Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives
Abstract
:1. Introduction
2. Search Strategies
3. Contamination Status of Bisphenols
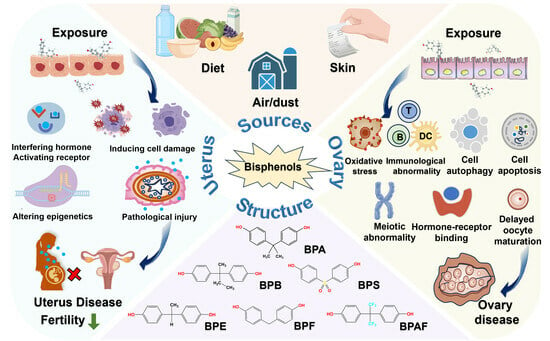
3.1. Sources of Bisphenols
3.2. Bisphenol Contamination in the Environment
| Sample Sources | Country | Measurement Time | BPA | BPS | BPF | BPB | BPAF | TBBPA | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (Range/Mean) | DR. (%) | Conc. (Range/Mean) | DR. (%) | Conc. (Range/Mean) | DR. (%) | Conc. (Range/Mean) | DR. (%) | Conc. (Range/Mean) | DR. (%) | Conc. (Range/Mean) | DR. (%) | ||||
| Bay water | America | 2017 | 12 ng/L | 97 | 8.8 ng/L | 41 | Shimabuku I et al., 2021 [42] | ||||||||
| Surface water | Romania | 2018–2019 | 74.5–135 ng/L | 6.15–8.23 ng/L | Chiriac FL et al., 2020 [47] | ||||||||||
| River | United Kingdom | 2015 | 38.1 ng/L | Petrie B et al., 2018 [48] | |||||||||||
| Lake | China | 2016 | 26 ng/L | 100 | 16 ng/L | 100 | 78 ng/L | 100 | 20 ng/L | 100 | 110 ng/L | 100 | Liu Y et al., 2017 [43] | ||
| Rainfall | China | 2015 | 1480 ng/L | 100 | 3.72 ng/L | 100 | 56.7 ng/L | 100 | 1.38 ng/L | 76 | Huang Z et al., 2020 [44] | ||||
| Drinking water | China | 2017 | 1.6 ng/L | 40 | 0.1 ng/L | 25 | 0.04 ng/L | 5 | 0.2 ng/L | 10 | 0.4 ng/L | 30 | Zhang H et al., 2018 [51] | ||
| Coastal water | Malaysia | 2022 | 59.01 ng/L | 100 | 10.96 ng/L | 1144 | 17.65 ng/L | Zainuddin AH et al., 2023 [57] | |||||||
| Fresh water | Europe | NF | 29 ng/L | Staples C et al., 2018 [58] | |||||||||||
| Marine water | 7 ng/L | ||||||||||||||
| Oceanic sandy beaches | 26 countries around the world | 2014–2015 | 4247 μg/kg | Kwon BG et al., 2020 [54] | |||||||||||
| Soil | China | NF | 0.17 μg/g dw | 0.078 μg/g dw | 0.21 μg/g dw | Xu Y et al., 2021 [55] | |||||||||
| Dust | 12 countries around the world | 2012–2014 | 1000 μg/g | 220 μg/g | 1000 μg/g | <1 μg/g | 3.1 μg/g | 87 μg/g | Wang W et al., 2015 [56] | ||||||
3.3. Bisphenol Contamination in Humans
3.4. Exposure Patterns of Bisphenols in the Population
4. Toxic Effects of Bisphenols on the Uterus
4.1. Animal Studies
4.1.1. Changes in Uterine Morphology
4.1.2. Changes in Overall Fertility
Estrus Cycle and Intrauterine Implantation
Uterine Arteries and Reproductive Circulation
Intergenerational Transmission
4.1.3. Interference with Hormone Levels
4.1.4. Uterine Cancer
4.1.5. Epigenomic Changes
4.2. In Vitro Studies
4.2.1. Receptor-Mediated Biological Effects
4.2.2. Transcriptional Regulation Mediated Biological Effects
4.3. Epidemiological Research
4.3.1. Uterine Fibroids
4.3.2. Endometriosis and Endometrial Carcinoma
4.3.3. Reproductive Disorders
4.3.4. Miscarriage
5. Toxic Effects of Bisphenols on the Ovary
5.1. Animal Studies
5.1.1. Changes in Ovarian Morphology
5.1.2. Changes in Meiotic Division
5.1.3. Autophagy and Apoptosis
5.1.4. Changes in Ovarian Development and Oocyte Maturation
5.1.5. Interference with Hormone Levels
5.1.6. Oxidative Stress
5.1.7. Ovarian Cancer
5.2. In Vitro Studies
5.2.1. Follicle Formation and Development
5.2.2. Meiosis
5.2.3. Autophagy and Apoptosis
5.2.4. Oxidative Damage
5.2.5. Chromosomal and DNA Damage
5.2.6. Hormone Production
5.3. Epidemiological Research
5.3.1. Polycystic Ovary Syndrome
5.3.2. Poor Ovarian Response
5.3.3. Diminished Ovarian Reserve (DOR)
5.3.4. Precocious Puberty
6. Toxic Effects of Bisphenols on Other Female Reproductive Organs
6.1. Bisphenols as Fallopian Tube Poisons
6.2. Bisphenols as Vaginal Poisons
7. Conclusions
8. Challenges and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. Int. 2022, 29, 62067–62092. [Google Scholar] [CrossRef]
- Ng, K.Y.B.; Cherian, G.; Kermack, A.J.; Bailey, S.; Macklon, N.; Sunkara, S.K.; Cheong, Y. Systematic review and meta-analysis of female lifestyle factors and risk of recurrent pregnancy loss. Sci. Rep. 2021, 11, 7081. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Gaskins, A.J.; Souter, I.; Smith, K.W.; Dodge, L.E.; Ehrlich, S.; Meeker, J.D.; Calafat, A.M.; Williams, P.L.; EARTH Study Team. Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Women Undergoing in Vitro Fertilization: Results from the EARTH Study. Environ. Health Perspect. 2016, 124, 831–839. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Vimpani, G.V.; Robertson, E.F.; Baghurst, P.A.; Clark, P.D. The Port Pirie cohort study: Maternal blood lead and pregnancy outcome. J. Epidemiol. Community Health 1986, 40, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; Fini, J.B.; Hofer, T.; Steffensen, I.L.; Dirven, H.; Barouki, R.; Olea, N.; et al. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef]
- Russo, G.; Barbato, F.; Mita, D.G.; Grumetto, L. Occurrence of Bisphenol A and its analogues in some foodstuff marketed in Europe. Food Chem Toxicol. 2019, 131, 110575. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.S., Jr.; Menon, R.; Yu, G.F.B.; Amosco, M.D. Actions of Bisphenol A on Different Feto-Maternal Compartments Contributing to Preterm Birth. Int. J. Mol. Sci. 2022, 23, 2411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.H.; Pan, S.D.; Wang, J.L.; Zheng, Y.B.; Xu, J.J.; Zhao, Y.G.; Cai, Z.X.; Jin, M.C. Contamination status of bisphenol A and its analogues (bisphenol S, F and B) in foodstuffs and the implications for dietary exposure on adult residents in Zhejiang Province. Food Chem. 2019, 294, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Çiftçi, S.; Yalçın, S.S.; Samur, G. Comparison of daily bisphenol A intake based on dietary and urinary levels in breastfeeding women. Reprod. Toxicol. 2021, 106, 9–17. [Google Scholar] [CrossRef]
- Huang, R.P.; Liu, Z.H.; Yuan, S.F.; Yin, H.; Dang, Z.; Wu, P.X. Worldwide human daily intakes of bisphenol A (BPA) estimated from global urinary concentration data (2000–2016) and its risk analysis. Environ. Pollut. 2017, 230, 143–152. [Google Scholar] [CrossRef]
- Costa, S.A.; Severo, M.; Correia, D.; Carvalho, C.; Magalhães, V.; Vilela, S.; Cunha, S.; Casal, S.; Lopes, C.; Torres, D. Methodological approaches for the assessment of bisphenol A exposure. Food Res. Int. 2023, 173 Pt 1, 113251. [Google Scholar] [CrossRef]
- Czarny-Krzymińska, K.; Krawczyk, B.; Szczukocki, D. Bisphenol A and its substitutes in the aquatic environment: Occurrence and toxicity assessment. Chemosphere 2023, 315, 137763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Quan, Q.; Zhang, M.; Zhang, N.; Zhang, W.; Zhan, M.; Xu, W.; Lu, L.; Fan, J.; Wang, Q. Occurrence of bisphenol A and its alternatives in paired urine and indoor dust from Chinese university students: Implications for human exposure. Chemosphere 2020, 247, 125987. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. The Impact of Prenatal Exposure to Bisphenol A on Male Reproductive Function. Front. Endocrinol. 2020, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, C.; Soto, A.M. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. Mol. Biol. 1998, 65, 143–150. [Google Scholar] [CrossRef]
- Sohoni, P.; Sumpter, J.P. Several environmental oestrogens are also anti-androgens. J. Endocrinol. 1998, 158, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.D.; Brandt, J.Z.; Grassi, T.F.; Silveira, L.T.; Scarano, W.R.; Barbisan, L.F. Genistein reduces the noxious effects of in utero bisphenol A exposure on the rat prostate gland at weaning and in adulthood. Food Chem Toxicol. 2015, 84, 64–73. [Google Scholar] [CrossRef]
