Effects of Irrigation with Slightly Algae-Contaminated Water on Soil Moisture, Nutrient Redistribution, and Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample
2.2. Algal and Column Experimental Design
2.3. Chemical Analysis
2.4. Molecular Analysis
2.5. Data Analysis
3. Results
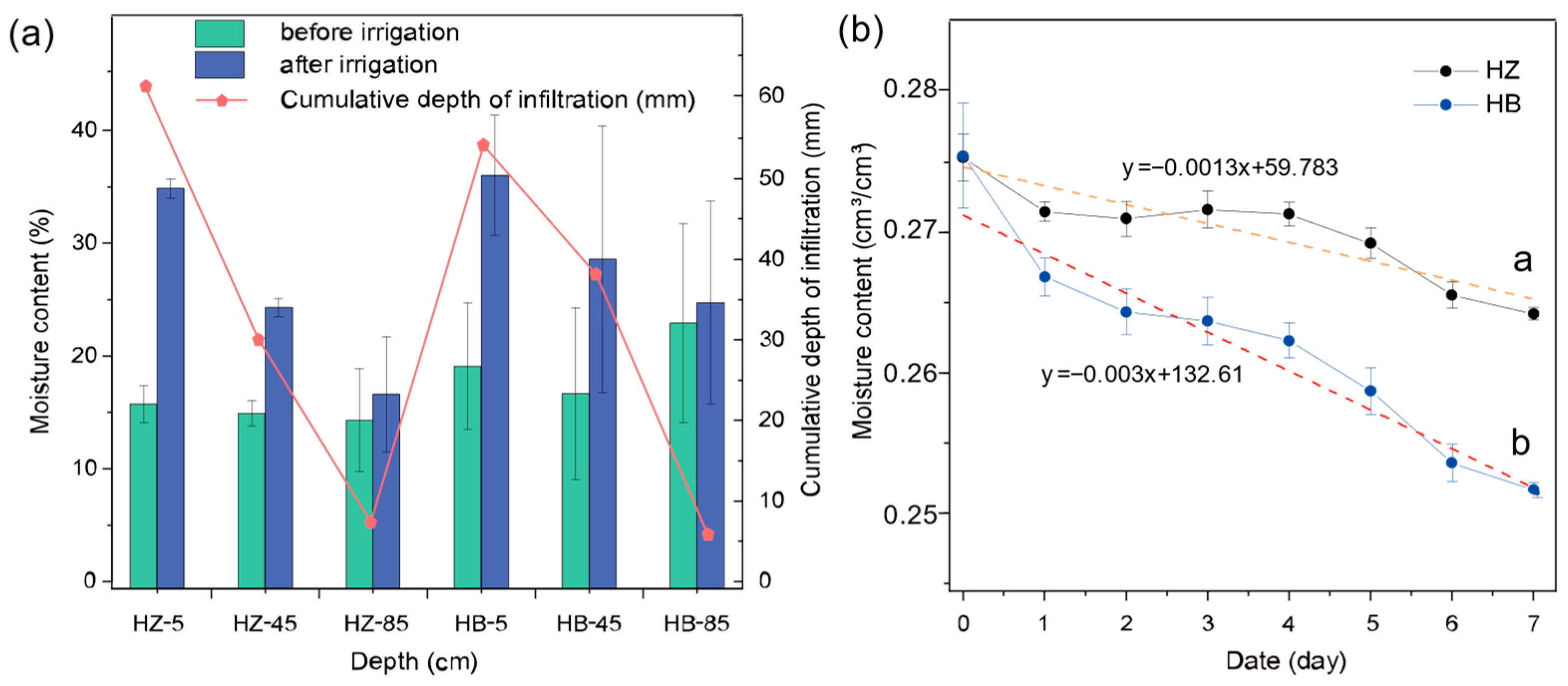
3.1. Soil Moisture Content Characteristics
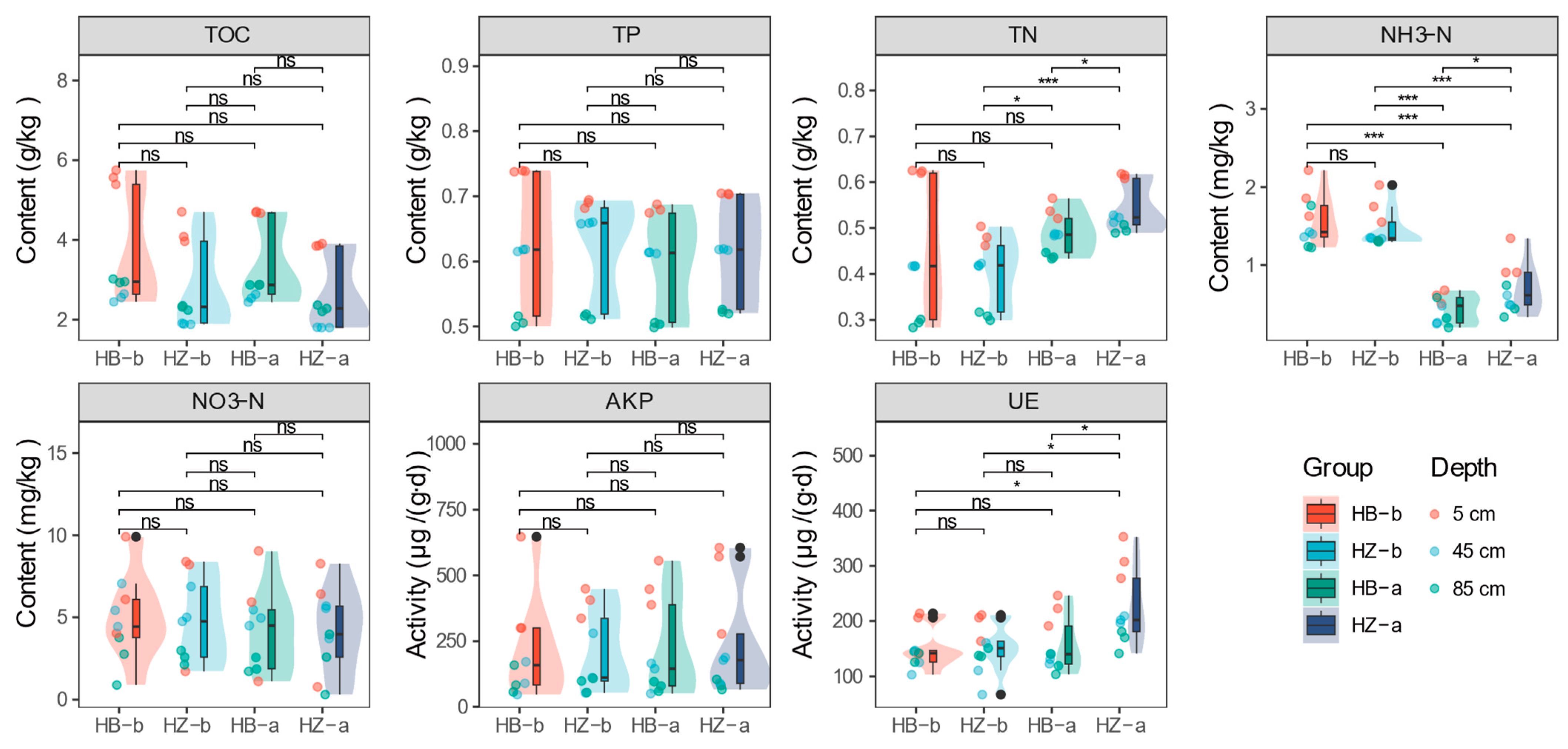
3.2. Soil Nutrient Characteristics
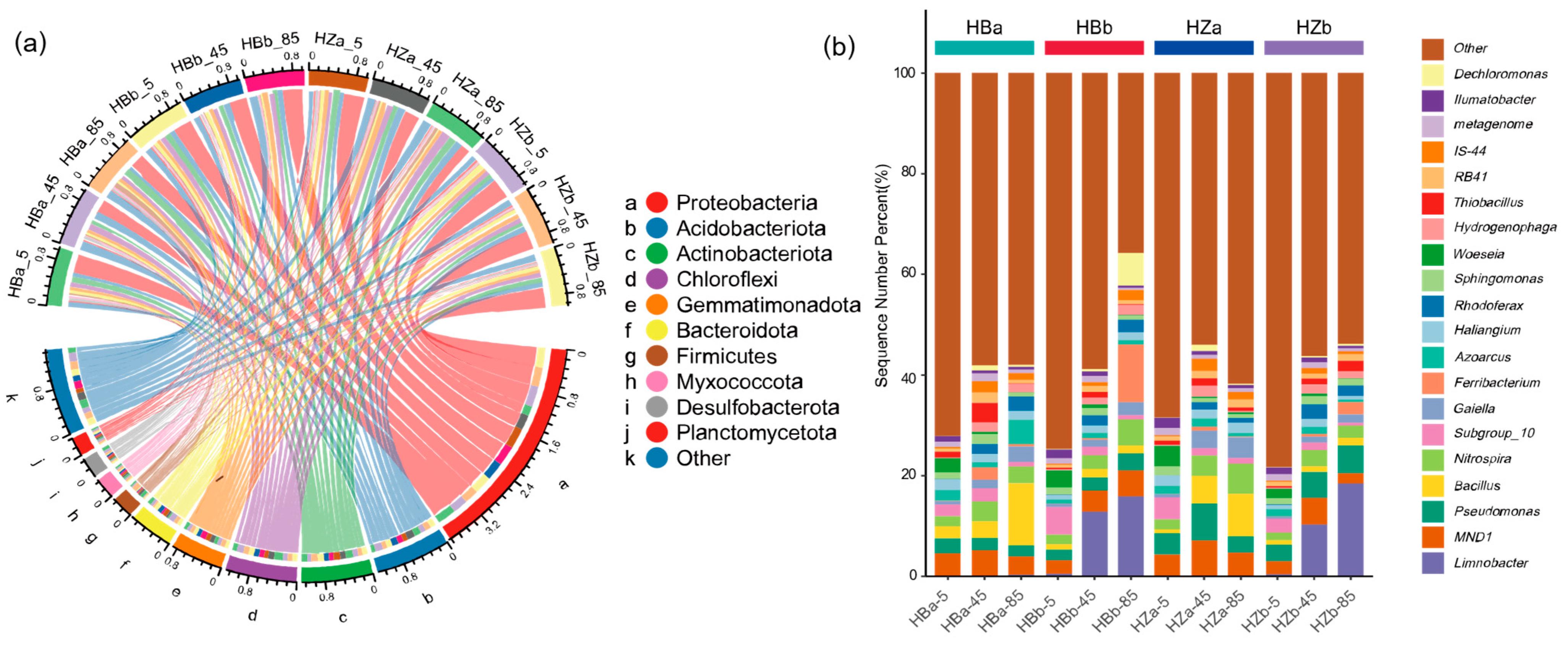
3.3. Soil Microbial Communities and Structure
3.4. Functional Genes Involved in the Soil Nitrogen Cycle
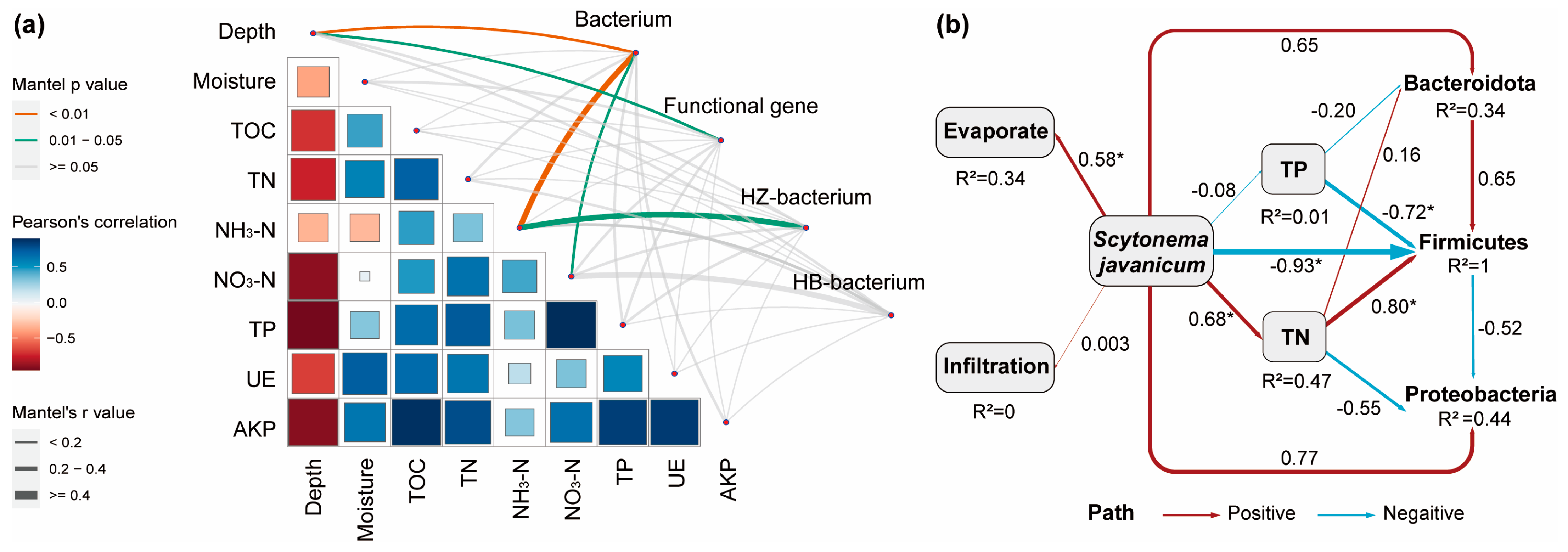
3.5. Factors Influencing Microbial Communities
4. Discussion
4.1. The Effect of Algae on the Physicochemical Properties of Soils
4.2. The Effect of Algae on Soil Microbial Communities and Functional Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Bo, Y.; Li, X.; Wang, S.; Zhou, G. Uncovering Current and Future Variations of Irrigation Water Use Across China Using Machine Learning. Earth’s Future 2024, 12, e2023EF003562. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar, M.; Alshehri, M.; Keshta, I.; Abugabah, A.; Sharma, S.K. Smart water management framework for irrigation in agriculture. Environ. Technol. 2024, 45, 2320–2334. [Google Scholar] [CrossRef]
- Siebert, S.; Kummu, M.; Porkka, M.; Doell, P.; Ramankutty, N.; Scanlon, B.R. A global data set of the extent of irrigated land from 1900 to 2005. Hydrol. Earth Syst. Sci. 2015, 19, 1521–1545. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Sadak, M.S.; Thabet, M. Induction of tolerance in groundnut plants against drought stress and Cercospora leaf spot disease with exogenous application of Arginine and Sodium nitroprusside under field condition. J. Soil Sci. Plant Nutr. 2023, 23, 6612–6631. [Google Scholar] [CrossRef]
- Chen, W.; Lu, S.; Jiao, W.; Wang, M.; Chang, A.C. Reclaimed water: A safe irrigation water source? Environ. Dev. 2013, 8, 74–83. [Google Scholar] [CrossRef]
- Lyu, S.; Chen, W. Soil quality assessment of urban green space under long-term reclaimed water irrigation. Environ. Sci. Pollut. Res. 2016, 23, 4639–4649. [Google Scholar] [CrossRef]
