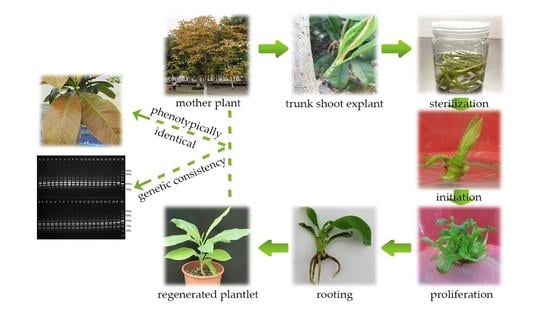
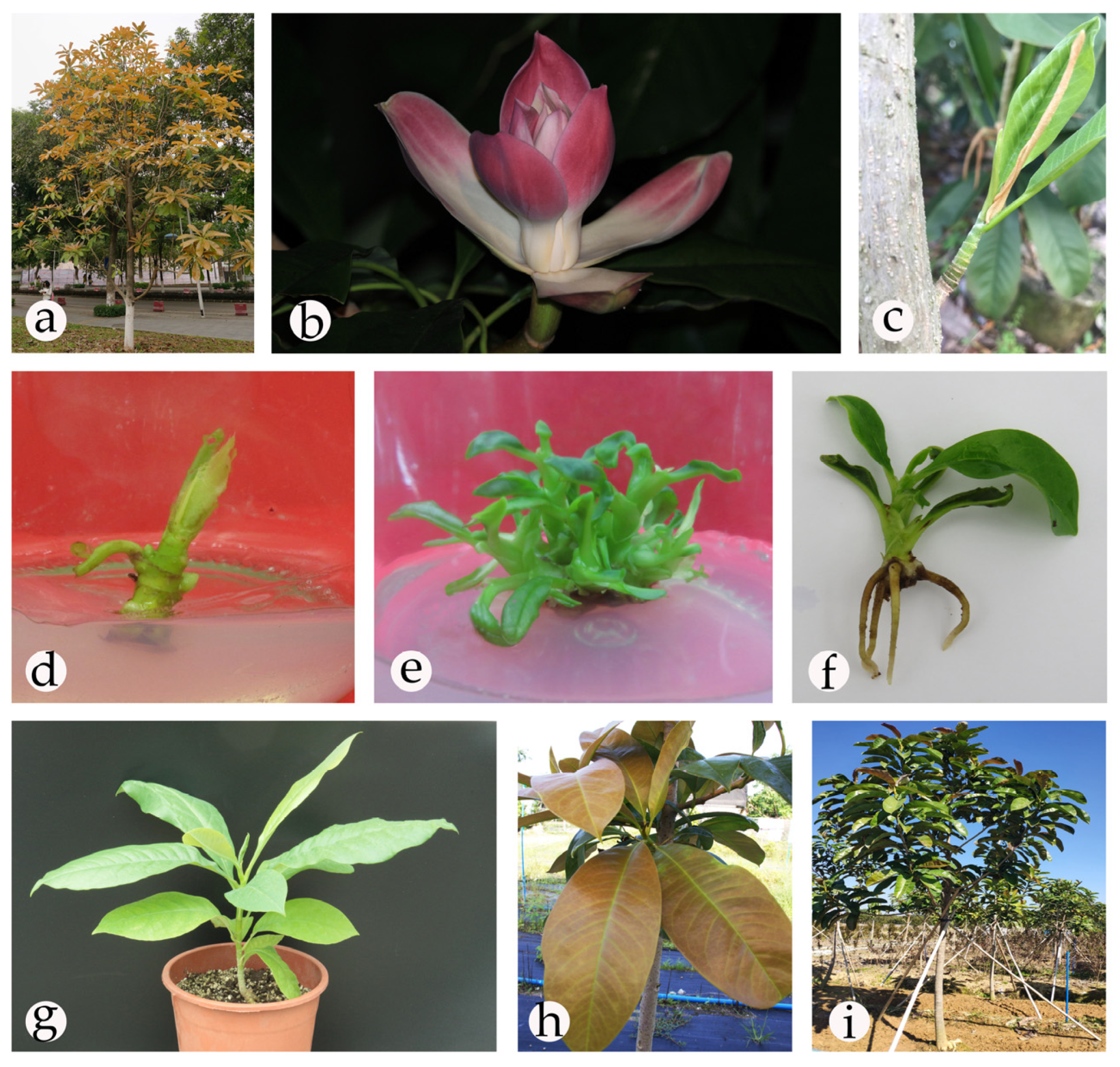
Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants
Abstract
:1. Introduction
2. Results
2.1. Establishment of an Aseptic System
2.2. Shoot Initiation
2.3. Shoot Proliferation
2.4. Rooting and Acclimatization
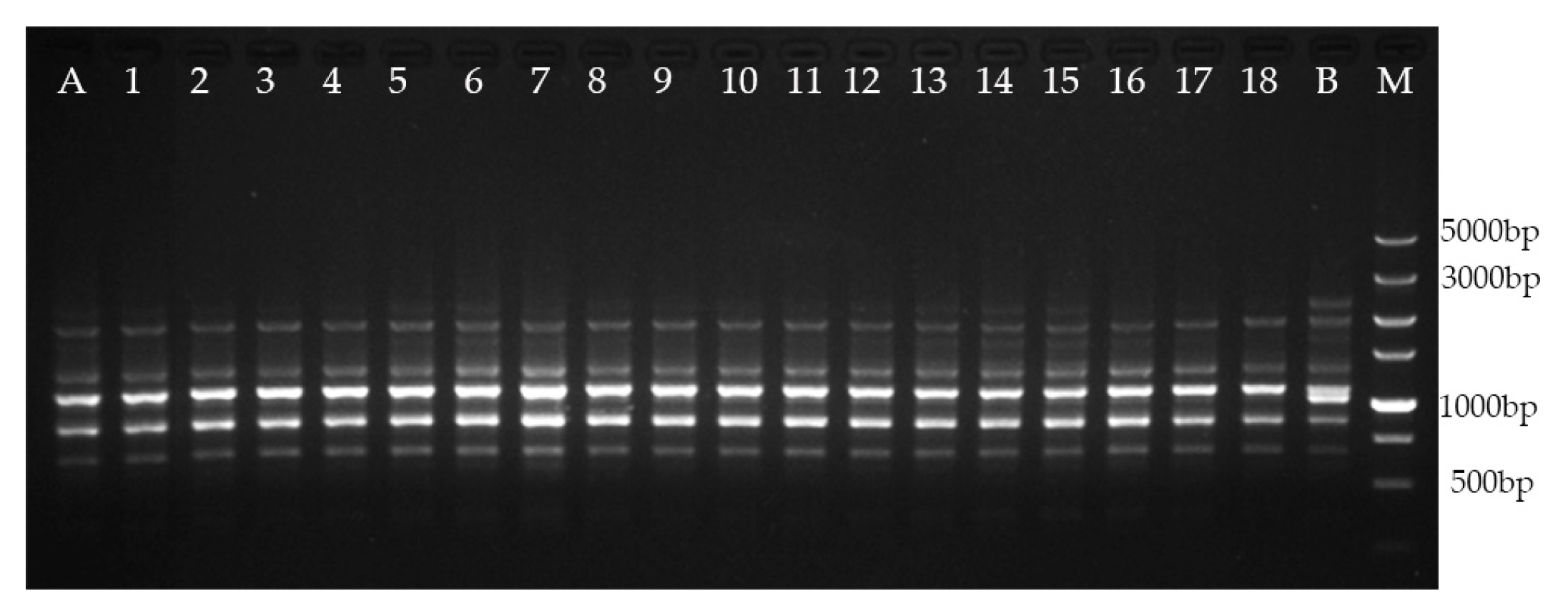
2.5. Genetic Fidelity Assessment
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sterilization
4.2. Media and Culture Conditions
4.3. Shoot Initiation
4.4. Shoot Proliferation
4.5. Rooting
4.6. Acclimatization
4.7. Genetic Fidelity Assessment
4.8. Statistical Analysis
- Induction rate (%) = number of induced explants/total number of explants × 100
- Multiplication rate (%) = total number of shoots ≥ 0.3 cm/number of initial shoots on subcultured explants × 100
- Number of shoots per explant = total number of shoots ≥ 0.5 cm/number of explants
- Rooting rate (%) = number of rooted explants/number of explants × 100
- Average root number = total number of roots/number of rooted explants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vu, Q.; Xia, N. Manglietia lucida (Magnoliaceae), a newly recorded species for Vietnam. J. Trop. Subtrop. Bot. 2010, 18, 43–46. [Google Scholar]
- Liu, Y. Magnolias of China; Beijing Sci. Technol. Press: Beijing, China, 2004. [Google Scholar]
- Deng, Y.; Lin, J.; Wu, Q.; Liu, T.; Liu, M.; Deng, X. Review on Magnolia tissue culture technology. J. For. Environ. Sci. 2018, 34, 118–124. [Google Scholar]
- Li, Z.; Guo, R. Review on propagation biology and analysis on endangered factors of endangered species of Manglietia. Life Sci. Res. 2014, 18, 90–94. [Google Scholar]
- González, M.S.; Urrego, L.E. Habitat and conservation status of molinillo (Magnolia sambuensis) and laurel arenillo (Magnolia katiorum), two endangered species from the lowland, Colombia. Trop. Conserv. Sci. 2016, 9, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Rivers, M.; Beech, E.; Murphy, L.; Oldfield, S. The Red List of Magnoliaceae Revised and Extended; Botanic Gardens Conservation International: Surrey, UK, 2016. [Google Scholar]
- Zeng, Z.; Deng, Y.; Liu, T.; Kang, L.; Guo, M.; Hong, W. Study on storage and germination characteristics of Magnolia lucida seeds. J. For. Environ. Sci. 2019, 35, 61–66. [Google Scholar]