- Stroheker, T.; Picard, K.; Lhuguenot, J.C.; Canivenc-Lavier, M.C.; Chagnon, M.C. Steroid activities comparison of natural and food wrap compounds in human breast cancer cell lines. Food Chem Toxicol. 2004, 42, 887–897. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Flaws, J.A. Evidence for bisphenol A-induced female infertility: A review (2007–2016). Fertil. Steril. 2016, 106, 827–856. [Google Scholar] [CrossRef]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.H.; VandeVoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Mao, J.; Jain, A.; Denslow, N.D.; Nouri, M.Z.; Chen, S.; Wang, T.; Zhu, N.; Koh, J.; Sarma, S.J.; Sumner, B.W.; et al. Bisphenol A and bisphenol S disruptions of the mouse placenta and potential effects on the placenta-brain axis. Proc. Natl. Acad. Sci. USA 2020, 117, 4642–4652. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jiang, P.; Li, Y.; Yan, W.; Yue, H. New insights into the effect of bisphenol AF exposure on maternal mammary glands at various stages of gestation in mice. Sci. Total Environ. 2022, 850, 157793. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, P.; Li, Y.; Su, L.; Yue, H.; Sang, N. Maternal Bisphenol B Exposure and Mammary Gland Development of Offspring: A Time-Series Analysis. Environ. Health 2023, 1, 278–290. [Google Scholar] [CrossRef]
- Serra, H.; Beausoleil, C.; Habert, R.; Minier, C.; Picard-Hagen, N.; Michel, C. Evidence for Bisphenol B Endocrine Properties: Scientific and Regulatory Perspectives. Environ. Health Perspect. 2019, 127, 106001. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; D’Andrea, S.; Bernabò, N.; Volle, D.H. Editorial: Bisphenols and Male Reproductive Health. Front. Endocrinol. 2020, 11, 597609. [Google Scholar] [CrossRef]
- Siddique, S.; Zhang, G.; Coleman, K.; Kubwabo, C. Investigation of the migration of bisphenols from baby bottles and sippy cups. Curr. Res. Food Sci. 2021, 4, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Petrarca, M.H.; Perez, M.A.F.; Tfouni, S.A.V. Bisphenol A and its structural analogues in infant formulas available in the Brazilian market: Optimisation of a UPLC-MS/MS method, occurrence, and dietary exposure assessment. Food Res. Int. 2022, 160, 111692. [Google Scholar] [CrossRef]
- Andaluri, G.; Manickavachagam, M.; Suri, R. Plastic toys as a source of exposure to bisphenol-A and phthalates at childcare facilities. Environ. Monit. Assess 2018, 190, 65. [Google Scholar] [CrossRef]
- Negev, M.; Berman, T.; Reicher, S.; Sadeh, M.; Ardi, R.; Shammai, Y. Concentrations of trace metals, phthalates, bisphenol A and flame-retardants in toys and other children’s products in Israel. Chemosphere 2018, 192, 217–224. [Google Scholar] [CrossRef]
- Freire, C.; Molina-Molina, J.M.; Iribarne-Durán, L.M.; Jiménez-Díaz, I.; Vela-Soria, F.; Mustieles, V.; Arrebola, J.P.; Fernández, M.F.; Artacho-Cordón, F.; Olea, N. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ. Int. 2019, 127, 592–600. [Google Scholar] [CrossRef]
- Frankowski, R.; Zgoła-Grześkowiak, A.; Grześkowiak, T.; Sójka, K. The presence of bisphenol A in the thermal paper in the face of changing European regulations—A comparative global research. Environ. Pollut. 2020, 265 Pt A, 114879. [Google Scholar] [CrossRef]
- Xu, Z.; Tian, L.; Liu, L.; Goodyer, C.G.; Hales, B.F.; Bayen, S. Food Thermal Labels are a Source of Dietary Exposure to Bisphenol S and Other Color Developers. Environ. Sci. Technol. 2023, 57, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Liu, Y.; Gong, X.; Zhang, T.; Sun, H. Widespread Occurrence of Bisphenol A in Daily Clothes and Its High Exposure Risk in Humans. Environ. Sci. Technol. 2019, 53, 7095–7102. [Google Scholar] [CrossRef]
- Česen, M.; Lenarčič, K.; Mislej, V.; Levstek, M.; Kovačič, A.; Cimrmančič, B.; Uranjek, N.; Kosjek, T.; Heath, D.; Dolenc, M.S. The occurrence and source identification of bisphenol compounds in wastewaters. Sci. Total Environ. 2018, 616–617, 744–752. [Google Scholar] [CrossRef]
- Drobna, Z.; Talarovicova, A.; Schrader, H.E.; Fennell, T.R.; Snyder, R.W.; Rissman, E.F. Bisphenol F has different effects on preadipocytes differentiation and weight gain in adult mice as compared with Bisphenol A and S. Toxicology 2019, 420, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Liu, X.; Okada, H.; Shimohigashi, M.; Shimohigashi, Y. Bisphenol AF is a full agonist for the estrogen receptor ERalpha but a highly specific antagonist for ERbeta. Environ. Health Perspect. 2010, 118, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Zhu, J.; Zhao, M.; Jin, H. Twenty bisphenol analogues in take-out polystyrene-made food containers: Concentration levels, simulated migration, and risk evaluation. Environ. Sci. Pollut. Res. Int. 2023, 30, 10516–10526. [Google Scholar] [CrossRef]
- Bello, A.; Xue, Y.; Bello, D. Urinary biomonitoring of occupational exposures to Bisphenol A Diglycidyl Ether (BADGE)—Based epoxy resins among construction painters in metal structure coating. Environ. Int. 2021, 156, 106632. [Google Scholar] [CrossRef]
- Lamprea, K.; Bressy, A.; Mirande-Bret, C.; Caupos, E.; Gromaire, M.C. Alkylphenol and bisphenol A contamination of urban runoff: An evaluation of the emission potentials of various construction materials and automotive supplies. Environ. Sci. Pollut. Res. Int. 2018, 25, 21887–21900. [Google Scholar] [CrossRef]
- Jia, L.L.; Zhang, Y.J.; Gao, C.J.; Guo, Y. Parabens and bisphenol A and its structural analogues in over-the-counter medicines from China. Environ. Sci. Pollut. Res. Int. 2021, 28, 45266–45275. [Google Scholar] [CrossRef]
- Shimabuku, I.; Chen, D.; Wu, Y.; Miller, E.; Sun, J.; Sutton, R. Occurrence and risk assessment of organophosphate esters and bisphenols in San Francisco Bay, California, USA. Sci. Total Environ. 2022, 813, 152287. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Song, N.; Guo, R.; Chen, M.; Mai, D.; Yan, Z.; Han, Z.; Chen, J. Occurrence, distribution and sources of bisphenol analogues in a shallow Chinese freshwater lake (Taihu Lake): Implications for ecological and human health risk. Sci. Total Environ. 2017, 599–600, 1090–1098. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, J.L.; Yang, Y.Y.; Jia, Y.W.; Zhang, Q.Q.; Chen, C.E.; Liu, Y.S.; Yang, B.; Xie, L.; Ying, G.G. Occurrence, mass loads and risks of bisphenol analogues in the Pearl River Delta region, South China: Urban rainfall runoff as a potential source for receiving rivers. Environ. Pollut. 2020, 263 Pt B, 114361. [Google Scholar] [CrossRef]
- Liu, Y.; Su, W.; Zhu, Y.; Xiao, L.; Hu, T. Endocrine disrupting compounds in the middle and lower reaches of the Lhasa River Basin: Occurrence, distribution, and risk assessment. Sci. Total Environ. 2020, 727, 138694. [Google Scholar] [CrossRef]
- Dueñas-Moreno, J.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, F.L.; Paun, I.; Pirvu, F.; Pascu, L.F.; Galaon, T. Occurrence and Fate of Bisphenol A and its Congeners in Two Wastewater Treatment Plants and Receiving Surface Waters in Romania. Environ. Toxicol. Chem. 2021, 40, 435–446. [Google Scholar] [CrossRef]