- Moreira, C.; Vasconcelos, V.; Antunes, A. Cyanobacterial Blooms: Current Knowledge and New Perspectives. Earth 2022, 3, 127–135. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fujiyama, H.; Miyamoto, K.; Hatanaka, J.; Wen, G.; Asae, A. Optimizing water cleaning system for microirrigation in the Tohaku irrigation project of Japan. In National Irrigation Symposium Proceedings of the 4th Decennial Symposium, Phoenix, Arizona, USA, November 14–16; American Society of Agricultural Engineers: St. Joseph, MO, USA, 2000. [Google Scholar]
- Cao, Q.; Steinman, A.D.; Su, X.; Xie, L. Effects of microcystins contamination on soil enzyme activities and microbial community in two typical lakeside soils. Environ. Pollut. 2017, 231, 134–142. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Tawfik, M.M.; Kabesh, M.O.; Sadak, M.S. Potential role of algae extract as a natural stimulating for wheat production under reduced nitrogen fertilizer rates and water deficit. Biocatal. Agric. Biotechnol. 2023, 51, 102794. [Google Scholar] [CrossRef]
- Xiao, B.; Hu, K.; Ren, T.; Li, B. Moss-dominated biological soil crusts significantly influence soil moisture and temperature regimes in semiarid ecosystems. Geoderma 2016, 263, 35–46. [Google Scholar] [CrossRef]
- Sandoval, P.A.; Lucia, C.S.; Manuel, M.N.; Garcia-Oliva, F.; Alarcon, A.; Adriana, M.S.; Esperon-Rodriguez, M. Biocrusts, inside and outside resource islands of Mimosa luisana (Leguminosae), improve soil carbon and nitrogen dynamics in a tropical semiarid ecosystem. Eur. J. Soil Biol. 2016, 74, 93–103. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, Q.; He, Q.; Yang, H.; Hu, C. Resource utilization of microalgae from biological soil crusts: Biodiesel production associated with desertification control. Biomass Bioenergy 2018, 116, 189–197. [Google Scholar] [CrossRef]
- Canton, Y.; Chamizo, S.; Rodriguez-Caballero, E.; Lazar, R.; Roncero-Ramos, B.; Raul, R.J.; Sole-Benet, A. Water Regulation in Cyanobacterial Biocrusts from Drylands: Negative Impacts of Anthropogenic Disturbance. Water 2020, 12, 720. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhao, L. Development of fungal community is a potential indicator for evaluating the stability of biological soil crusts in temperate desert revegetation. Appl. Soil Ecol. 2020, 147, 103404. [Google Scholar] [CrossRef]
- Fisher, K.; Jefferson, J.; Vaishampayan, P. Bacterial Communities of Mojave Desert Biological Soil Crusts Are Shaped by Dominant Photoautotrophs and the Presence of Hypolithic Niches. Front. Ecol. Evol. 2020, 7, 518. [Google Scholar] [CrossRef]
