plants of Dendrobium thyrsiflorum: A threatened, medicinal orchid. Plant. Cell Tiss. Org. 2015, 122, 535–550. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and In Vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. Study on the chemical composition and antioxidant activity of extracts from shoot culture and regenerated plants of Scutellaria altissima L. Acta Physiol. Plant. 2015, 37, 1736–1745. [Google Scholar] [CrossRef]
- Liu, B.L.; Fan, Z.B.; Liu, Z.Q.; Qiu, X.H.; Jiang, Y.H. Comparison of phytochemical and antioxidant activities in micropropagated and seed-derived Salvia miltiorrhiza plants. HortScience 2018, 53, 1038–1044. [Google Scholar] [CrossRef]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Hakiman, M.; Maziah, M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. J. Med. Plant. Res. 2009, 3, 120–131. [Google Scholar]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent. Am. J. Enolo. Viticult. 1965, 16, 144–158. [Google Scholar]
- Wong, S.P.; Lai, P.L.; Jen, H.W.K. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharm. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
| Treatment | Shoot Regeneration (%) | Number of Shoots | Length of Shoots (cm) | Number of Leaves | Leaves Fresh Weight (mg) |
|---|---|---|---|---|---|
| MS0 | 88.89 b | 1.00 e | 1.41 d | 2.22 d | 4.00 e |
| 4 µM BAP | 88.89 b | 1.30 c | 1.95 b | 3.44 c | 8.67 bc |
| 8 µM BAP | 100 a | 1.43 b | 1.92 b | 3.67 bc | 9.00 bb |
| 12 µM BAP | 100 a | 1.30 c | 2.29 a | 4.78 a | 16.00 a |
| 16 µM BAP | 100 a | 1.43 b | 1.96 b | 3.78 bc | 8.67 bc |
| 4 µM kinetin | 88.89 b | 1.10 d | 1.60 c | 2.11 d | 4.00 e |
| 8 µM kinetin | 100 a | 1.57 a | 1.67 c | 3.89 b | 6.00 de |
| 12 µM kinetin | 100 a | 1.30 c | 1.92 b | 3.44 c | 7.33 bcd |
| 16 µM kinetin | 100 a | 1.30 c | 1.88 b | 3.67 bc | 6.33 cde |
| Treatment (MS + 12 µM BAP + Auxin) | Shoot Regeneration (%) | Number of Shoots | Length of Shoots (cm) | Number of Leaves | Leaves Fresh Weight (mg) | Callus Fresh Weight (mg) |
|---|---|---|---|---|---|---|
| Control | 100 a | 1.67 a | 3.23 a | 7.34 a | 21.57 a | 10.00 d |
| 2 µM IBA | 77.78 c | 1.11 bc | 1.32 d | 3.22 bc | 3.43 bc | 37.57 bcd |
| 4 µM IBA | 88.89 b | 1.44 ab | 1.76 b | 4.66 b | 6.90 b | 42.23 abcd |
| 6 µM IBA | 66.67 d | 0.78 cd | 1.35 d | 2.33 bc | 3.53 bc | 53.10 abc |
| 8 µM IBA | 44.44 f | 0.44 e | 1.03 f | 0.89 c | 1.33 c | 69.00 ab |
| 2 µM NAA | 88.89 b | 1.11 bc | 1.59 c | 3.44 bc | 3.53 bc | 15.13 cd |
| 4 µM NAA | 77.78 c | 0.89 cd | 1.27 de | 1.67 c | 2.10 c | 81.43 a |
| 6 µM NAA | 77.78 c | 0.89 cd | 1.28 de | 2.22 bc | 1.77 c | 63.53 ab |
| 8 µM NAA | 55.56 e | 0.67 d | 1.13 ef | 1.67 c | 2.23 c | 60.53 ab |
| MS Basal Medium Strength | Shoot Regeneration (%) | Number of Shoots | Length of Shoots (cm) | Number of Leaves | Leaves Fresh Weight (mg) |
|---|---|---|---|---|---|
| Half-strength | 44.44 b | 0.67 b | 1.35 c | 3.00 b | 1.78 c |
| Full-strength | 100 a | 1.67 a | 3.23 a | 7.33 a | 21.57 a |
| Double-strength | 100 a | 1.44 a | 2.83 b | 6.67 a | 10.33 b |
| Sucrose Concentration (g/L) | Shoot Regeneration (%) | Number of Shoots | Length of Shoots (cm) | Number of Leaves | Leaves Fresh Weight (mg) |