- Petrie, B.; Lopardo, L.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Assessment of bisphenol-A in the urban water cycle. Sci. Total Environ. 2019, 650 Pt 1, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Kiejza, D.; Kotowska, U.; Polińska, W.; Karpińska, J. USAEME-GC/MS Method for Easy and Sensitive Determination of Nine Bisphenol Analogues in Water and Wastewater. Molecules 2022, 27, 4977. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Kannan, K. Mass flows and removal of eight bisphenol analogs, bisphenol A diglycidyl ether and its derivatives in two wastewater treatment plants in New York State, USA. Sci. Total Environ. 2019, 648, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Li, J.; Yang, M. Occurrence and exposure assessment of bisphenol analogues in source water and drinking water in China. Sci. Total Environ. 2019, 655, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, J.; Ren, J.; Shen, J.; Fan, J.; Xi, R.; Chen, W.; Chen, Q. Occurrence, Distribution and Ecological Risk of Bisphenol Analogues in the Surface Water from a Water Diversion Project in Nanjing, China. Int. J. Environ. Res. Public Health 2019, 16, 3296. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, D.; Qi, Z.; Long, C.; Li, G.; Yu, Y. A large-scale nationwide study of urinary phenols in the Chinese population. Sci. Total Environ. 2023, 894, 164850. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Chung, S.Y.; Saido, K. Sandy beaches as hotspots of bisphenol A. Environ. Res. 2020, 191, 110175. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, A.; Li, Y.; He, Y.; Xu, J.; Lu, Z. Determination and occurrence of bisphenol A and thirteen structural analogs in soil. Chemosphere 2021, 277, 130232. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Abualnaja, K.O.; Asimakopoulos, A.G.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.B.; et al. A comparative assessment of human exposure to tetrabromobisphenol A and eight bisphenols including bisphenol A via indoor dust ingestion in twelve countries. Environ. Int. 2015, 83, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zainuddin, A.H.; Roslan, M.Q.J.; Razak, M.R.; Yusoff, F.M.; Haron, D.E.M.; Aris, A.Z. Occurrence, distribution, and ecological risk of bisphenol analogues in marine ecosystem of urbanized coast and estuary. Mar. Pollut. Bull. 2023, 192, 115019. [Google Scholar] [CrossRef]
- Staples, C.; van der Hoeven, N.; Clark, K.; Mihaich, E.; Woelz, J.; Hentges, S. Distributions of concentrations of bisphenol A in North American and European surface waters and sediments determined from 19 years of monitoring data. Chemosphere 2018, 201, 448–458. [Google Scholar] [CrossRef]
- Philips, E.M.; Santos, S.; Steegers, E.A.P.; Asimakopoulos, A.G.; Kannan, K.; Trasande, L.; Jaddoe, V.W.V. Maternal bisphenol and phthalate urine concentrations and weight gain during pregnancy. Environ. Int. 2020, 135, 105342. [Google Scholar] [CrossRef]
- Sol, C.M.; van Zwol-Janssens, C.; Philips, E.M.; Asimakopoulos, A.G.; Martinez-Moral, M.P.; Kannan, K.; Jaddoe, V.W.V.; Trasande, L.; Santos, S. Maternal bisphenol urine concentrations, fetal growth and adverse birth outcomes: A population-based prospective cohort. Environ. Health 2021, 20, 60. [Google Scholar] [CrossRef]
- Kim, S.; Park, E.; Park, E.K.; Lee, S.; Kwon, J.A.; Shin, B.H.; Kang, S.; Park, E.Y.; Kim, B. Urinary Concentrations of Bisphenol Mixtures during Pregnancy and Birth Outcomes: The MAKE Study. Int. J. Environ. Res. Public Health 2021, 18, 10098. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; Kim, G.H.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Lin, B.G.; Zhou, B.; Cao, W.C.; Chen, P.P.; Deng, Y.L.; Hou, J.; Sun, S.Z.; Zheng, T.Z.; Lu, W.Q.; et al. Sex-specific associations of prenatal exposure to bisphenol A and its alternatives with fetal growth parameters and gestational age. Environ. Int. 2021, 146, 106305. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, L.; Liu, B.; Wu, M.; Liu, Y.; Bi, J.; Liu, Q.; Chen, K.; Cao, Z.; Xu, S.; et al. Prenatal exposure to bisphenol S and altered newborn mitochondrial DNA copy number in a baby cohort study: Sex-specific associations. Chemosphere 2021, 263, 128019. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Warasally, M.Z.; Magwai, T.; Naidoo, R.; Chuturgoon, A. A pilot study: Relationship between Bisphenol A, Bisphenol A glucuronide and sex steroid hormone levels in cord blood in A South African population. Reprod. Toxicol. 2021, 100, 83–89. [Google Scholar] [CrossRef]
- Gaylord, A.; Barrett, E.S.; Sathyanarayana, S.; Swan, S.H.; Nguyen, R.H.N.; Bush, N.R.; Carroll, K.; Day, D.B.; Kannan, K.; Trasande, L. Prenatal bisphenol A and S exposure and atopic disease phenotypes at age 6. Environ. Res. 2023, 226, 115630. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, S.; Liu, T.; Yang, C.; Wu, Y.; Jennifer Tan, H.J.; Wei, B.; Ma, X.; Feng, B.; Jiang, Q.; et al. Association of prenatal exposure to bisphenols and birth size in Zhuang ethnic newborns. Chemosphere 2020, 252, 126422. [Google Scholar] [CrossRef]
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Kim, B.N.; Lim, Y.H. Association of bisphenol A, bisphenol F, and bisphenol S with ADHD symptoms in children. Environ. Int. 2022, 161, 107093. [Google Scholar] [CrossRef]
- Silva, C.C.V.; Jaddoe, V.W.V.; Sol, C.M.; El Marroun, H.; Martinez-Moral, M.P.; Kannan, K.; Trasande, L.; Santos, S. Phthalate and Bisphenol Urinary Concentrations, Body Fat Measures, and Cardiovascular Risk Factors in Dutch School-Age Children. Obesity 2021, 29, 409–417. [Google Scholar] [CrossRef]
- Tkalec, Ž.; Kosjek, T.; Snoj Tratnik, J.; Stajnko, A.; Runkel, A.A.; Sykiotou, M.; Mazej, D.; Horvat, M. Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: Urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 2021, 146, 106172. [Google Scholar] [CrossRef]
- Youssef, M.M.; El-Din, E.; AbuShady, M.M.; El-Baroudy, N.R.; Abd El Hamid, T.A.; Armaneus, A.F.; El Refay, A.S.; Hussein, J.; Medhat, D.; Latif, Y.A. Urinary bisphenol A concentrations in relation to asthma in a sample of Egyptian children. Hum. Exp. Toxicol. 2018, 37, 1180–1186. [Google Scholar] [CrossRef]
- Guo, C.; Ren, F.; Jin, J.; Zhang, H.; Wang, L.; Zhang, H.; Chen, J. Internal exposure of Chinese children from a typical coastal city to bisphenols and possible association with thyroid hormone levels. Environ. Int. 2021, 156, 106759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Aimuzi, R.; Nian, M.; Zhang, Y.; Luo, K.; Zhang, J. Bisphenol A substitutes and sex hormones in children and adolescents. Chemosphere 2021, 278, 130396. [Google Scholar] [CrossRef] [PubMed]
- Gys, C.; Bastiaensen, M.; Bruckers, L.; Colles, A.; Govarts, E.; Martin, L.R.; Verheyen, V.; Koppen, G.; Morrens, B.; Den Hond, E.; et al. Determinants of exposure levels of bisphenols in flemish adolescents. Environ. Res. 2021, 193, 110567. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.; Huh, D.A.; Moon, K.W. Urinary bisphenol concentrations and its association with metabolic disorders in the US and Korean populations. Environ. Pollut. 2022, 295, 118679. [Google Scholar] [CrossRef] [PubMed]
- Husøy, T.; Andreassen, M.; Hjertholm, H.; Carlsen, M.H.; Norberg, N.; Sprong, C.; Papadopoulou, E.; Sakhi, A.K.; Sabaredzovic, A.; Dirven, H.A.A.M. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ. Int. 2019, 132, 105103. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ahn, Y.A.; Choi, K.; Park, J.; Moon, H.B.; Choi, G.; Lee, J.J.; Suh, E.; Kim, H.J.; Eun, S.H.; et al. Bisphenol A in infant urine and baby-food samples among 9- to 15-month-olds. Sci. Total Environ. 2019, 697, 133861. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Meng, T.; Chen, L.; Wu, Y.; Xiong, S.; Ding, L. Determination of four bisphenol environmental hormone residues in infant serum by liquid chromatography-tandem mass spectrometry. Se Pu 2020, 38, 1381–1387. [Google Scholar]
- Chen, Y.; Miao, M.; Wang, Z.; Ji, H.; Zhou, Y.; Liang, H.; He, G.; Yuan, W. Prenatal bisphenol exposure and intelligence quotient in children at six years of age: A prospective cohort study. Chemosphere 2023, 334, 139023. [Google Scholar] [CrossRef]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef]
- Rubin, A.M.; Seebacher, F. Bisphenols impact hormone levels in animals: A meta-analysis. Sci. Total Environ. 2022, 828, 154533. [Google Scholar] [CrossRef]
- Ayazgök, B.; Tüylü Küçükkılınç, T. Low-dose bisphenol A induces RIPK1-mediated necroptosis in SH-SY5Y cells: Effects on TNF-α and acetylcholinesterase. J. Biochem. Mol. Toxicol. 2018, 33, e22233. [Google Scholar] [CrossRef] [PubMed]