- Petrosyan, A.A. Variation in walnuts propagated by seed. Sadovodstvo 1970, 10, 26–27. [Google Scholar]
- Huai, H.; Ma, L.; Jia, Z. Research progress in tissue culture of Magnoliaceae. Pract. For. Technol. 2010, 8, 27–29. [Google Scholar]
- Borah, R.; Kumaria, S.; Choudhury, H. In vitro plant regeneration of Magnolia punduana: An endemic and threatened plant species. Plant Tissue Cult. Biotechnol. 2017, 27, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An efficient micropropagation protocol for an endangered ornamental tree species (Magnolia sirindhorniae Noot. & Chalermglin) and assessment of genetic uniformity through DNA markers. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Mata-Rosas, M.N.; Jiménez-Rodríguez, A.N.; Chávez-Avila, V.M. Somatic embryogenesis and organogenesis in Magnolia dealbata Zucc. (Magnoliaceae), an endangered, endemic Mexican species. HortScience 2006, 41, 1325–1329. [Google Scholar] [CrossRef]
- Hussain, S.A.; Ahmad, N.; Anis, M. Synergetic effect of TDZ and BA on minimizing the post-exposure effects on axillary shoot proliferation and assessment of genetic fidelity in Rauvolfia tetraphylla (L.). Rend. Lincei. Sci. Fis. Nat. 2018, 29, 109–115. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Amiri, S.; Fotovat, R.; Tarinejhad, A.; Panahi, B.; Mohammadi, S.A. Optimization of hormonal combinations for in vitro regeneration of lesser periwinkle (Vinca minor L.) and assessment of genetic homogeneity. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 1–7. [Google Scholar] [CrossRef]
- Bennici, A.; Anzidei, M.; Vendramin, G.G. Genetic stability and uniformity of Foeniculum vulgare Mill. regenerated plants through organogenesis and somatic embryogenesis. Plant Sci. 2004, 166, 221–227. [Google Scholar] [CrossRef]
- Smýkal, P.; Valledor, L.; Rodriguez, R.; Griga, M. Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.). Plant Cell. Rep. 2007, 26, 1985–1998. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell. Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propag. Soc. 1980, 30, 421–427. [Google Scholar]
- Tsay, H.S.; Lee, C.Y.; Agrawal, D.C.; Basker, S. Influence of ventilation closure, gelling agent and explant type on shoot bud proliferation and hyperhydricity in Scrophularia yoshimurae—A medicinal plant. In Vitro Cell. Dev. Biol. Plant 2006, 42, 445–449. [Google Scholar] [CrossRef]