- Coban, O.; De, D.G.B.; van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 2022, 375, abe0725. [Google Scholar] [CrossRef] [PubMed]
- Kheirfam, H. Increasing soil potential for carbon sequestration using microbes from biological soil crusts. J. Arid. Environ. 2020, 172, 104022. [Google Scholar] [CrossRef]
- Housman, D.C.; Powers, H.H.; Collins, A.D.; Belnap, J. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. J. Arid. Environ. 2006, 66, 620–634. [Google Scholar] [CrossRef]
- Zhang, Y. The microstructure and formation of biological soil crusts in their early developmental stage. Chin. Sci. Bull. 2005, 50, 117–121. [Google Scholar] [CrossRef]
- Yoon, S.; Cruz-Garcia, C.; Sanford, R.; Ritalahti, K.M.; Loeffler, F.E. Denitrification versus respiratory ammonification: Environmental controls of two competing dissimilatory NO3–/NO2– reduction pathways in Shewanella loihica strain PV-4. ISME J. 2015, 9, 1093–1104. [Google Scholar] [CrossRef]
- Neumann, D.; Heuer, A.; Hemkemeyer, M.; Martens, R.; Tebbe, C.C. Importance of soil organic matter for the diversity of microorganisms involved in the degradation of organic pollutants. ISME J. 2014, 8, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Qu, L.; Ma, K.; Yuan, X. Soil microbial properties under different vegetation types on Mountain Han. Sci. China-Life Sci. 2013, 56, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, F.; Hu, C.; Liu, B. Metagenomics reveals taxon-specific responses of the nitrogen-cycling microbial community to long-term nitrogen fertilization. Soil Biol. Biochem. 2021, 156, 108214. [Google Scholar] [CrossRef]
- Grassmann, C.S.; Mariano, E.; Diniz, P.P.; Borges, B.M.F.; Borges, C.D.; Tsai, S.M.; Rosolem, C.A. Functional N-cycle genes in soil and N2O emissions in tropical grass-maize intercropping systems. Soil Biol. Biochem. 2022, 169, 108655. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wu, L.; Li, X.; Li, G.; Li, J.; Li, C.; Zhao, C.; Wang, F.; Du, C.; Deng, C.; et al. Effects of ambient temperature on the redistribution efficiency of nutrients by desert cyanobacteria- Scytonema javanicum. Sci. Total Environ. 2020, 737, 139733. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.J.; Wu, H.Y.; Zhang, X.D.; Liang, Y.B.; Shi, D.Y.; Wang, L.; Li, H.B. Comparative analysis of chlorine-resistant bacteria after chlorination and chloramination in drinking water treatment plants. J. Hazard. Mater. 2024, 4, 100138. [Google Scholar] [CrossRef]
