Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector
Abstract
:1. Introduction
2. Results
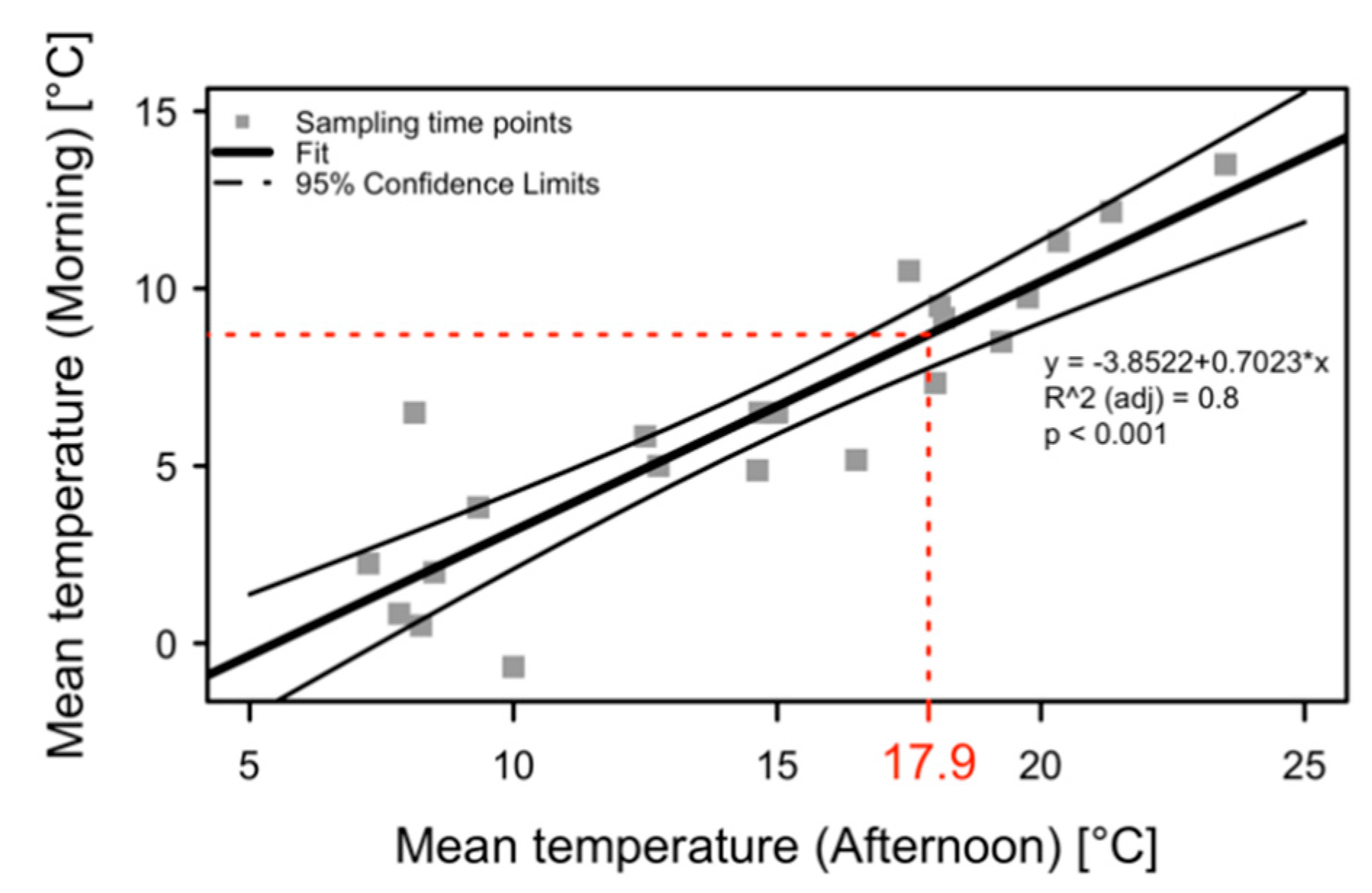
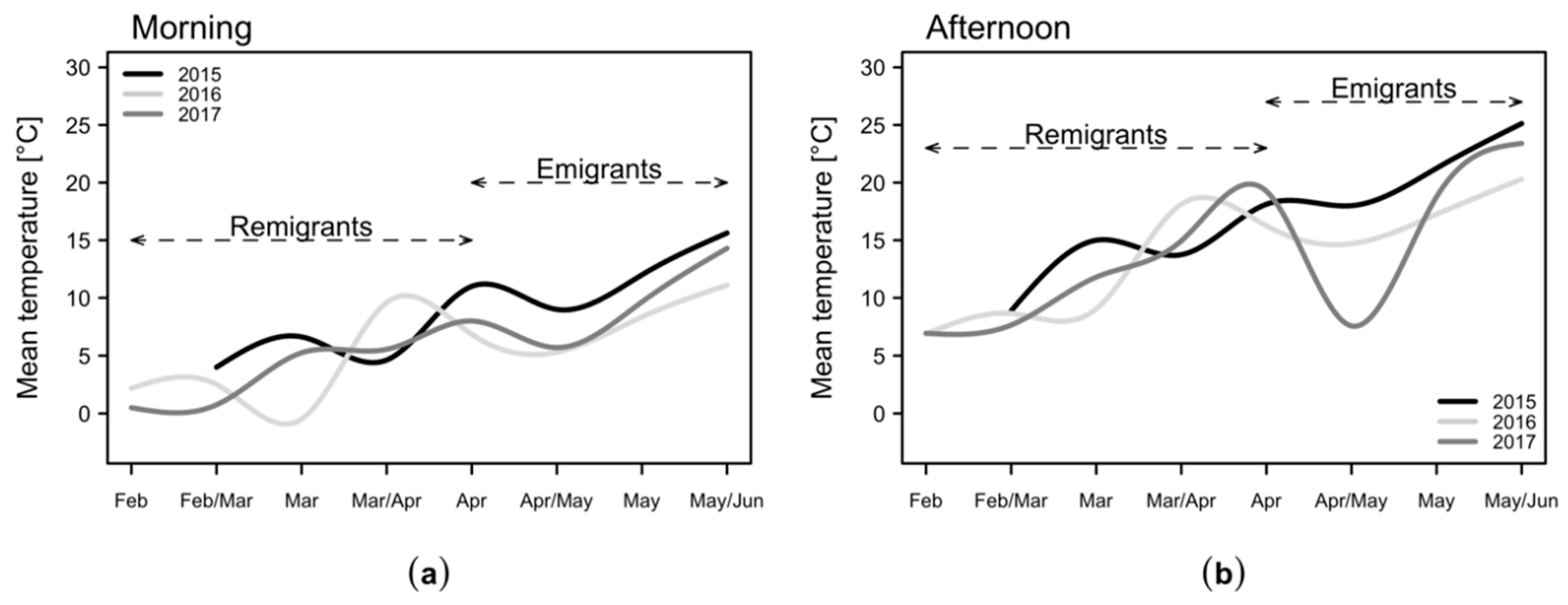
2.1. Temperatures in the Apple Orchard
2.2. Psyllid Community
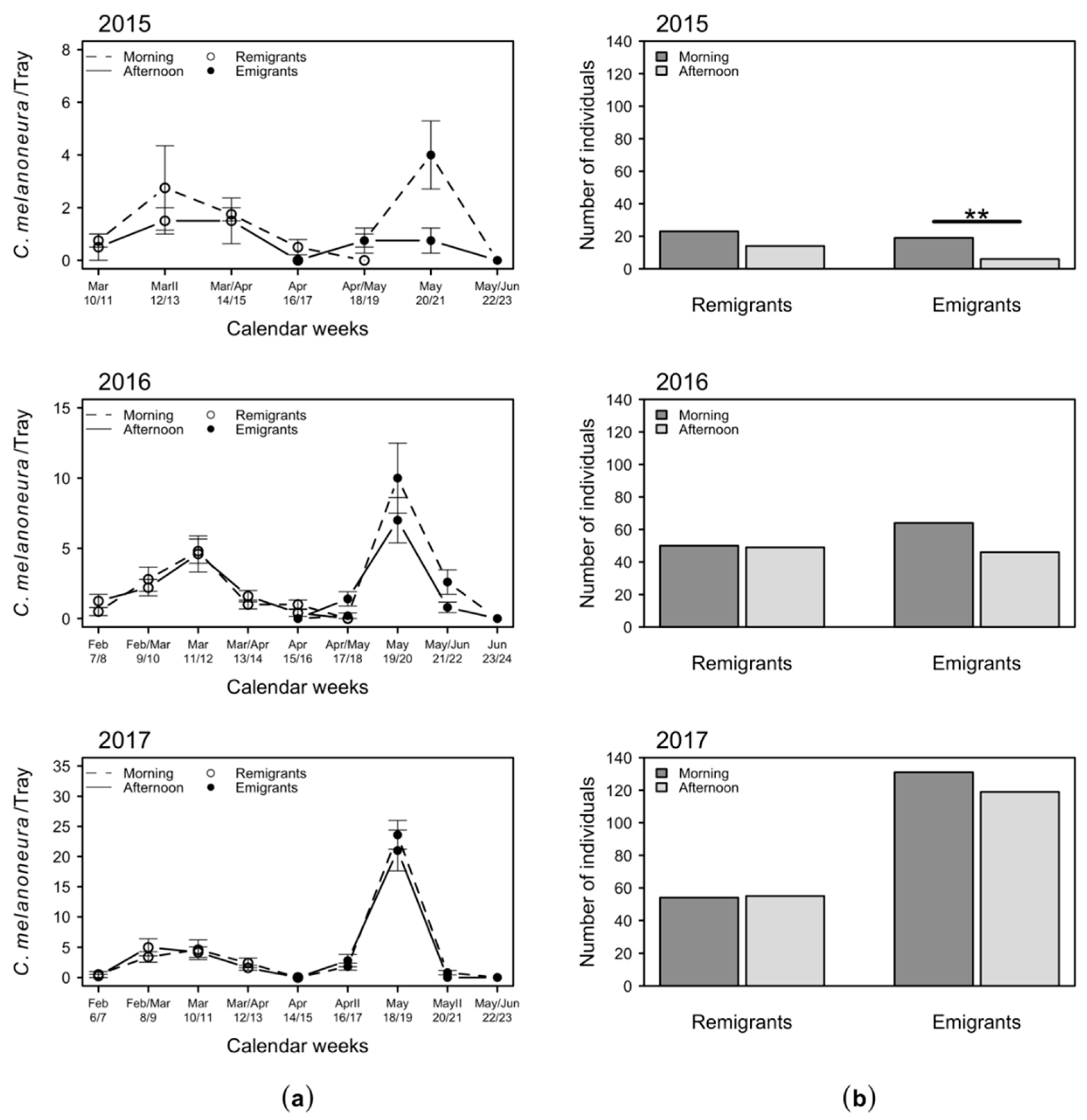
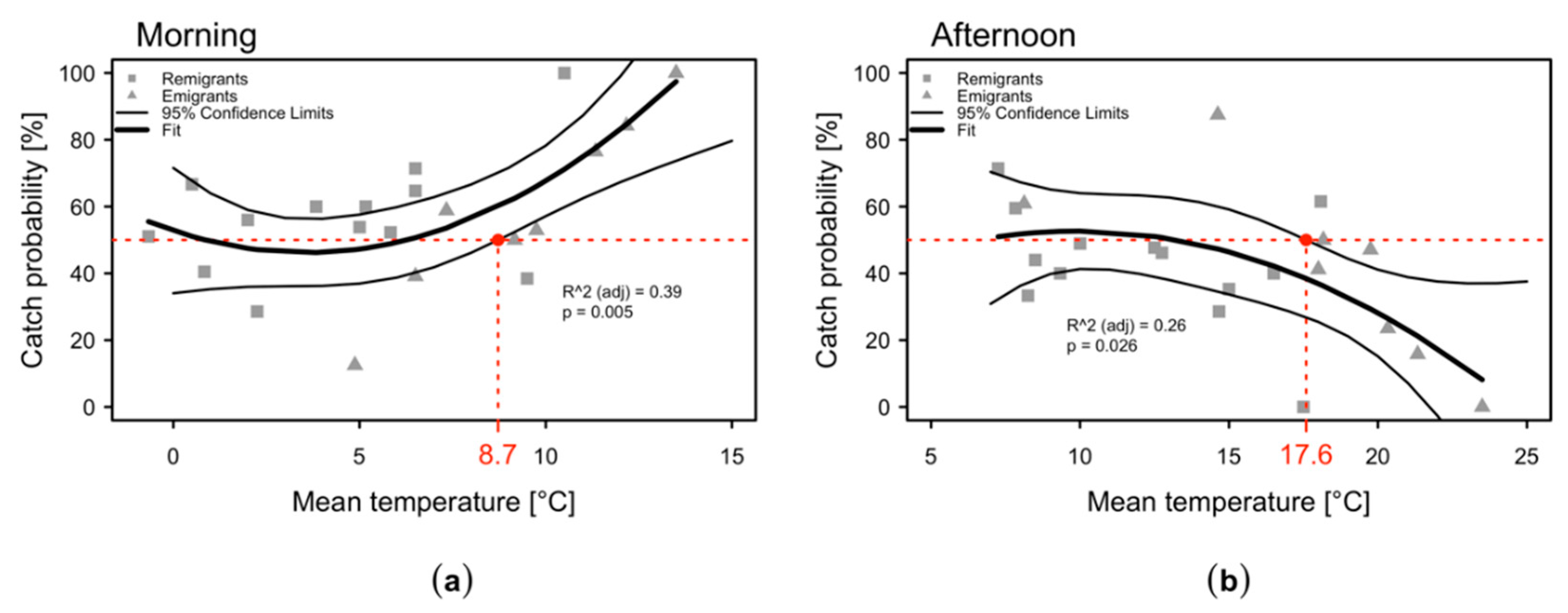
2.3. Daytime Variation in the Numbers of C. melanoneura Specimens
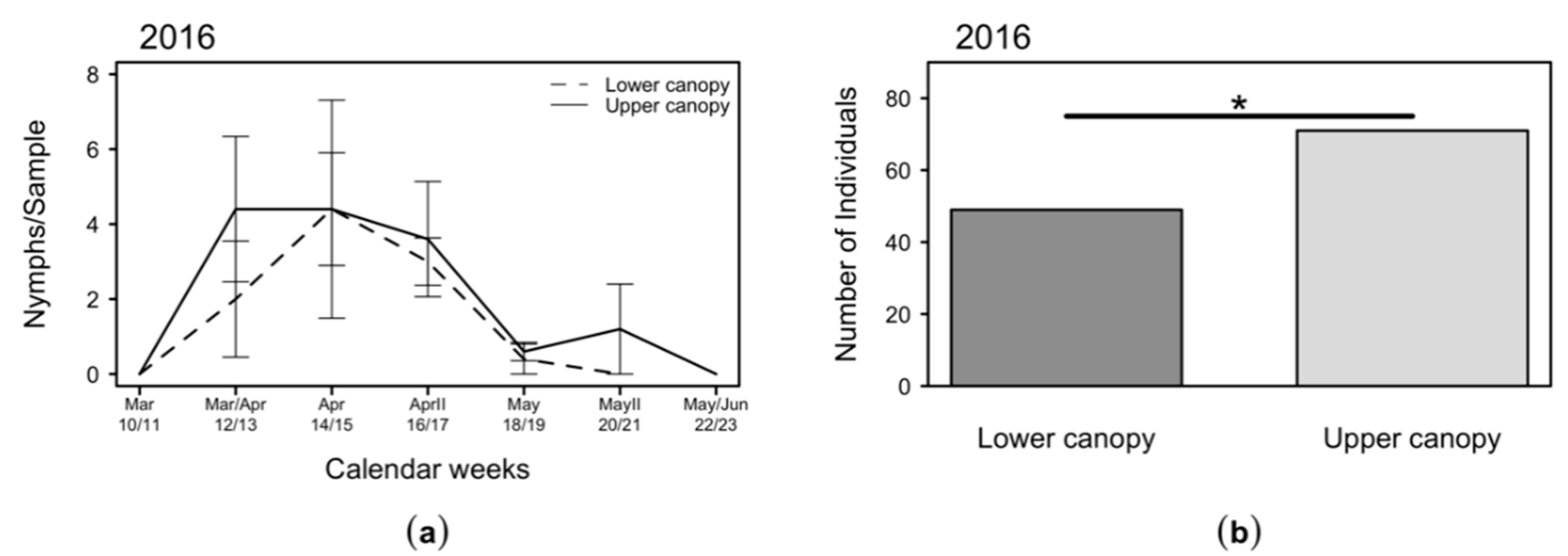
2.4. Variation of C. melanoneura Densities at Different Canopy Heights
2.5. Sampling of C. picta
2.6. Infection Rate with ‘Ca. P. mali’ in Selected Psylloidea Species
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Insect Sampling
4.3. Material Identification
4.4. Detection of ‘Ca. P. mali’ in Psyllids
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Month 2015 | Mar | MarII | Mar/Apr | Apr | Apr/May | May | May/Jun | |
| Calendar Weeks 2015 | 10/11 | 12/13 | 14/15 | 16/17 | 18/19 | 20/21 | 22/23 | |
| Minmorning [°C] | 3 | 5 | 3.5 | 8 | 8.5 | 10.5 | 13.5 | |
| Maxmorning [°C] | 4.5 | 7.5 | 7 | 12.5 | 10.5 | 14.5 | 16.5 | |
| Meanmorning [°C] | 4 | 6.6 | 4.6 | 11 | 9 | 12 | 15.6 | |
| Minafternoon [°C] | 7.5 | 13.5 | 9.5 | 12.5 | 16 | 21 | 22.5 | |
| Maxafternoon [°C] | 11 | 16.5 | 16.5 | 20 | 21 | 22 | 28 | |
| Meanafternoon [°C] | 8.9 | 15 | 13.8 | 18.1 | 18 | 21.3 | 25.1 | |
| Month 2016 | Feb | Feb/Mar | Mar | Mar/Apr | Apr | Apr/May | May | May/Jun |
| Calendar Weeks 2016 | 7/8 | 9/10 | 11/12 | 13/14 | 15/16 | 17/18 | 19/20 | 21/22 |
| Minmorning [°C] | 1.5 | 1 | −2.3 | 8.5 | 4 | −1 | 3.5 | 10 |
| Maxmorning [°C] | 3.3 | 4 | 0.5 | 10.5 | 8.5 | 9 | 10.5 | 13 |
| Meanmorning [°C] | 2.2 | 2.5 | −0.5 | 9.6 | 6.9 | 5.3 | 8.4 | 11.1 |
| Minafternoon [°C] | 6.5 | 7.5 | 6 | 17 | 7.5 | 8 | 13.5 | 16.5 |
| Maxafternoon [°C] | 8 | 9.5 | 12.5 | 19.5 | 19 | 19 | 24 | 23 |
| Meanafternoon [°C] | 6.9 | 8.7 | 9.1 | 18.1 | 16.3 | 14.7 | 17.3 | 20.3 |
| Month 2017 | Feb | Feb/Mar | Mar | Mar/Apr | Apr | AprII | May | MayII |
| Calendar Weeks 2017 | 6/7 | 8/9 | 10/11 | 12/13 | 14/15 | 16/17 | 18/19 | 20/21 |