- Cassells, A.C. Pathogen and microbial contamination management in micropropagation—An overview. In Pathogen and Microbial Contamination Management in Micropropagation; Cassells, A.C., Ed.; Springer: Dordrecht, The Netherlands, 1997; pp. 1–13. [Google Scholar]
- Pinto, A.; Demattê, M.; Creste, S.; Barbosa, J. Seed and seedling surface-sterilization for in vitro culture of Tillandsia gardneri (Bromeliaceae). In Proceedings of the VII International Symposium on In Vitro Culture and Horticultural Breeding, Ghent, Belgium, 18 September 2011; Volume 961, pp. 383–389. [Google Scholar]
- Murashige, T. Plant propagation through tissue cultures. Annu. Rev. Plant Physiol. 1974, 25, 135–166. [Google Scholar] [CrossRef]
- Enjalric, F.; Carron, M.-P.; Lardet, L. Contamination of primary cultures in tropical areas: The case of Hevea brasiliensis. Bact. Bact.-like Contam. Plant Tissue Cult. 1988, 225, 57–66. [Google Scholar] [CrossRef]
- Rakesh, K.; Bangarwa, K.; Yadava, R. Study on effect of different sterilization treatments on culture establishment in Eucalyptus tereticornis. J. Trop. For. 2012, 28, 72–75. [Google Scholar]
- Garg, R.K.; Srivastava, V.; Kaur, K.; Gosal, S. Effect of sterilization treatments on culture establishment in Jatropha curcas L. Karnataka J. Agric. Sci. 2014, 27, 190–192. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- De Diego, N.; Montalbán, I.; Fernández de Larrinoa, E.; Moncaleán, P. In vitro regeneration of Pinus pinaster adult trees. Can. J. For. Res. 2008, 38, 2607–2615. [Google Scholar] [CrossRef]
- Tuskan, G.; Sargent, W.; Rensema, T.; Walla, J. Influence of plant growth regulators, basal media and carbohydrate levels on the in vitro development of Pinus ponderosa (Dougl. ex Law.) cotyledon explants. Plant Cell Tissue Organ. Cult. 1990, 20, 47–52. [Google Scholar] [CrossRef]
- Ivanova, M.; Staden, J.V. Nitrogen source, concentration, and NH4 +:NO3 ratio influence shoot regeneration and hyperhydricity in tissue cultured Aloe polyphylla. Plant Cell Tissue Organ. Cult. 2009, 99, 167–174. [Google Scholar] [CrossRef]
- Feng, F.; Li, H.; Xie, J. The occurrence and prevention of vitrification of test-tube seedlings in aloe tissue culture. J. Southwest Univ. (Nat. Sci. Ed.) 2001, 23, 449–451. [Google Scholar]