- Liu, Y.X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. iMeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.; He, B.; Wu, X.; Du, Y. Nitrate loss by runoff in response to rainfall amount category and different combinations of fertilization and cultivation in sloping croplands. Agric. Water Manag. 2022, 273, 107916. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, M.; Han, B.; Butterly, C.; Hu, H.; He, J.; Griffith, D.W.T.; Chen, D. NH3 and greenhouse gas emissions during co-composting of lignite and poultry wastes and the following amendment of co-composted products in soil. Environ. Technol. 2024, 1, 1–14. [Google Scholar]
- Lan, T.; He, X.; Wang, Q.; Deng, O.; Zhou, W.; Luo, L.; Chen, G.; Zeng, J.; Yuan, S.; Zeng, M.; et al. Synergistic effects of biological nitrification inhibitor, urease inhibitor, and biochar on NH3 volatilization, N leaching, and nitrogen use efficiency in a calcareous soil–wheat system. Appl. Soil Ecol. 2022, 174, 104412. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Xia, M.; Wang, Q. Soil properties and functional genes in nitrogen removal process of bioretention. Environ. Technol. 2024, 45, 2268–2283. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.J.; Saggar, S.; Berben, P.; Palmada, T.; Lopez-Villalobos, N.; Pal, P. Use of a urease inhibitor to mitigate ammonia emissions from urine patches. Environ. Technol. 2021, 42, 20–31. [Google Scholar] [CrossRef] [PubMed]
- González Fernández, L.A.; Castillo Ramos, V.; Sánchez Polo, M.; Medellín Castillo, N.A. Fundamentals in applications of algae biomass: A review. J. Environ. Manag. 2023, 338, 117830. [Google Scholar] [CrossRef] [PubMed]
- Pak, T.; Gomari, K.E.; Bose, S.; Tonon, T.; Hughes, D.; Gronnow, M.; Macquarrie, D. Biochar from brown algae: Production, activation, and characterisation. Bioresour. Technol. Rep. 2023, 24, 101688. [Google Scholar] [CrossRef]
- Yue, Y.; Cheng, L.; Sun, Y.; Pang, Y.; Wu, B.; Shi, L.; He, J.; Jia, X. Changes of soil water budget in the area covered by biological soil crusts in Mu Us sandy land, China. J. Appl. Ecol. 2022, 33, 1861–1870. [Google Scholar]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria Inoculation Improves Soil Stability and Fertility on Different Textured Soils: Gaining Insights for Applicability in Soil Restoration. Front. Environ. Sci. 2018, 6, 49. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Freudenberger, D.; Koen, T.B. Diversity and abundance of biological soil crust taxa in relation to fine and coarse-scale disturbances in a grassy eucalypt woodland in eastern Australia. Plant Soil 2006, 281, 255–268. [Google Scholar] [CrossRef]