| Minmorning [°C] | 0.5 | −0.5 | 4 | 3 | 6.5 | 4.5 | 9.5 | 11.5 |
| Maxmorning [°C] | 0.5 | 2 | 8 | 6.5 | 9.5 | 8.5 | 10 | 15.5 |
| Meanmorning [°C] | 0.5 | 0.8 | 5.3 | 5.6 | 8 | 5.7 | 9.7 | 14.3 |
| Minafternoon [°C] | 6.5 | 6 | 8 | 9.5 | 18.5 | 6.8 | 16.5 | 23 |
| Maxafternoon [°C] | 10 | 11.5 | 19 | 22 | 20 | 9.5 | 23 | 24 |
| Meanafternoon [°C] | 6.9 | 7.7 | 11.8 | 15 | 19.3 | 7.6 | 18.8 | 23.4 |
References
- Prokopy, R.J.; Wirth, C.B.; Leskey, T.C. Movement of plum curculio adults toward host trees and traps: Flight versus walking. Entomol. Exp. Appl. 1999, 91, 385–392. [Google Scholar] [CrossRef]
- Southwood, T.R.E. Ecological Methods. In With the Particular Reference to the Study of Insect Populations, 2nd ed.; Springer: Dordrecht, The Netherlands, 1978. [Google Scholar]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark; Brill: Leiden, The Netherlands, 1992. [Google Scholar]
- Ouvrard, D. Psyl’list. Available online: https://www.hemiptera-databases.org/psyllist/ (accessed on 26 April 2020).
- Hodkinson, I.D. Life cycle variation and adaptation in jumping plant lice (Insecta Hemiptera: Psylloidea): A global synthesis. J. Nat. Hist. 2009, 43, 65–179. [Google Scholar] [CrossRef]
- Burckhardt, D.; Ouvrard, D.; Queiroz, D.; Percy, D. Psyllid Host-Plants (Hemiptera: Psylloidea): Resolving a Semantic Problem. Fla. Entomol. 2014, 97, 242–246. [Google Scholar] [CrossRef]
- Čermák, V.; Lauterer, P. Overwintering of psyllids in South Moravia (Czech Republic) with respect to the vectors of the apple proliferation cluster phytoplasmas. Bull. Insectol. 2008, 61, 147–148. [Google Scholar]
- Pizzinat, A.; Tedeschi, R.; Alma, A.; Bertaccini, A.; Maini, S. Cacopsylla melanoneura (Foerster): Aestivation and overwintering habitats in Northwest Italy. Bull. Insectol. 2011, 64, S135–S136. [Google Scholar]
- Lauterer, P. Results of the investigation on Hemiptera in Moravia, made by the Moravian museum (Psylloidea 2). Acta Mus. Morav. Sci. Biol. 1999, 84, 71–151. [Google Scholar]
- Mattedi, L.; Forno, F.; Cainelli, C.; Grando, M.S.; Jarausch, W. Research on Candidatus Phytoplasma mali transmission by insect vectors in Trentino. Acta Hortic. 2008, 369–374. [Google Scholar] [CrossRef]