- Bairu, M.W.; Stirk, W.A.; Staden, J.V. Factors contributing to In Vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tissue Organ. Cult. 2009, 98, 239–248. [Google Scholar] [CrossRef]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant growth regulators II: Cytokinins, their analogues and antagonists. In Plant Propagation by Tissue Culture; George, E.F., Hall, M.A., De Klerk, G.-J., Eds.; Springer: Basel, Switzerland, 2008; Volume 1, pp. 205–226. [Google Scholar]
- Perveen, S.; Varshney, A.; Anis, M.; Aref, I.M. Influence of cytokinins, basal media and pH on adventitious shoot regeneration from excised root cultures of Albizia lebbeck. J. For. Res. 2011, 22, 47–52. [Google Scholar] [CrossRef]
- Koroch, A.; Kapteyn, J.; Juliani, H.; Simon, J. In vitro regeneration of Echinacea pallida from leaf explants. In Vitro Cell. Dev. Biol. Plant 2003, 39, 415–418. [Google Scholar] [CrossRef]
- Mamun, A.; Matin, M.; Bari, M.; Siddique, N.; Sultana, R.; Rahman, M.; Musa, A. Micropropagation of woody legume (Albizia lebbeck) through tissue culture. Pak. J. Biol. Sci. 2004, 7, 1099–1103. [Google Scholar]
- Greenway, M.B.; Phillips, I.C.; Lloyd, M.N.; Hubstenberger, J.F.; Phillips, G.C. A nutrient medium for diverse applications and tissue growth of plant species in vitro. In Vitro Cell. Dev. Biol. Plant 2012, 48, 403–410. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, X.; Tang, J.; Xin, P.; Zhao, X.L.W.; Yu, F. Research progress on tissue culture of Magnoliaceae in China. Jiangxi Sci. 2013, 31, 53–57. [Google Scholar]
- Druart, P.; Kevers, C.; Boxus, P.; Gaspar, T. In vitro promotion of root formation by apple shoots through darkness effect on endogenous phenols and peroxidases. Z. Pflanzenphysiol. 1982, 108, 429–436. [Google Scholar] [CrossRef]
- Pan, M.; Van Staden, J. The effect of activated charcoal and auxins on root formation by hypocotyl segments of Daucus carota. S. Afr. J. Bot. 2002, 68, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Deng, X. A Study on Tissue Culture and Rapid Propagation of Parakmeria Lotungensis (Chun et C. Tsoong) Law, Sinomanglietia Glauca Z.X. Yu et Q.Y. Zhang and Photinia × Fraseri ’Red Robin’. Ph.D. Thesis, Nanjing Forestry University, Nanjing, China, 2004. [Google Scholar]
- Domínguez, F.; Chávez, M.; Garduño-Ramírez, M.L.; Chávez-Avila, V.M.; Mata, M.; Cruz-Sosa, F. Honokiol and magnolol production by in vitro micropropagated plants of Magnolia dealbata, an endangered endemic Mexican species. Nat. Prod. Commun. 2010, 5, 1934578X1000500213. [Google Scholar] [CrossRef] [Green Version]