- Robles-Aguilera, V.; Gálvez-Ontiveros, Y.; Rodrigo, L.; Salcedo-Bellido, I.; Aguilera, M.; Zafra-Gómez, A.; Monteagudo, C.; Rivas, A. Factors Associated with Exposure to Dietary Bisphenols in Adolescents. Nutrients 2021, 13, 1553. [Google Scholar] [CrossRef] [PubMed]
- Schiano, M.E.; Sodano, F.; Magli, E.; Corvino, A.; Fiorino, F.; Rimoli, M.G.; Seccia, S.; Albrizio, S. Quantitative determination of BPA, BPB, BPF and BPS levels in canned legumes from Italian market. Food Chem. 2023, 416, 135642. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Inácio, T.; Almada, M.; Ferreira, R.; Fernandes, J.O. Gas chromatography-mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Food Res. Int. 2020, 135, 109293. [Google Scholar] [CrossRef]
- Pacyga, D.C.; Sathyanarayana, S.; Strakovsky, R.S. Dietary Predictors of Phthalate and Bisphenol Exposures in Pregnant Women. Adv. Nutr. 2019, 10, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wattar, N.; Field, C.J.; Dinu, I.; Dewey, D.; Martin, J.W.; APrON study team. Exposure and dietary sources of bisphenol A (BPA) and BPA-alternatives among mothers in the APrON cohort study. Environ. Int. 2018, 119, 319–326. [Google Scholar] [CrossRef]
- Kim, S.; Lee, I.; Lim, J.E.; Lee, A.; Moon, H.B.; Park, J.; Choi, K. Dietary contribution to body burden of bisphenol A and bisphenol S among mother-children pairs. Sci. Total Environ. 2020, 744, 140856. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zheng, J.; Pineda, M.; Yargeau, V.; Furlong, D.; Chevrier, J.; Bornman, R.; Obida, M.; Gates Goodyer, C.; Bayen, S. Targeted screening of 11 bisphenols and 7 plasticizers in food composites from Canada and South Africa. Food Chem. 2022, 385, 132675. [Google Scholar] [CrossRef]
- Makowska, K.; Staniszewska, M.; Bodziach, K.; Calka, J.; Gonkowski, S. Concentrations of bisphenol a (BPA) in fresh pork loin meat under standard stock-farming conditions and after oral exposure—A preliminary study. Chemosphere 2022, 295, 133816. [Google Scholar] [CrossRef]
- Russo, G.; Varriale, F.; Barbato, F.; Grumetto, L. Are Canned Beverages Industries Progressively Switching to Bisphenol AF? J. Food Sci. 2019, 84, 3303–3311. [Google Scholar] [CrossRef]
- Wan, Y.P.; Ma, Q.G.; Hayat, W.; Liu, Z.H.; Dang, Z. Ten bisphenol analogues in Chinese fresh dairy milk: High contribution ratios of conjugated form, importance of enzyme hydrolysis and risk evaluation. Environ. Sci. Pollut. Res. Int. 2023, 30, 88049–88059. [Google Scholar] [CrossRef]
- Heinälä, M.; Ylinen, K.; Tuomi, T.; Santonen, T.; Porras, S.P. Assessment of Occupational Exposure to Bisphenol A in Five Different Production Companies in Finland. Ann. Work Expo. Health 2017, 61, 44–55. [Google Scholar]
- Zhuang, W.; Wu, K.; Wang, Y.; Zhu, H.; Deng, Z.; Peng, L.; Zhu, G. Association of serum bisphenol-A concentration and male reproductive function among exposed workers. Arch. Environ. Contam. Toxicol. 2015, 68, 38–45. [Google Scholar] [CrossRef]
- Pan, Y.; Han, L.; Chen, X.; Wei, X.; Zhou, X.; Liang, D.; Yin, R.; Jiao, X.; Li, H.; Li, A.J.; et al. Occurrence of emerging bisphenol S analogues in urine from five occupational populations in South China. Environ. Int. 2023, 172, 107773. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, S.; Kim, K.T.; Kim, S.; Park, S.; Lee, H.; Jeong, Y.; Lim, J.E.; Moon, H.B.; Choi, K. Bisphenol A exposure through receipt handling and its association with insulin resistance among female cashiers. Environ. Int. 2018, 117, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Cai, M.; Wu, C.C.; Bao, L.J.; Li, J. Distribution profiles of bisphenols in school supplies and implications for human exposure. Sci. Total Environ. 2022, 849, 157938. [Google Scholar] [CrossRef]
- Lu, S.; Yu, Y.; Ren, L.; Zhang, X.; Liu, G.; Yu, Y. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact. Sci. Total Environ. 2018, 621, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Souza, M.C.O.; González, N.; Marquès, M.; Barbosa, F.; Domingo, J.L.; Nadal, M.; Rovira, J. Dermal exposure to bisphenols in pregnant women’s and baby clothes: Risk characterization. Sci. Total Environ. 2023, 878, 163122. [Google Scholar] [CrossRef]
- Li, A.J.; Kannan, K. Elevated Concentrations of Bisphenols, Benzophenones, and Antimicrobials in Pantyhose Collected from Six Countries. Environ. Sci. Technol. 2018, 52, 10812–10819. [Google Scholar] [CrossRef]
- Ferrey, M.L.; Coreen Hamilton, M.; Backe, W.J.; Anderson, K.E. Pharmaceuticals and other anthropogenic chemicals in atmospheric particulates and precipitation. Sci. Total Environ. 2018, 612, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Kawamura, K. Ubiquity of bisphenol A in the atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Y.; Guo, J.; Yu, T.; Sun, L.; Xiao, X.; Zhu, D.; Nakanishi, T.; Hiromori, Y.; Li, J.; et al. Fluorene-9-bisphenol is anti-oestrogenic and may cause adverse pregnancy outcomes in mice. Nat. Commun. 2017, 8, 14585. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, X.; Tian, Y.; Xu, P.; Yue, H.; Sang, N. Bisphenol B and bisphenol AF exposure enhances uterine diseases risks in mouse. Environ. Int. 2023, 173, 107858. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Ekman, G.C.; Kaur, J.; Davila, J.; Bagchi, I.C.; Clark, S.G.; Dziuk, P.J.; Hayashi, K.; Bartol, F.F. Brief exposure to progesterone during a critical neonatal window prevents uterine gland formation in mice. Biol. Reprod. 2012, 86, 63. [Google Scholar] [CrossRef]
- Cooke, P.S.; Spencer, T.E.; Bartol, F.F.; Hayashi, K. Uterine glands: Development, function and experimental model systems. Mol. Hum. Reprod. 2013, 19, 547–558. [Google Scholar] [CrossRef]
- Ke, Q.; Yang, L.; Cui, Q.; Diao, W.; Zhang, Y.; Xu, M.; He, B. Ciprofibrate attenuates airway remodeling in cigarette smoke-exposed rats. Respir. Physiol. Neurobiol. 2020, 271, 103290. [Google Scholar] [CrossRef]
- Zhang, W.; Li, A.; Pan, Y.; Wang, F.; Li, M.; Liang, Y.; Yao, X.; Song, J.; Song, M.; Jiang, G. Tetrabromobisphenol A induces THR β-mediated inflammation and uterine injury in mice at environmentally relevant exposure concentrations. J. Hazard Mater. 2021, 407, 124859. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Li, A.; Song, M. Tetrachlorobisphenol A induced immunosuppression and uterine injury in mice. Ecotoxicol. Environ. Saf. 2021, 207, 111527. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Conley, J.M.; Hannas, B.R.; Furr, J.R.; Wilson, V.S.; Gray, L.E., Jr. A Demonstration of the Uncertainty in Predicting the Estrogenic Activity of Individual Chemicals and Mixtures from an In Vitro Estrogen Receptor Transcriptional Activation Assay (T47D-KBluc) to the In Vivo Uterotrophic Assay Using Oral Exposure. Toxicol. Sci. 2016, 153, 382–395. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Yu, T.; Zhou, L.; Fan, X.; Xiao, H.; Wang, Y.; Yang, L.; Lv, J.; Jia, X.; et al. Bisphenol AP is anti-estrogenic and may cause adverse effects at low doses relevant to human exposure. Environ. Pollut. 2018, 242 Pt B, 1625–1632. [Google Scholar] [CrossRef]
- Sato, J.; Nasu, M.; Tsuchitani, M. Comparative histopathology of the estrous or menstrual cycle in laboratory animals. J. Toxicol. Pathol. 2016, 29, 155–162. [Google Scholar] [CrossRef]
- Ahsan, N.; Ullah, H.; Ullah, W.; Jahan, S. Comparative effects of Bisphenol S and Bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 2018, 197, 336–343. [Google Scholar] [CrossRef] [PubMed]
- ECHA, ECHA Database. 2018. Available online: http://www.echa.europa.eu/ (accessed on 26 October 2023).