- Fischnaller, S.; Parth, M.; Messner, M.; Stocker, R.; Kerschbamer, C.; Reyes-Dominguez, Y.; Janik, K. Occurrence of different Cacopsylla species in apple orchards in South Tyrol (Italy) and detection of apple proliferation phytoplasma in Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psylloidea). Cicadina 2017, 17, 37–51. [Google Scholar]
- Frisinghelli, C.; Delaiti, L.; Grando, M.S.; Forti, D.; Vindimian, M.E. Cacopsylla costalis (Flor 1861), as a vector of apple proliferation in Trentino. J. Phytopathol. 2000, 148, 425–431. [Google Scholar] [CrossRef]
- Tedeschi, R.; Alma, A. Transmission of apple proliferation phytoplasma by Cacopsylla melanoneura (Homoptera: Psyllidae). J. Econ. Entomol. 2004, 97, 8–13. [Google Scholar] [CrossRef]
- Jarausch, B.; Tedeschi, R.; Sauvion, N.; Gross, J.; Jarausch, W. Psyllid Vectors. In Phytoplasmas: Plant Pathogenic Bacteria—II.; Bertaccini, A., Weintraub, P.G., Rao, G.P., Mori, N., Eds.; Springer: Singapore, 2019; pp. 53–78. [Google Scholar]
- Oppedisano, T.; Panassiti, B.; Pedrazzoli, F.; Mittelberger, C.; Bianchedi, P.L.; Angeli, G.; de Cristofaro, A.; Janik, K.; Anfora, G.; Ioriatti, C. Importance of psyllids’ life stage in the epidemiology of apple proliferation phytoplasma. J Pest Sci 2019, 57, 135. [Google Scholar] [CrossRef]
- Seemüller, E.; Schneider, B. ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 2004, 54, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Seemüller, E. Apple proliferation. In Compendium of Apple and Pear Diseases; Jones, A.L., Aldwinkel, H.S., Eds.; APS Press: St. Paul, MN, USA, 1990; pp. 67–68. [Google Scholar]
- Seemüller, E.; Carraro, L.; Jarausch, W.; Schneider, B. CHAPTER 14: Apple Proliferation Phytoplasma. In Virus and Virus-Like Diseases of Pome and Stone Fruits; Hadidi, A., Barba, M., Candresse, T., Jelkmann, W., Eds.; APS Press: St. Paul, MN, USA, 2011; pp. 67–73. [Google Scholar] [CrossRef] [Green Version]