- Giri, C.; Shyamkumar, B.; Anjaneyulu, C. Progress in tissue culture, genetic transformation and applications of biotechnology to trees: An overview. Trees 2004, 18, 115–135. [Google Scholar] [CrossRef]
- Kumar, S.; Mangal, M.; Dhawan, A.; Singh, N. Assessment of genetic fidelity of micropropagated plants of Simmondsia chinensis (Link) Schneider using RAPD and ISSR markers. Acta Physiol. Plant. 2011, 33, 2541–2545. [Google Scholar] [CrossRef]
- Chavan, J.; Gaikwad, N.; Kshirsagar, P.; Umdale, S.; Bhat, K.; Dixit, G.; Yadav, S. Highly efficient In Vitro proliferation and genetic stability analysis of micropropagated Ceropegia evansii by RAPD and ISSR markers: A critically endangered plant of Western Ghats. Plant Biosyst. 2015, 149, 442–450. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Yu, H.-H.; Yang, Z.-L.; Sun, B.; Liu, R.-N. Genetic diversity and relationship of endangered plant Magnolia officinalis (Magnoliaceae) assessed with ISSR polymorphisms. Biochem. Syst. Ecol. 2011, 39, 71–78. [Google Scholar] [CrossRef]
- Zhou, H.; Shao, M.; Wu, H.; Yin, J.; Ji, K. Genetic diversity among 10 genera 17 species of Magnoliaceae by RAPD marker. Biotechnology 2011, 6, 46–49. [Google Scholar]

| No. | Treatment Composition (Duration) | Survival (%) | ||
|---|---|---|---|---|
| Ethanol 75% (s) | Benzalkonium Bromide 1% (min) | Mercuric Chloride 0.1% (min) | ||
| 1 | - | 5 | - | 4 |
| 2 | - | - | 5 | 36 |
| 3 | 30 | - | - | 27 |
| 4 | 30 | 5 | 1 | 56 |
| 5 | 30 | 5 | 3 | 68 |
| 6 | 30 | 5 | 5 | 78 |
| 7 | 30 | 5 | 7 | 61 |
| No. | Medium | BA (mg/L) | NAA (mg/L) | IBA (mg/L) | Induction Rate (%) (Mean ± SE; n = 3) | Observation |
|---|---|---|---|---|---|---|
| 1 | ML | 1 | 0.05 | - | 95.56 ± 3.14 a | Robust and green shoots |
| 2 | ML | 1 | - | 0.05 | 85.56 ± 1.57 b | Robust and green shoots |
| 3 | ML | 1 | - | - | 60.00 ± 2.72 de | Slow growth of shoots |
| 4 | DCR | 1 | 0.05 | - | 74.44 ± 4.16 c | Green shoots |
| 5 | DCR | 1 | - | 0.05 | 63.33 ± 2.72 d | Slow growth of shoots |
| 6 | DCR | 1 | - | - | 48.89 ± 3.14 ghi | Slow growth of shoots |
| 7 | WPM | 1 | 0.05 | - | 57.78 ± 4.16 def | Slow growth of shoots |
| 8 | WPM | 1 | - | 0.05 | 51.11 ± 5.67 fgh | Slow growth of shoots |