- Signorile, P.G.; Spugnini, E.P.; Mita, L.; Mellone, P.; D’Avino, A.; Bianco, M.; Diano, N.; Caputo, L.; Rea, F.; Viceconte, R.; et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen. Comp. Endocrinol. 2010, 168, 318–325. [Google Scholar] [CrossRef]
- Signorile, P.G.; Spugnini, E.P.; Citro, G.; Viceconte, R.; Vincenzi, B.; Baldi, F.; Baldi, A. Endocrine disruptors in utero cause ovarian damages linked to endometriosis. Front. Biosci. 2012, 4, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.G.; Foster, W.G.; deCatanzaro, D. Bisphenol-A exposure during the period of blastocyst implantation alters uterine morphology and perturbs measures of estrogen and progesterone receptor expression in mice. Reprod. Toxicol. 2010, 30, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Varayoud, J.; Ramos, J.G.; Bosquiazzo, V.L.; Lower, M.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology 2011, 152, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Diao, H.; Smith, M.A.; Song, X.; Ye, X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod. Toxicol. 2011, 32, 434–441. [Google Scholar] [CrossRef]
- Barberio, L.; Paulesu, L.; Canesi, L.; Grasselli, E.; Mandalà, M. Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth. Int. J. Mol. Sci. 2021, 22, 6912. [Google Scholar] [CrossRef]
- Varberg, K.M.; Soares, M.J. Paradigms for investigating invasive trophoblast cell development and contributions to uterine spiral artery remodeling. Placenta 2021, 113, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.E.; Meyer, N.; Santamaria, C.G.; Schumacher, A.; Luque, E.H.; Zenclussen, M.L.; Rodriguez, H.A.; Zenclussen, A.C. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci. Rep. 2018, 8, 9196. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yannan, Z.; Ren, L.; Qi, S.; Wei, W.; Lihong, J. Adverse reproductive function induced by maternal BPA exposure is associated with abnormal autophagy and activating inflamation via mTOR and TLR4/NF-κB signaling pathways in female offspring rats. Reprod. Toxicol. 2020, 96, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ziv-Gal, A.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol. Appl. Pharmacol. 2015, 284, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peña, A.A.; Rivera-Baños, J.; Méndez-Carrillo, L.L.; Ramírez-Solano, M.I.; Galindo-Bustamante, A.; Páez-Franco, J.C.; Morimoto, S.; González-Mariscal, L.; Cruz, M.E.; Mendoza-Rodríguez, C.A. Perinatal administration of bisphenol A alters the expression of tight junction proteins in the uterus and reduces the implantation rate. Reprod. Toxicol. 2017, 69, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Allard, P.; Colaiácovo, M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 2010, 107, 20405–20410. [Google Scholar] [CrossRef]
- Yin, G.; Zhu, X.; Guo, C.; Yang, Y.; Han, T.; Chen, L.; Yin, W.; Gao, P.; Zhang, H.; Geng, J.; et al. Differential expression of estradiol and estrogen receptor α in severe preeclamptic pregnancies compared with normal pregnancies. Mol. Med. Rep. 2013, 7, 981–985. [Google Scholar] [CrossRef]
- Gokina, N.I.; Chan, S.L.; Chapman, A.C.; Oppenheimer, K.; Jetton, T.L.; Cipolla, M.J. Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Front. Physiol. 2013, 4, 184. [Google Scholar] [CrossRef]
- Li, Q.; Davila, J.; Kannan, A.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Chronic Exposure to Bisphenol A Affects Uterine Function During Early Pregnancy in Mice. Endocrinology 2016, 157, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Davila, J.; Bagchi, M.K.; Bagchi, I.C. Chronic exposure to bisphenol a impairs progesterone receptor-mediated signaling in the uterus during early pregnancy. Receptors Clin. Investig. 2016, 3, e1369. [Google Scholar]
- Vigezzi, L.; Bosquiazzo, V.L.; Kass, L.; Ramos, J.G.; Muñoz-de-Toro, M.; Luque, E.H. Developmental exposure to bisphenol A alters the differentiation and functional response of the adult rat uterus to estrogen treatment. Reprod. Toxicol. 2015, 52, 83–92. [Google Scholar] [CrossRef]
- Aldad, T.S.; Rahmani, N.; Leranth, C.; Taylor, H.S. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil. Steril. 2011, 96, 175–179. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.M.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, X.; Wang, Z.; Wang, X.; Dou, Z.; Li, Z. Bisphenol a influences blastocyst implantation via regulating integrin β3 and trophinin expression levels. Int. J. Clin. Exp. Med. 2015, 8, 20035–20045. [Google Scholar] [PubMed]
- Salleh, N.; Giribabu, N.; Feng, A.O.; Myint, K. Bisphenol A, Dichlorodiphenyltrichloroethane (DDT) and Vinclozolin Affect ex-vivo Uterine Contraction in Rats via Uterotonin (Prostaglandin F2α, Acetylcholine and Oxytocin) Related Pathways. Int. J. Med. Sci. 2015, 12, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Deshpande, S.B. Bisphenol A decreases the spontaneous contractions of rat uterus in vitro through a nitrergic mechanism. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, H.; Chen, K.; Ding, B.; Xu, J.; Ren, M.; Zhang, C.; Shen, Y. Effects of bisphenol A on uterine leiomyoma: In vitro and in vivo evaluation with mechanistic insights related to XBP1. Ecotoxicol. Environ. Saf. 2022, 247, 114201. [Google Scholar] [CrossRef] [PubMed]
- Othman, E.R.; Al-Adly, D.M.; Elgamal, D.A.; Ghandour, N.; El-Sharkawy, S. Bisphenol A Concentrates Preferentially in Human Uterine Leiomyoma and Induces Proliferation in Rat Myometrium. Reprod. Sci. 2016, 23, 508–514. [Google Scholar] [CrossRef]
- Neff, A.M.; Blanco, S.C.; Flaws, J.A.; Bagchi, I.C.; Bagchi, M.K. Chronic Exposure of Mice to Bisphenol-A Alters Uterine Fibroblast Growth Factor Signaling and Leads to Aberrant Epithelial Proliferation. Endocrinology 2019, 160, 1234–1246. [Google Scholar] [CrossRef]
- Leung, Y.K.; Biesiada, J.; Govindarajah, V.; Ying, J.; Kendler, A.; Medvedovic, M.; Ho, S.M. Low-Dose Bisphenol A in a Rat Model of Endometrial Cancer: A CLARITY-BPA Study. Environ. Health Perspect. 2020, 128, 127005. [Google Scholar] [CrossRef]
- National Toxicology Program. Toxicology Studies of Tetrabromobisphenol A in F344/NTac Rats and B6C3F1/N Mice and Toxicology and Carcinogenesis Studies of Tetrabromobisphenol A in Wistar Han [Crl:WI(Han)] Rats and B6C3F1/N Mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 2014, 587, NTP-TR-587. [Google Scholar] [CrossRef]
- Harvey, J.B.; Osborne, T.S.; Hong, H.H.; Bhusari, S.; Ton, T.V.; Pandiri, A.R.; Masinde, T.; Dunnick, J.; Peddada, S.; Elmore, S.; et al. Uterine Carcinomas in Tetrabromobisphenol A-exposed Wistar Han Rats Harbor Increased Tp53 Mutations and Mimic High-grade Type I Endometrial Carcinomas in Women. Toxicol. Pathol. 2015, 43, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Dunnick, J.K.; Sanders, J.M.; Kissling, G.E.; Johnson, C.L.; Boyle, M.H.; Elmore, S.A. Environmental chemical exposure may contribute to uterine cancer development: Studies with tetrabromobisphenol A. Toxicol. Pathol. 2015, 43, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, D.S.; Rager, J.E.; Haws, L.C.; Borghoff, S.J. A high dose mode of action for tetrabromobisphenol A-induced uterine adenocarcinomas in Wistar Han rats: A critical evaluation of key events in an adverse outcome pathway framework. Regul. Toxicol. Pharmacol. 2016, 77, 143–159. [Google Scholar] [CrossRef]
- Jones, R.L.; Lang, S.A.; Kendziorski, J.A.; Greene, A.D.; Burns, K.A. Use of a Mouse Model of Experimentally Induced Endometriosis to Evaluate and Compare the Effects of Bisphenol A and Bisphenol AF Exposure. Environ. Health Perspect. 2018, 126, 127004. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ. Health Perspect. 2009, 117, 879–885. [Google Scholar] [CrossRef]
- Singh, S.; Li, S.S. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int. J. Mol. Sci. 2012, 13, 10143–10153. [Google Scholar] [CrossRef]
- Mileva, G.; Baker, S.L.; Konkle, A.T.; Bielajew, C. Bisphenol-A: Epigenetic reprogramming and effects on reproduction and behavior. Int. J. Environ. Res. Public Health 2014, 11, 7537–7561. [Google Scholar] [CrossRef]
- Bromer, J.G.; Zhou, Y.; Taylor, M.B.; Doherty, L.; Taylor, H.S. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. Faseb, J. 2010, 24, 2273–2280. [Google Scholar] [CrossRef]
- Hiyama, M.; Choi, E.K.; Wakitani, S.; Tachibana, T.; Khan, H.; Kusakabe, K.T.; Kiso, Y. Bisphenol-A (BPA) affects reproductive formation across generations in mice. J. Vet. Med. Sci. 2011, 73, 1211–1215. [Google Scholar] [CrossRef]
- Hashimoto, H.; Vertino, P.M.; Cheng, X. Molecular coupling of DNA methylation and histone methylation. Epigenomics 2010, 2, 657–669. [Google Scholar] [CrossRef]
- Xiong, Y.; Wen, X.; Liu, H.; Zhang, M.; Zhang, Y. Bisphenol A affects endometrial stromal cells decidualization, involvement of epigenetic regulation. J. Steroid Biochem. Mol. Biol. 2020, 200, 105640. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yao, X.; Ting, G.; Ling, J.; Huimin, L.; Yuan, Q.; Chun, Z.; Ming, Z.; Yuanzhen, Z. BPA modulates the WDR5/TET2 complex to regulate ERβ expression in eutopic endometrium and drives the development of endometriosis. Environ. Pollut. 2021, 268 Pt B, 115748. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhang, Z.; Wang, D.; Wang, H.; Yan, G.; Wang, Y. Bisphenol-A Dose-dependently Regulates the Development of Ovary and Uterus through Differential Expression of METTL3. 2020. Available online: https://www.researchsquare.com/article/rs-16090/v1 (accessed on 26 October 2023).