- Strauss, E. Phytoplasma research begins to bloom. Science 2009, 325, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, P.G.; Beanland, L. Insect vectors of phytoplasmas. Ann. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, R.; Baldessari, M.; Mazzoni, V.; Trona, F.; Angeli, G. Population dynamics of Cacopsylla melanoneura (Hemiptera: Psyllidae) in Northeast Italy and its role in the apple proliferation epidemiology in apple orchards. J. Econ. Entomol. 2012, 105, 322–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, R.; Lauterer, P.; Brusetti, L.; Tota, F.; Alma, A. Composition, abundance and phytoplasma infection in the hawthorn psyllid fauna of northwestern Italy. Eur. J. Plant Pathol. 2009, 123, 301–310. [Google Scholar] [CrossRef]
- Baric, S.; Öttl, S.; Dalla Via, J. Infection rates of natural psyllid populations with ‘Candidatus Phytoplasma mali’ in South Tyrol (Northern Italy). Julius-Kühn-Archiv 2010, 427, 189–192. [Google Scholar]
- Miñarro, M.; Somoano, A.; Moreno, A.; García, R.R. Candidate insect vectors of apple proliferation in Northwest Spain. Springerplus 2016, 5, 1240. [Google Scholar] [CrossRef] [Green Version]
- Krysan, J.L.; Horton, D.R. Seasonality of Catch of Pear Psylla Cacopsylla pyricola (Homoptera Psyllidae) on Yellow Traps. Environ. Entomol. 1991, 20, 626–634. [Google Scholar] [CrossRef]
- Tedeschi, R.; Bosco, D.; Alma, A. Population dynamics of Cacopsylla melanoneura (Homoptera: Psyllidae), a vector of apple proliferation phytoplasma in northwestern Italy. J. Econ. Entomol. 2002, 95, 544–551. [Google Scholar] [CrossRef]
- Jarausch, B.; Burckhardt, D.; Lauterer, P.; Jarausch, W. Psyllids (Hemiptera, Psylloidea) captured in commercial apple and stone fruit orchards in southwest Germany, eastern France and northwest Switzerland. Mitt. Schweiz. Entomol. Ges. 2009, 82, 205–215. [Google Scholar]
- Burts, E.C.; Retan, A.H. Detection of Pear Psylla; Cooperative Extension Service, College of Agriculture, Washington State University: Pullman, WA, USA, 1969. [Google Scholar]