- David, Q.; Jonah, M.G.; John, M.; Jason, C.Q. Geographical assessment of open pond algal productivity and evaporation losses across the United States. Algal Res. 2021, 60, 102483. [Google Scholar]
- Sun, L.; Bai, Z.; Yang, Q.; Fu, R.; Li, H.; Li, X. In situ assessment of the initial phase of wastewater biofilm formation: Effect of the presence of algae in an aerobic bacterial biofilm system. Water Res. 2024, 253, 121283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lamusa, A. Influence mechanism of development of biological soil crust on evaporation process. J. Arid Land Resour. Environ. 2011, 25, 193–200. [Google Scholar]
- Chamizo, S.; Canton, Y.; Rodriguez-Caballero, E.; Domingo, F. Biocrusts positively affect the soil water balance in semiarid ecosystems. Ecohydrology 2016, 9, 1208–1221. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Gardner, R.D. Cyanobacteria-based soil amendments in the soil-plant system: Effects of inoculations on soil nutrient and microbial dynamics under spring wheat growth. Algal Res. 2024, 77, 103326. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Budel, B.; Andreae, M.O.; Poschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Jassey, V.E.J.; Walcker, R.; Kardol, P.; Geisen, S.; Heger, T.; Lamentowicz, M.; Hamard, S.; Lara, E. Contribution of soil algae to the global carbon cycle. New Phytol. 2022, 234, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.; Ouyang, H.; Wu, L.; Zhang, D.; Hu, C. Biological soil crust community types differ in photosynthetic pigment composition, fluorescence and carbon fixation in Shapotou region of China. Appl. Soil Ecol. 2017, 111, 9–16. [Google Scholar] [CrossRef]
- Khoshru, B.; Khoshmanzar, E.; Asgari Lajayer, B.; Ghorbanpour, M. Soil moisture–mediated changes in microorganism biomass and bioavailability of nutrients in paddy soil. In Plant Stress Mitigators; Ghorbanpour, M., Adnan Shahid, M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 479–494. [Google Scholar]