- Aghajanova, L.; Giudice, L.C. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod. Biomed. Online 2011, 22, 249–256. [Google Scholar] [CrossRef]
- Shen, Y.; Ren, M.L.; Feng, X.; Cai, Y.L.; Gao, Y.X.; Xu, Q. An evidence in vitro for the influence of bisphenol A on uterine leiomyoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 80–83. [Google Scholar] [CrossRef]
- Benjamin, K.; Marquez, C.M.; Morta, M.; Reyes, E.M.; Aragones, L.; Velarde, M. Bisphenol S Increases Cell Number and Stimulates Migration of Endometrial Epithelial Cells. J. ASEAN Fed. Endocr. Soc. 2023, 38, 13–22. [Google Scholar] [CrossRef]
- Olson, M.R.; Su, R.; Flaws, J.A.; Fazleabas, A.T. Bisphenol A impairs decidualization of human uterine stromal fibroblasts. Reprod. Toxicol. 2017, 73, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, Q.; Ding, B.; Xu, J.; Shen, Y. Bisphenol A promotes the proliferation of leiomyoma cells by GPR30-EGFR signaling pathway. J. Obstet. Gynaecol. Res. 2019, 45, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Park, S.B.; Park, J.W.; Oh, S.R.; Han, M. Bisphenol A modulates inflammation and proliferation pathway in human endometrial stromal cells by inducing oxidative stress. Reprod. Toxicol. 2018, 81, 41–49. [Google Scholar] [CrossRef]
- Chou, W.C.; Lee, P.H.; Tan, Y.Y.; Lin, H.C.; Yang, C.W.; Chen, K.H.; Chuang, C.Y. An integrative transcriptomic analysis reveals bisphenol A exposure-induced dysregulation of microRNA expression in human endometrial cells. Toxicol. Vitr. 2017, 41, 133–142. [Google Scholar] [CrossRef]
- Yu, L.; Das, P.; Vall, A.J.; Yan, Y.; Gao, X.; Sifre, M.I.; Bortner, C.D.; Castro, L.; Kissling, G.E.; Moore, A.B. Bisphenol A induces human uterine leiomyoma cell proliferation through membrane-associated ERα36 via nongenomic signaling pathways. Mol. Cell. Endocrinol. 2019, 484, 59–68. [Google Scholar] [CrossRef]
- Kang, S.Y.; Kim, M.R.; Kim, J.H.; Cho, H.H. Gene expression profiles of the normal myometrium after 48 and 96 hours of exposure to BPA. BioChip J. 2015, 9, 293–299. [Google Scholar] [CrossRef]
- Li, Z.; Yin, H.; Shen, Y.; Ren, M.; Xu, X. The influence of phenolic environmental estrogen on the transcriptome of uterine leiomyoma cells: A whole transcriptome profiling-based analysis. Ecotoxicol. Environ. Saf. 2021, 211, 111945. [Google Scholar] [CrossRef]
- Wang, K.H.; Kao, A.P.; Chang, C.C.; Lin, T.C.; Kuo, T.C. Bisphenol A-induced epithelial to mesenchymal transition is mediated by cyclooxygenase-2 up-regulation in human endometrial carcinoma cells. Reprod. Toxicol. 2015, 58, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Castro, L.; Clayton, N.P.; Bushel, P.; Li, S.; Yu, L.; Flagler, N.D.; Scappini, E.; Yan, Y.; Dixon, D. Bisphenol A and its analogues induce Fibrosis in a 3D Human Uterine Fibroid Model through TGF-beta Signaling. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ma, X.F.; Zhang, J.; Shuai, H.L.; Guan, B.Z.; Luo, X.; Yan, R.L. IKKβ/NF-κB mediated the low doses of bisphenol A induced migration of cervical cancer cells. Arch. Biochem. Biophys. 2015, 573, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mauskopf, J.; Flynn, M.; Thieda, P.; Spalding, J.; Duchane, J. The economic impact of uterine fibroids in the United States: A summary of published estimates. J. Womens Health 2005, 14, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.R.; Clark, A.D.; Banks, N.K.; Henne, M.B.; Stegmann, B.J.; Segars, J.H. The estimated annual cost of uterine leiomyomata in the United States. Am. J. Obstet. Gynecol. 2012, 206, 211.e1–211.e9. [Google Scholar] [CrossRef]
- McWilliams, M.M.; Chennathukuzhi, V.M. Recent Advances in Uterine Fibroid Etiology. Semin. Reprod. Med. 2017, 35, 181–189. [Google Scholar] [CrossRef]
- Pavone, D.; Clemenza, S.; Sorbi, F.; Fambrini, M.; Petraglia, F. Epidemiology and Risk Factors of Uterine Fibroids. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 3–11. [Google Scholar] [CrossRef]
- Pollack, A.Z.; Buck Louis, G.M.; Chen, Z.; Sun, L.; Trabert, B.; Guo, Y.; Kannan, K. Bisphenol A, benzophenone-type ultraviolet filters, and phthalates in relation to uterine leiomyoma. Environ. Res. 2015, 137, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Hanane, Y. Bisphenol A Exposure Is Linked with Uterine Fibroma in Western Algerian Women of Childbearing Age. Acta HealthMedica 2018, 3, 239. [Google Scholar]
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Dutta, S.; Banu, S.K.; Arosh, J.A. Endocrine disruptors and endometriosis. Reprod. Toxicol. 2023, 115, 56–73. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Witz, C.A. Current concepts in the pathogenesis of endometriosis. Clin. Obstet. Gynecol. 1999, 42, 566–585. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; D’Armiento, M.; De Falco, M.; Boccellino, M.; Quagliuolo, L.; Baldi, A. New evidence of the presence of endometriosis in the human fetus. Reprod. Biomed. Online 2010, 21, 142–147. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; Viceconte, R.; Bulzomi, P.; D’Armiento, M.; D’Avino, A.; Baldi, A. Embryologic origin of endometriosis: Analysis of 101 human female fetuses. J. Cell. Physiol. 2012, 227, 1653–1656. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, A. New evidence in endometriosis. Int. J. Biochem. Cell Biol. 2015, 60, 19–22. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen Signaling in Endometrial Cancer: A Key Oncogenic Pathway with Several Open Questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P.; Wijayaratne, A.; Chang, C.Y.; Norris, J.D. Elucidation of the molecular mechanism of action of selective estrogen receptor modulators. Am. J. Cardiol. 2002, 90, 35f–43f. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, B.H.; Amanlou, M.; Lak, T.B.; Ghazizadeh, M.; Eslami, B. A case-control study of bisphenol A and endometrioma among subgroup of Iranian women. J. Res. Med. Sci. 2017, 22, 7. [Google Scholar] [PubMed]
- Peinado, F.M.; Lendínez, I.; Sotelo, R.; Iribarne-Durán, L.M.; Fernández-Parra, J.; Vela-Soria, F.; Olea, N.; Fernández, M.F.; Freire, C.; León, J.; et al. Association of Urinary Levels of Bisphenols A, F, and S with Endometriosis Risk: Preliminary Results of the EndEA Study. Int. J. Environ. Res. Public Health 2020, 17, 1194. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Colacurci, N.; Trabucco, E.; Carpentiero, C.; Grumetto, L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009, 23, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Aquino, C.I.; Troisi, J.; D’Antonio, A.; Giugliano, L.; Raffone, A.; Sarno, L.; Saccone, G.; Scala, G.; Guida, M. Endometrial Carcinoma and Bisphenol A: A Pilot Case-Control Study. Biomed. J. Sci. Tech. Res. 2019, 21, 16073–16079. [Google Scholar]
- Upson, K.; Sathyanarayana, S.; De Roos, A.J.; Koch, H.M.; Scholes, D.; Holt, V.L. A population-based case-control study of urinary bisphenol A concentrations and risk of endometriosis. Hum. Reprod. 2014, 29, 2457–2464. [Google Scholar] [CrossRef]
- Wen, X.; Xiong, Y.; Jin, L.; Zhang, M.; Huang, L.; Mao, Y.; Zhou, C.; Qiao, Y.; Zhang, Y. Bisphenol A Exposure Enhances Endometrial Stromal Cell Invasion and Has a Positive Association with Peritoneal Endometriosis. Reprod. Sci. 2020, 27, 704–712. [Google Scholar] [CrossRef]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Ye, X.; Calafat, A.M.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum. Reprod. 2012, 27, 3583–3592. [Google Scholar] [CrossRef]