- Horton, D.R. Relationship among sampling methods in density estimates of pear psylla (Homoptera: Psyllidae): Implications of sex, reproductive maturity, and sampling location. Ann. Entomol. Soc. Am. 1994, 87, 583–591. [Google Scholar] [CrossRef]
- Taylor, R.A.; Mordecai, E.A.; Gilligan, C.A.; Rohr, J.R.; Johnson, L.R. Mathematical models are a powerful method to understand and control the spread of Huanglongbing. PeerJ 2016, 4, e2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapatos, E.T.; Stratopoulou, E.T. Duration times of the immature stages of Cacopsylla pyri L. (Hom., Psyllidae), estimated under field conditions, and their relationship to ambient temperature. J. Appl. Entomol. 1999, 123, 555–559. [Google Scholar] [CrossRef]
- Panassiti, B.; Sander, N.; Mazzoni, V.; Fischnaller, S.; Parth, M.; Messner, M.; Janik, K.; Hartig, F. A temperature-based model for predicting the immigration of Cacopsylla melanoneura and C. picta, vectors of the apple proliferation disease, in South Tyrol, Northern Italy (Hemiptera: Psylloidea). Cicadina 2019, 18, 1–11. [Google Scholar]
- Zartaloudis, Z.; Navrozidis, E.; Copland, M. Sampling method affects estimates of population density and reproductive maturity of summer-form pistachio psyllids. N. Z. J. Zool. 2007, 34, 303–310. [Google Scholar] [CrossRef]
- Stratopoulou, E.T.; Kapatos, E.T. Distribution of Population of Immature Stages of Pear Psylla, Cacopsylla pyri, within the Tree and Development of Sampling Strategy. Entomol. Hell. 1992, 10, 5–10. [Google Scholar] [CrossRef]
- Horton, D.R.; Burts, E.C.; Unruh, T.R.; Krysan, J.L.; Coop, L.B.; Croft, B.A. Intraorchard changes in distribution of winterform pear psylla (Homoptera: Psyllidae) associated with leaf fall in pear. Ann. Entomol. Soc. Am. 1993, 86, 599–608. [Google Scholar] [CrossRef]
- Sétamou, M.; Sanchez, A.; Patt, J.M.; Nelson, S.D.; Jifon, J.; Louzada, E.S. Diurnal patterns of flight activity and effects of light on host finding behavior of the Asian citrus psyllid. J. Insect Behav. 2012, 25, 264–276. [Google Scholar] [CrossRef]