- Liu, M.; Xia, L.; Liu, R.; Gao, Z.; Han, C.; Feng, J.; Wang, J.; Qu, W.; Xing, T. Degradation of High-Concentration Nitrate Nitrogen in Groundwater: A Laboratory Study. J. Chem. 2021, 2021, 4797946. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, L.; Zhang, Y.; Zhang, B.; Zhao, Z.; Zhang, Y.; Li, M.; Jiang, X. Nitrogen Fixation Occurring in Sediments: Contribution to the Nitrogen Budget of Lake Taihu, China. J. Geophys. Res.-Biogeosci. 2018, 123, 2661–2674. [Google Scholar] [CrossRef]
- Shatwell, T.; Koehler, J. Decreased nitrogen loading controls summer cyanobacterial blooms without promoting nitrogen-fixing taxa: Long-term response of a shallow lake. Limnol. Oceanogr. 2019, 64, S166–S178. [Google Scholar] [CrossRef]
- Xu, H.; McCarthy, M.J.; Paerl, H.W.; Brookes, J.D.; Zhu, G.; Hall, N.S.; Qin, B.; Zhang, Y.; Zhu, M.; Hampel, J.J.; et al. Contributions of external nutrient loading and internal cycling to cyanobacterial bloom dynamics in Lake Taihu, China: Implications for nutrient management. Limnol. Oceanogr. 2021, 66, 1492–1509. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, C.; Vadiveloo, A.; Montes, M.L.; Xia, L.; Song, S.; Fernandez, M.A.; Lan, S. Efficient nutrient recycling from wastewater to deserts: A comparative study on biocrust cyanobacteria performance. Chem. Eng. J. 2024, 491, 151927. [Google Scholar] [CrossRef]
- Liu, J.; Macrae, M.L.; Elliott, J.A.; Baulch, H.M.; Wilson, H.F.; Kleinman, P.J.A. Impacts of Cover Crops and Crop Residues on Phosphorus Losses in Cold Climates: A Review. J. Environ. Qual. 2019, 48, 850–868. [Google Scholar] [CrossRef]
- Sun, D.; Jiang, X.; Wu, Q.; Zhou, N. Intragenomic Heterogeneity of 16S rRNA Genes Causes Overestimation of Prokaryotic Diversity. Appl. Environ. Microbiol. 2013, 79, 5962–5969. [Google Scholar] [CrossRef]
- Bothe, H.; Schmitz, O.; Yates, M.G.; Newton, W.E. Nitrogen Fixation and Hydrogen Metabolism in Cyanobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 529–551. [Google Scholar] [CrossRef]
- Nguyen, M.L.; Westerhoff, P.; Baker, L.; Hu, Q.; Esparza-Soto, M.; Sommerfeld, M. Characteristics and reactivity of algae-produced dissolved organic carbon. J. Environ. Eng. 2005, 131, 1574–1582. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Su, Y.; Jia, R. Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: A four-year field study. Catena 2012, 97, 119–126. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Chen, Y.; Zhang, Z.; Li, Y. Carbon fixation of cyanobacterial–algal crusts after desert fixation and its implication to soil organic carbon accumulation in desert. Land Degrad. Dev. 2013, 24, 342–349. [Google Scholar]