- Ehrlich, S.; Williams, P.L.; Missmer, S.A.; Flaws, J.A.; Berry, K.F.; Calafat, A.M.; Ye, X.; Petrozza, J.C.; Wright, D.; Hauser, R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ. Health Perspect. 2012, 120, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Mok-Lin, E.; Ehrlich, S.; Williams, P.L.; Petrozza, J.; Wright, D.L.; Calafat, A.M.; Ye, X.; Hauser, R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010, 33, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Yuan, W.; Yang, F.; Liang, H.; Zhou, Z.; Li, R.; Gao, E.; Li, D.K. Associations between Bisphenol A Exposure and Reproductive Hormones among Female Workers. Int. J. Environ. Res. Public Health 2015, 12, 13240–13250. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, W.; Zhu, W.; Chen, L.; Wang, W.; Tian, Y.; Shen, L.; Zhang, J.; Shanghai Birth Cohort Study. Associations of female exposure to bisphenol A with fecundability: Evidence from a preconception cohort study. Environ. Int. 2018, 117, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Dwivedi, A.K.; Alvarado, L.; Kupesic-Plavsic, S. Exposure of U.S. population to endocrine disruptive chemicals (Parabens, Benzophenone-3, Bisphenol-A and Triclosan) and their associations with female infertility. Environ. Pollut. 2020, 265 Pt A, 114763. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, Y.; Jiang, J.; Liu, Y.; Luo, X.; Shen, Z.; Chen, X.; Wang, Y.; Dai, Y.; Zhao, J.; et al. Higher urinary bisphenol A concentration is associated with unexplained recurrent miscarriage risk: Evidence from a case-control study in eastern China. PLoS ONE 2015, 10, e0127886. [Google Scholar] [CrossRef] [PubMed]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef]
- Huang, M.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere 2020, 253, 126707. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Guo, Z.; Chen, M.; Wang, C.; He, C.; Zuo, Z. A pilot study on polycystic ovarian syndrome caused by neonatal exposure to tributyltin and bisphenol A in rats. Chemosphere 2019, 231, 151–160. [Google Scholar] [CrossRef]
- Saddick, S.Y. Light and Transmission Electron Microscopic Studies on Subacute Toxicity of Bisphenol A on the Rat Ovary. Anal. Quant. Cytopathol. Histpathol. 2015, 37, 227–234. [Google Scholar]
- Yue, H.; Tian, Y.; Wu, X.; Yang, X.; Xu, P.; Zhu, H.; Sang, N. Exploration of the damage and mechanisms of BPS exposure on the uterus and ovary of adult female mice. Sci. Total Environ. 2023, 868, 161660. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, H.; Feng, Z.; Zhang, S.; Wang, L.; Zhang, J.; Liu, Q.; Zhao, X.; Feng, D.; Feng, X. Fluorene-9-bisphenol exposure induces cytotoxicity in mouse oocytes and causes ovarian damage. Ecotoxicol. Environ. Saf. 2019, 180, 168–178. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, X.; Fan, W.; Liu, S.; Li, M.; Miao, Y.; Ding, C.; Tang, Z.; Yan, L.; Liu, G. Bisphenol A and genistein have opposite effects on adult chicken ovary by acting on ERα/Nrf2-Keap1-signaling pathway. Chem. Biol. Interact. 2021, 347, 109616. [Google Scholar] [CrossRef]
- Biswas, S.; Ghosh, S.; Samanta, A.; Das, S.; Mukherjee, U.; Maitra, S. Bisphenol A impairs reproductive fitness in zebrafish ovary: Potential involvement of oxidative/nitrosative stress, inflammatory and apoptotic mediators. Environ. Pollut. 2020, 267, 115692. [Google Scholar] [CrossRef]
- Rivera, O.E.; Varayoud, J.; Rodríguez, H.A.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod. Toxicol. 2011, 32, 304–312. [Google Scholar] [CrossRef]
- Hunt, P.A.; Lawson, C.; Gieske, M.; Murdoch, B.; Smith, H.; Marre, A.; Hassold, T.; VandeVoort, C.A. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc. Natl. Acad. Sci. USA 2012, 109, 17525–17530. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Whorton, A.; Hayashi, K. Prenatal Exposure to Bisphenol A Analogues on Female Reproductive Functions in Mice. Toxicol. Sci. 2019, 168, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, C.; Li, S.; Cui, Y.; Bao, Y.; Shi, W. Maternal exposure to bisphenol A during pregnancy interferes ovaries development of F1 female mice. Theriogenology 2020, 142, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Babić, S.; Barišić, J.; Bielen, A.; Bošnjak, I.; Sauerborn Klobučar, R.; Ujević, I.; Strunjak-Perović, I.; Topić Popović, N.; Čož-Rakovac, R. Multilevel ecotoxicity assessment of environmentally relevant bisphenol A concentrations using the soil invertebrate Eisenia fetida. J. Hazard Mater. 2016, 318, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Liu, J.; Wang, W.; Li, H.; Zhu, J.; Weng, S.; Xiao, S.; Wu, T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod. Toxicol. 2014, 44, 33–40. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Beckett, E.M.; Abi Salloum, B.; Ye, W.; Padmanabhan, V. Developmental programming: Prenatal BPA treatment disrupts timing of LH surge and ovarian follicular wave dynamics in adult sheep. Toxicol. Appl. Pharmacol. 2014, 279, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Mostari, M.H.; Rahaman, M.M.; Akhter, M.A.; Ali, M.H.; Sasanami, T.; Tokumoto, T. Transgenerational effects of bisphenol A on zebrafish reproductive tissues and sperm motility. Reprod. Toxicol. 2022, 109, 31–38. [Google Scholar] [CrossRef]
- Hunt, P.A.; Koehler, K.E.; Susiarjo, M.; Hodges, C.A.; Ilagan, A.; Voigt, R.C.; Thomas, S.; Thomas, B.F.; Hassold, T.J. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 2003, 13, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Susiarjo, M.; Hassold, T.J.; Freeman, E.; Hunt, P.A. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007, 3, e5. [Google Scholar] [CrossRef]
- Vimal, D.; Saini, S.; Kristipati, R.R.; Chowdhuri, D.K. Atrazine or bisphenol A mediated negative modulation of mismatch repair gene, mlh1 leads to defective oogenesis and reduced female fertility in Drosophila melanogaster. Chemosphere 2019, 225, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.; Gieske, M.; Murdoch, B.; Ye, P.; Li, Y.; Hassold, T.; Hunt, P.A. Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A. Biol. Reprod. 2011, 84, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, S.; Jampy, A.; Moison, D.; Wieckowski, M.; Messiaen, S.; Martini, E.; Campalans, A.; Radicella, J.P.; Rouiller-Fabre, V.; Livera, G.; et al. Foetal exposure to the bisphenols BADGE and BPAF impairs meiosis through DNA oxidation in mouse ovaries. Environ. Pollut. 2023, 317, 120791. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Tian, Y.; Yan, Z.H.; Li, W.D.; Zang, C.J.; Li, L.; Sun, X.F.; Shen, W.; Cheng, S.F. Maternal Bisphenol S exposure affects the reproductive capacity of F1 and F2 offspring in mice. Environ. Pollut. 2020, 267, 115382. [Google Scholar] [CrossRef]
- Ma, S.; Li, R.; Gong, X.; Shi, W.; Zhong, X. Lycopene reduces in utero bisphenol A exposure-induced mortality, benefits hormones, and development of reproductive organs in offspring mice. Environ. Sci. Pollut. Res. Int. 2018, 25, 24041–24051. [Google Scholar] [CrossRef]