| 9 | WPM | 1 | - | - | 42.22 ± 1.57 i | Slow growth of shoots |
| 10 | MS | 1 | 0.05 | - | 53.33 ± 5.44 efg | Hyperhydricity |
| 11 | MS | 1 | - | 0.05 | 43.33 ± 4.71 hi | Hyperhydricity |
| 12 | MS | 1 | - | - | 33.33 ± 5.44 j | Hyperhydricity, browning |
| No. | BA (mg/L) | NAA (mg/L) | Multiplication Rate (%) (Mean ± SE, n = 3) | Number of Shoots per Explant (≥ 0.5 cm) (Mean ± SE, n = 3) | Growth State of Buds |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 98.87 ± 4.93 l | 1.20 ± 0.08 h | Shorter shoots |
| 2 | 0.2 | 0.02 | 247.85 ± 5.65 ef | 4.07 ± 0.21 d | Shorter shoots |
| 3 | 0.2 | 0.04 | 253.54 ± 3.27 de | 4.60 ± 0.14 b | Shorter shoots |
| 4 | 0.2 | 0.06 | 235.91 ± 3.18 g | 3.63 ± 0.12 e | Shorter shoots |
| 5 | 0.2 | 0.08 | 215.90 ± 6.27 hi | 3.00 ± 0.08 f | Partial callus |
| 6 | 0.4 | 0.02 | 274.69 ± 3.15 b | 4.70 ± 0.08 b | Robust shoots |
| 7 | 0.4 | 0.04 | 284.56 ± 3.88 a | 5.17 ± 0.12 a | Robust shoots |
| 8 | 0.4 | 0.06 | 267.85 ± 5.82 bc | 4.50 ± 0.22 b | Robust shoots |
| 9 | 0.4 | 0.08 | 250.69 ± 4.52 def | 4.43 ± 0.21 bc | Partial shoots |
| 10 | 0.6 | 0.02 | 259.58 ± 8.67 cd | 4.37 ± 0.21 bcd | Robust shoots |
| 11 | 0.6 | 0.04 | 268.81 ± 2.76 bc | 5.30 ± 0.08 a | Robust shoots |
| 12 | 0.6 | 0.06 | 251.68 ± 8.26 def | 4.40 ± 0.22 bcd | Robust shoots |
| 13 | 0.6 | 0.08 | 241.84 ± 4.12 fg | 4.10 ± 0.29 cd | Slightly crinkled leaf, callus |
| 14 | 0.8 | 0.02 | 208.79 ± 2.54 ij | 3.67 ± 0.09 e | Crinkled leaf |
| 15 | 0.8 | 0.04 | 224.23 ± 3.70 h | 3.30 ± 0.08 f | Crinkled leaf |
| 16 | 0.8 | 0.06 | 204.14 ± 2.12 j | 3.27 ± 0.17 f | Flavescence, partial callus |
| 17 | 0.8 | 0.08 | 177.42 ± 2.40 k | 2.57 ± 0.05 g | Flavescence, callus |
| Group | No. | NAA (mg/L) | IBA (mg/L) | Percentage of Rooting (%) (Mean ± SE, n = 3) | Average Root Number (Mean ± SE, n = 3) |
|---|---|---|---|---|---|
| Directly cultured by light | 1 | 0.3 | 0 | 0 j | 0 i |
| 2 | 0.3 | 0.5 | 28.89 ± 3.14 gh | 1.34 ± 0.07 gh | |
| 3 | 0.3 | 1 | 36.67 ± 7.20 fg | 1.53 ± 0.07 efg | |
| 4 | 0.6 | 0 | 0 j | 0 i | |
| 5 | 0.6 | 0.5 | 41.11 ± 6.85 def | 1.81 ± 0.20 e | |
| 6 | 0.6 | 1 | 52.22 ± 5.67 cd | 2.59 ± 0.14 d | |
| 7 | 0.9 | 0 | 13.33 ± 2.72 i | 1.15 ± 0.11 h | |
| 8 | 0.9 | 0.5 | 27.78 ± 4.16 gh | 1.74 ± 0.20 ef | |
| 9 | 0.9 | 1 | 20.00 ± 2.72 hi | 1.44 ± 0.14 fgh | |
| Darkness for 7 days | 1 | 0.3 | 0 | 27.78 ± 4.16 gh | 1.78 ± 0.13 e |