- Nissinen, A.; Lina, K.; Anderbrant, O. Physiological state of female and light intensity affect the host-plant selection of carrot psyllid, Trioza apicalis (Hemiptera: Triozidae). Eur. J. Entomol. 2008, 105, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Rygg, T. Biological investigations on the carrot psyllid Trioza apicalis Foerster (Homoptera, Triozidae). Meld. Nor. Landbrukshogsk. 1977, 56, 1–20. [Google Scholar]
- Dell, A.I.; Pawar, S.; van Savage, M. Systematic variation in the temperature dependence of physiological and ecological traits. Proc. Natl. Acad. Sci. USA 2011, 108, 10591–10596. [Google Scholar] [CrossRef] [Green Version]
- Amarasekare, P.; Savage, V. A framework for elucidating the temperature dependence of fitness. Am. Nat. 2012, 179, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Hodkinson, I.D.; Bird, M.J. Psyllidae (Jumping plant-lice, psyllids). In Greenland Entomofauna, 1st ed.; Bocher, J., Kristiensen, N.P., Pape, T., Wilhelmson, L., Eds.; Brill: Leiden, The Netherlands, 2015; pp. 113–119. [Google Scholar]
- Kappel, F.; Quamme, H.A. Orchard training systems influence early canopy development and light microclimate within apple tree canopies. Can. J. Plant Sci. 1993, 73, 237–248. [Google Scholar] [CrossRef]
- Tanny, J.; Cohen, S.; Grava, A.; Naor, A.; Lukyanov, V. The effect of shading screens on microclimate of apple orchards. Acta Hortic. 2009, 807, 103–108. [Google Scholar] [CrossRef]
- Jenser, G.; Szita, E.; Balint, J. Measuring pear psylla population density (Cacopsylla pyri L. and C. pyricola Forster): Review of previous methods and evaluation of a new technique. North-West. J. Zool. 2010, 6, 54–62. [Google Scholar]
- Hodkinson, I.D.; White, I.M. Homoptera Psylloidea. Handbooks for the Identification of British Insects; Royal Entomological Society of London: London, UK, 1979; Volume II, Part 5(a). [Google Scholar]
- Burckhardt, D. Phylogenetische Verhältnisse in der Gattung Psylla s.l. (Sternorrhyncha, Psyllodea) mit besonderer Berücksichtigung von Psylla colorata Löw. Mitt. Schweiz. Entomol. Ges. 1979, 52, 109–115. [Google Scholar] [CrossRef]