- Song, X.; Bo, Y.; Feng, Y.; Tan, Y.; Zhou, C.; Yan, X.; Ruan, R.; Xu, Q.; Cheng, P. Potential applications for multifunctional microalgae in soil improvement. Front. Environ. Sci. 2022, 10, 1035332. [Google Scholar] [CrossRef]
- Redmile-Gordon, M.; Gregory, A.S.; White, R.P.; Watts, C.W. Soil organic carbon, extracellular polymeric substances (EPS), and soil structural stability as affected by previous and current land-use. Geoderma 2020, 363, 114143. [Google Scholar] [CrossRef]
- Li, J.; Xu, M.; Wang, J.; Lan, C.; Lai, J. Effects of nutrient limitation on cell growth, exopolysaccharide secretion and TEP production of Phaeocystis globosa. Mar. Environ. Res. 2023, 183, 105801. [Google Scholar] [CrossRef]
- Kappaun, K.; Piovesan, A.R.; Carlini, C.R.; Ligabue-Braun, R. Ureases: Historical aspects.; catalytic.; and non-catalytic properties—A review. J. Adv. Res. 2018, 13, 3–17. [Google Scholar] [CrossRef]
- Yahya, M.N.; Gökçekuş, H.; Orhon, D.; Keskinler, B.; Karagunduz, A.; Omwene, P.I. A Study on the Hydrolysis of Urea Contained in Wastewater and Continuous Recovery of Ammonia by an Enzymatic Membrane Reactor. Processes 2021, 9, 1703. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Q.; Sun, H.; Jia, L.; Zhao, L.; Wu, W. Metagenomic analyses of microbial structure and metabolic pathway in solid-phase denitrification systems for advanced nitrogen removal of wastewater treatment plant effluent: A pilot-scale study. Water Res. 2021, 196, 117067. [Google Scholar] [CrossRef]
- Yuan, D.; Zheng, L.; Liu, Y.X.; Cheng, H.; Ding, A.; Wang, X.; Tan, Q.; Wang, X.; Xing, Y.; Xie, E.; et al. Nitrifiers Cooperate to Produce Nitrous Oxide in Plateau Wetland Sediments. Environ. Sci. Technol. 2023, 57, 810–821. [Google Scholar] [CrossRef]
- Shrewsbury, L.H.; Smith, J.L.; Huggins, D.R.; Carpenter-Boggs, L.; Reardon, C.L. Denitrifier abundance has a greater influence on denitrification rates at larger landscape scales but is a lesser driver than environmental variables. Soil Biol. Biochem. 2016, 103, 221–231. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, H.; Sun, X.; Zhu, Y.; Yang, L. Nitrification and denitrification by algae-attached and free-living microorganisms during a cyanobacterial bloom in Lake Taihu.; a shallow Eutrophic Lake in China. Biogeochemistry 2016, 131, 135–146. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, R.; Sun, B.; Che, C.; Hou, L. Effects of Sea Animal Activities on Tundra Soil Denitrification and nirS- and nirK-Encoding Denitrifier Community in Maritime Antarctica. Front. Microbiol. 2020, 11, 573302. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, J.; Liu, Y.; Zhao, J.; Ma, J.; Yu, Q.; Zou, P.; Lin, H.; Wang, Q. Differential responses of soil nirS- and nirK-type denitrifying microbial communities to long-term application of biogas slurry in a paddy soil. Appl. Soil Ecol. 2023, 182, 104711. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Hu, H.; Cai, Z.; Lei, Y.; Li, W.; Zhang, M.; Li, Z.; Zhu, Y.; Cui, L. Changes of the denitrifying communities in a multi-stage free water surface constructed wetland. Sci. Total Environ. 2019, 650, 1419–1425. [Google Scholar] [CrossRef]
- Shi, R.; Xu, S.; Qi, Z.; Huang, H.; Liang, Q. Seasonal patterns and environmental drivers of nirS- and nirK-encoding denitrifiers in sediments of Daya Bay.; China. Oceanologia 2019, 61, 308–320. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, L.; Liu, M.; Gao, J.; Yin, G.; Li, X.; Deng, F.; Lin, X.; Jiang, X.; Chen, F.; et al. Diversity.; Abundance.; and Distribution of nirS-Harboring Denitrifiers in Intertidal Sediments of the Yangtze Estuary. Microb. Ecol. 2015, 70, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.R.H.; Zhao, M.; Jones, C.M.; Hallin, S. Soil type overrides plant effect on genetic and enzymatic N2O production potential in arable soils. Soil Biol. Biochem. 2016, 100, 125–128. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.; Wang, X.; Huang, C.; Bao, Z.; Zhao, X.; Tan, Z.; Xie, E. Effects of Irrigation with Slightly Algae-Contaminated Water on Soil Moisture, Nutrient Redistribution, and Microbial Community. Processes 2024, 12, 1639. https://doi.org/10.3390/pr12081639
Zheng H, Wang X, Huang C, Bao Z, Zhao X, Tan Z, Xie E. Effects of Irrigation with Slightly Algae-Contaminated Water on Soil Moisture, Nutrient Redistribution, and Microbial Community. Processes. 2024; 12(8):1639. https://doi.org/10.3390/pr12081639
Chicago/Turabian StyleZheng, Han, Xiaofei Wang, Chen Huang, Zhe Bao, Xiaohui Zhao, Zhanming Tan, and En Xie. 2024. "Effects of Irrigation with Slightly Algae-Contaminated Water on Soil Moisture, Nutrient Redistribution, and Microbial Community" Processes 12, no. 8: 1639. https://doi.org/10.3390/pr12081639