|---|---|---|---|---|---|
| 20 | 100 a | 1.33 b | 2.71b | 6.97 ab | 16.67 b |
| 25 | 100 a | 1.56 ab | 3.06 a | 8.00 a | 14.67 bc |
| 30 | 100 a | 1.67 a | 3.23 a | 7.33 a | 21.57 a |
| 35 | 100 a | 1.67 a | 2.78 b | 6.23 b | 11.67 c |
| 40 | 77.78 b | 1.00 c | 2.39 c | 4.57 c | 6.00 d |
| 45 | 66.67 c | 0.78 d | 1.70 d | 2.80 d | 5.00 d |
| 50 | 33.33 d | 0.33 e | 1.18 e | 0.90 e | 0.68 e |
| Source of Leaves | Aqueous Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Polyphenols (mg GAE/g DW) | Phenolic Acids (mg GAE/g DW) | Flavonoids (mg RE/g DW) | |||||||
| 25 | 100 | Mean | 25 | 100 | Mean | 25 | 100 | Mean | |
| Tissue culture | 2.15 b | 2.77 a | 2.46 A | 3.13 b | 4.29 a | 3.71 A | 9.10 b | 21.76 a | 15.43 A |
| Conventional | 1.54 c | 1.55 c | 1.55 B | 2.12 d | 2.76 c | 2.44 B | 5.26 d | 6.68 c | 5.97 B |
| Mean | 1.85 B | 2.16 A | 2.63 B | 3.53 A | 7.18 B | 14.22 A | |||
| Source of Leaves | Aqueous Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| DPPH (mg TE/g DW) | ABTS (mg TE/g DW) | FRAP (mg TE/g DW) | |||||||
| 25 | 100 | Mean | 25 | 100 | Mean | 25 | 100 | Mean | |
| Tissue culture | 2.98 b | 3.20 a | 3.10 A | 1.48 b | 1.50 a | 1.49 A | 7.13 b | 9.76 a | 8.45 A |
| Conventional | 2.72 c | 2.44 d | 2.58 B | 1.47 b | 1.46 b | 1.47 B | 4.27 d | 5.31 c | 4.79 B |
| Mean | 2.85 A | 2.82 B | 1.48 A | 1.48 A | 5.70 B | 7.54 A | |||
| Source of Leaves | Aqueous Temperature (°C) | |||||
|---|---|---|---|---|---|---|
| Superoxide Inhibition (%) | Iron (II) Chelating Activity (%) | |||||
| 25 | 100 | Mean | 25 | 100 | Mean | |
| Tissue culture | 52.22 b | 53.74 a | 52.98 A | 54.33 b | 53.50 b | 53.92 B |
| Conventional | 39.84 d | 48.97 c | 44.41 B | 69.24 a | 51.33 b | 60.29 A |
| Mean | 46.03 B | 51.36 A | 61.79 A | 52.42 B | ||
| Variable | TPP | TPC | TFC | DPPH | FRAP | ABTS | O2− | Fe2+ |
|---|---|---|---|---|---|---|---|---|
| TPP | 1 | |||||||
| TPC | 0.95 ** | 1 | ||||||
| TFC | 0.95 ** | 0.96 ** | 1 | |||||
| DPPH | 0.92 ** | 0.76 ** | 0.81 ** | 1 | ||||
| FRAP | 0.98 ** | 0.99 ** | 0.95 ** | 0.84 ** | 1 | |||
| ABTS | 0.86 ** | 0.78 ** | 0.86 ** | 0.85 ** | 0.81 ** | 1 | ||
| O2− | 0.76 ** | 0.86 ** | 0.69 ** | 0.53 ns | 0.85 ** | 0.46 ns | 1 | |
| Fe2+ | −0.39 ns | −0.58 ns | −0.38 ns | −0.08 ns | −0.52 ns | −0.11 ns | −0.82 ** | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haida, Z.; Nakasha, J.J.; Hakiman, M. In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass). Plants 2020, 9, 1030. https://doi.org/10.3390/plants9081030
Haida Z, Nakasha JJ, Hakiman M. In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass). Plants. 2020; 9(8):1030. https://doi.org/10.3390/plants9081030
Chicago/Turabian StyleHaida, Zainol, Jaafar Juju Nakasha, and Mansor Hakiman. 2020. "In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass)" Plants 9, no. 8: 1030. https://doi.org/10.3390/plants9081030
APA StyleHaida, Z., Nakasha, J. J., & Hakiman, M. (2020). In Vitro Responses of Plant Growth Factors on Growth, Yield, Phenolics Content and Antioxidant Activities of Clinacanthus nutans (Sabah Snake Grass). Plants, 9(8), 1030. https://doi.org/10.3390/plants9081030