| 2 | 0.3 | 0.5 | 51.11 ± 6.85 cd | 2.59 ± 0.15 d | |
| 3 | 0.3 | 1 | 60.00 ± 7.20 c | 3.35 ± 0.17 c | |
| 4 | 0.6 | 0 | 37.78 ± 4.16 efg | 2.34 ± 0.11 d | |
| 5 | 0.6 | 0.5 | 71.11 ± 6.29 b | 4.26 ± 0.27 b | |
| 6 | 0.6 | 1 | 87.78 ± 4.16 a | 4.89 ± 0.13 a | |
| 7 | 0.9 | 0 | 42.22 ± 6.85 def | 1.52 ± 0.23 efg | |
| 8 | 0.9 | 0.5 | 54.44 ± 8.31 c | 3.40 ± 0.30 c | |
| 9 | 0.9 | 1 | 48.89 ± 5.67 cde | 2.24 ± 0.14 d |
| Primer Code | Sequence (5′–3′) | No. of Scorable Bands | Range of Amplification (bp) |
|---|---|---|---|
| RAPD | |||
| S10 | CTGCTGGGAC | 6 | 750–3000 |
| S11 | GTAGACCCGT | 5 | 1000–3000 |
| S17 | AGGGAACGAG | 3 | 500–3000 |
| S18 | CCACAGCAGT | 5 | 750–3000 |
| S22 | TGCCGAGCTG | 5 | 750–3000 |
| S31 | CAATCGCCGT | 4 | 250–2000 |
| S38 | CTGGGGCTGA | 5 | 750–3000 |
| S40 | GTTGCGATCC | 4 | 1000–3000 |
| S69 | CTCACCGTCC | 5 | 1000–3000 |
| S144 | GTGACATGCC | 5 | 750–3000 |
| S154 | TGCGGCTGAG | 4 | 750–2000 |
| S155 | ACGCACAACC | 4 | 1000–3000 |
| S160 | AACGGTGACC | 4 | 750–2000 |
| S163 | GGACTGCAGA | 5 | 2000–3000 |
| S173 | CTGGGGCTGA | 3 | 750–2000 |
| S174 | CTGGGGCTGA | 3 | 1000–2000 |
| Total | 70 | ||
| ISSR | |||
| UBC811 | (GA)8G | 5 | 500–3000 |
| UBC835 | (SG)8YC | 4 | 750–3000 |
| UBC840 | (GA)8YT | 5 | 500–3000 |
| UBC842 | (GA)8YG | 6 | 750–3000 |
| UBC844 | (CA)8RG | 5 | 1000–3000 |
| UBC864 | (ATG)6 | 6 | 750–5000 |
| Total | 31 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, L.; Zheng, K.; Xie, Y.; Deng, Y.; Yu, Y.; Zhu, M.; Xi, R.; Deng, X. Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants. Plants 2020, 9, 997. https://doi.org/10.3390/plants9080997
Kang L, Zheng K, Xie Y, Deng Y, Yu Y, Zhu M, Xi R, Deng X. Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants. Plants. 2020; 9(8):997. https://doi.org/10.3390/plants9080997
Chicago/Turabian StyleKang, Lu, Keyuan Zheng, Yuqing Xie, Yanwen Deng, Yina Yu, Mulan Zhu, Ruchun Xi, and Xiaomei Deng. 2020. "Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants" Plants 9, no. 8: 997. https://doi.org/10.3390/plants9080997
APA StyleKang, L., Zheng, K., Xie, Y., Deng, Y., Yu, Y., Zhu, M., Xi, R., & Deng, X. (2020). Efficient Tissue Culture Protocol for Magnolia lucida (Magnoliaceae) and Confirmation of Genetic Stability of the Regenerated Plants. Plants, 9(8), 997. https://doi.org/10.3390/plants9080997