- Oettl, S.; Schlink, K. Molecular Identification of Two Vector Species, Cacopsylla melanoneura and Cacopsylla picta (Hemiptera: Psyllidae), of Apple Proliferation Disease and Further Common Psyllids of Northern Italy. J. Econ. Entomol. 2015, 108, 2174–2183. [Google Scholar] [CrossRef]
- Mittelberger, C.; Obkircher, L.; Oettl, S.; Oppedisano, T.; Pedrazzoli, F.; Panassiti, B.; Kerschbamer, C.; Anfora, G.; Janik, K. The insect vector Cacopsylla picta vertically transmits the bacterium ‘Candidatus Phytoplasma mali’ to its progeny. Plant Pathol. 2017, 66, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Martini, M.; Tedeschi, R. EvaGreen real-time PCR protocol for specific ‘Candidatus Phytoplasma mali’ detection and quantification in insects. Mol. Cell. Probes 2013, 27, 129–136. [Google Scholar] [CrossRef]

| 2015 | 2016 | 2017 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Family | Species | n | n Female | n | n Female | n | n Female | n | [%] |
| Psyllidae | Cacopsylla melanoneura | 62 | 37 | 209 | 125 | 359 | 207 | 630 | 61.7 |
| Baeopelma colorata | 66 | 25 | 26 | 14 | 45 | 25 | 137 | 13.4 | |
| Cacopsylla pruni | 19 | 9 | 27 | 15 | 17 | 9 | 63 | 6.2 | |
| Cacopsylla brunneipennis | 30 | 26 | 23 | 22 | 53 | 5.2 | |||
| Cacopsylla breviantennata | 8 | 5 | 4 | 2 | 5 | 2 | 17 | 1.7 | |
| Cacopsylla picta | 9 | 4 | 4 | 0 | - | - | 13 | 1.3 | |
| Cacopsylla crataegi | 1 | 1 | 5 | 1 | 1 | 0 | 7 | 0.7 | |
| Cacopsylla affinis | 1 | 1 | 2 | 0 | 3 | 1 | 6 | 0.6 | |
| Cacopsylla pulchra | 3 | 2 | 2 | 0 | 5 | 0.5 | |||
| Baeopelma foersteri | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 0.3 | |
| Cacopsylla pulchella | 1 | 1 | 1 | 1 | 2 | 0.2 | |||
| Cacopsylla mali | 2 | 1 | 2 | 0.2 | |||||
| Cacopsylla spp. * | 15 | 12 | 2 | 2 | 17 | 1.7 | |||
| Psylla alni | 1 | 0 | 1 | 0.1 | |||||
| Cacopsylla peregrina | 1 | 0 | 1 | 0.1 | |||||
| Triozidae | Trioza urticae | 9 | 4 | 4 | 3 | 8 | 5 | 21 | 2.1 |
| Trioza remota | 2 | 1 | 5 | 2 | 2 | 2 | 9 | 0.9 | |
| Trioza rhamni | 7 | 5 | 7 | 0.7 | |||||
| Lauritrioza alacris | 3 | 1 | 3 | 0.3 | |||||
| Bactericera nigricornis | 1 | 1 | 2 | 2 | 3 | 0.3 | |||
| Bactericera curvatinervis | 2 | 1 | 2 | 0.2 | |||||
| Bactericera acutipennis | 1 | 1 | 1 | 1 | 2 | 0.2 | |||
| Trioza anthrisci | 1 | 1 | 1 | 0.1 | |||||
| Trioza rotundata | 1 | 1 | 1 | 0.1 | |||||
| Trioza spp. ** | 1 | 1 | 0.1 | ||||||
| Aphalaridae | Rhinocola aceris | 2 | 1 | 1 | 1 | 5 | 2 | 8 | 0.8 |
| Aphalara polygoni | 2 | 0 | 2 | 0.2 | |||||
| Homotomidae | Homotoma ficus | 2 | 0 | 2 | 0.2 | ||||
| Liviidae | Camarotoscena speciosa | 1 | 0 | 1 | 0.1 | ||||
| Psyllopsis fraxinicola | 1 | 1 | 1 | 0.1 | |||||
| Number of species | 18 | 21 | 17 | 28 | |||||
| Number of specimens | 207 | 331 | 483 | 1021 | |||||
| 2015 | 2016 | 2017 | ||||
|---|---|---|---|---|---|---|
| AP+/Analysed/Sampled | AP+ | AP+/Analysed/Sampled | AP+ | AP+/Analysed/Sampled | AP+ | |
| Species | [n] | [%] | [n] | [%] | [n] | [%] |
| C. melanoneura | 1/62/62 | 1.51 | 2/208/209 | 0.96 | 4/77/359 | 5.19 |
| C. picta | 2/9/9 | 22.22 | 0/4/4 | 0 | 0/0/0 | 0 |
| B. colorata | 0/66/66 | 0 | 0/26/26 | 0 | ||
| C. brunneipennis | 0/27/30 | 0 | ||||
| C. pruni | 0/19/19 | 0 | 0/27/27 | 0 | ||
| C. breviantennata | 0/8/8 | 0 | 0/4/4 | 0 | ||
| T. urticae | 0/9/9 | 0 | 0/4/4 | 0 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthel, D.; Kerschbamer, C.; Panassiti, B.; Malenovský, I.; Janik, K. Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector. Plants 2020, 9, 1168. https://doi.org/10.3390/plants9091168
Barthel D, Kerschbamer C, Panassiti B, Malenovský I, Janik K. Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector. Plants. 2020; 9(9):1168. https://doi.org/10.3390/plants9091168
Chicago/Turabian StyleBarthel, Dana, Christine Kerschbamer, Bernd Panassiti, Igor Malenovský, and Katrin Janik. 2020. "Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector" Plants 9, no. 9: 1168. https://doi.org/10.3390/plants9091168
APA StyleBarthel, D., Kerschbamer, C., Panassiti, B., Malenovský, I., & Janik, K. (2020). Effect of Daytime and Tree Canopy Height on Sampling of Cacopsylla melanoneura, a ‘Candidatus Phytoplasma mali’ Vector. Plants, 9(9), 1168. https://doi.org/10.3390/plants9091168