- Shi, M.; Sekulovski, N.; MacLean, J.A.; Hayashi, K. Effects of bisphenol A analogues on reproductive functions in mice. Reprod. Toxicol. 2017, 73, 280–291. [Google Scholar] [CrossRef]
- Rankin, S.M.; Grosjean, E.M. Effects of bisphenol A in the ring-legged earwig, Euborellia annulipes. Ecotoxicology 2010, 19, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.I.; An, Y.J. Assessing potential indicator of endocrine-disrupting property of chemicals using soil invertebrates. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 245, 109036. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ru, S.; Wang, L.; Wei, S.; Zhang, J.; Qin, J.; Liu, R.; Zhang, X. Bisphenol S exposure alters behavioral parameters in adult zebrafish and offspring. Sci. Total Environ. 2020, 741, 140448. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, H.; Yang, M.; Yu, Y.; Yan, M.; Zhou, L.; Liu, X.; Xiao, S.; Yang, Y.; Wang, Y. Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere 2019, 221, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, X.; Cheng, H.; Zhang, Y.; Li, H.; Zhu, Y.; Wang, R.; Li, W.; Wang, R.; Wu, F. Bisphenol A interferes with lncRNA Fhadlos2 and RUNX3 association in adolescent mouse ovary. Ecotoxicol. Environ. Saf. 2023, 259, 115060. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, J.Y.; Chung, J.Y.; Kim, Y.J.; Park, J.E.; Oh, S.; Yoon, Y.D.; Yoo, K.S.; Yoo, Y.H.; Kim, J.M. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect. 2013, 121, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Ther, L.; Gao, L.; Wang, W.; Ziv-Gal, A.; Flaws, J.A. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod. Toxicol. 2017, 74, 150–157. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Luense, L.J.; Christenson, L.K.; Padmanabhan, V. Developmental programming: Gestational bisphenol-A treatment alters trajectory of fetal ovarian gene expression. Endocrinology 2013, 154, 1873–1884. [Google Scholar] [CrossRef]
- Risalde, M.A.; Molina, A.M.; Lora, A.J.; Ayala, N.; Gómez-Villamandos, J.C.; Moyano, M.R. Immunohistochemical expression of aromatase cyp19a1a and cyp19a1b in the ovary and brain of zebrafish (Danio rerio) exposed to different concentrations of bisphenol A. Aquat. Toxicol. 2021, 237, 105876. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Li, J.; Chen, M.; Peng, D.; Liang, Y.; Song, M.; Zhang, J.; Jiang, G. Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. Environ. Toxicol. 2016, 31, 285–294. [Google Scholar] [CrossRef]
- Forner-Piquer, I.; Beato, S.; Piscitelli, F.; Santangeli, S.; Di Marzo, V.; Habibi, H.R.; Maradonna, F.; Carnevali, O. Effects of BPA on zebrafish gonads: Focus on the endocannabinoid system. Environ. Pollut. 2020, 264, 114710. [Google Scholar] [CrossRef]
- Zhang, T.; Guan, Y.; Wang, S.; Wang, L.; Cheng, M.; Yuan, C.; Liu, Y.; Wang, Z. Bisphenol A induced abnormal DNA methylation of ovarian steroidogenic genes in rare minnow Gobiocypris rarus. Gen. Comp. Endocrinol. 2018, 269, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Zhu, L.; Ran, B.; Wang, Z. Bisphenol A disturbs transcription of steroidogenic genes in ovary of rare minnow Gobiocypris rarus via the abnormal DNA and histone methylation. Chemosphere 2020, 240, 124935. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qu, X.; Ming, Z.; Yao, Y.; Zhang, Y. The correlation between exposure to BPA and the decrease of the ovarian reserve. Int. J. Clin. Exp. Pathol. 2018, 11, 3375–3382. [Google Scholar] [PubMed]
- Téteau, O.; Liere, P.; Pianos, A.; Desmarchais, A.; Lasserre, O.; Papillier, P.; Vignault, C.; Lebachelier de la Riviere, M.E.; Maillard, V.; Binet, A.; et al. Bisphenol S Alters the Steroidome in the Preovulatory Follicle, Oviduct Fluid and Plasma in Ewes with Contrasted Metabolic Status. Front. Endocrinol. 2022, 13, 892213. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Ru, S.; Qin, J.; Wang, W.; Zhang, J.; Wei, S.; Wang, J.; Zhang, X. Transgenerational effects of parental bisphenol S exposure on zebrafish (Danio rerio) reproduction. Food Chem. Toxicol. 2022, 165, 113142. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Hussein, M.A.; Suleiman, A.H.; Abohassan, M.G.; Ahmed, M.M.; Moustafa, A.A.; Moumen, A.F.; Abdel-Aziz Swelum, A. Ameliorative effect of ginseng extract on phthalate and bisphenol A reprotoxicity during pregnancy in rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 21205–21215. [Google Scholar] [CrossRef] [PubMed]
- Nourian, A.; Soleimanzadeh, A.; Shalizar Jalali, A.; Najafi, G. Effects of bisphenol-S low concentrations on oxidative stress status and in vitro fertilization potential in mature female mice. Vet. Res. Forum. 2017, 8, 341–345. [Google Scholar]
- Eldefrawy, F.; Xu, H.S.; Pusch, E.; Karkoura, A.; Alsafy, M.; Elgendy, S.; Williams, S.M.; Navara, K.; Guo, T.L. Modulation of folliculogenesis in adult laying chickens by bisphenol A and bisphenol S: Perspectives on ovarian morphology and gene expression. Reprod. Toxicol. 2021, 103, 181–190. [Google Scholar] [CrossRef]
- Bujnakova Mlynarcikova, A.; Scsukova, S. Bisphenol analogs AF and S: Effects on cell status and production of angiogenesis-related factors by COV434 human granulosa cell line. Toxicol. Appl. Pharmacol. 2021, 426, 115634. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Craig, Z.R.; Wang, W.; Flaws, J.A. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod. Toxicol. 2013, 42, 58–67. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Qin, X.S.; Zhou, Y.; Zhang, X.F.; Wang, L.Q.; De Felici, M.; Chen, H.; Qin, G.Q.; Shen, W. Di-(2-ethylhexyl) phthalate and bisphenol A exposure impairs mouse primordial follicle assembly in vitro. Environ. Mol. Mutagen. 2014, 55, 343–353. [Google Scholar] [CrossRef]
- Ganesan, S.; Keating, A.F. Bisphenol A-Induced Ovotoxicity Involves DNA Damage Induction to Which the Ovary Mounts a Protective Response Indicated by Increased Expression of Proteins Involved in DNA Repair and Xenobiotic Biotransformation. Toxicol. Sci. 2016, 152, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Desmarchais, A.; Téteau, O.; Papillier, P.; Jaubert, M.; Druart, X.; Binet, A.; Maillard, V.; Elis, S. Bisphenol S Impaired In Vitro Ovine Early Developmental Oocyte Competence. Int. J. Mol. Sci. 2020, 21, 1238. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Kerslake, R.; Kyrou, I.; Randeva, H.S.; Sisu, C.; Karteris, E. Impact of Environmentally Relevant Concentrations of Bisphenol A (BPA) on the Gene Expression Profile in an In Vitro Model of the Normal Human Ovary. Int. J. Mol. Sci. 2022, 23, 5334. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, S.W.; Wang, L.; Sun, Y.; Xu, F.; He, H.; Wang, S.; Zhang, Z.; Pan, X. Interfering effects of bisphenol A on in vitro growth of preantral follicles and maturation of oocyes. Clin. Chim. Acta 2018, 485, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Trapphoff, T.; Heiligentag, M.; El Hajj, N.; Haaf, T.; Eichenlaub-Ritter, U. Chronic exposure to a low concentration of bisphenol A during follicle culture affects the epigenetic status of germinal vesicles and metaphase II oocytes. Fertil. Steril. 2013, 100, 1758–1767.e1. [Google Scholar] [CrossRef] [PubMed]
- Brieño-Enríquez, M.A.; Robles, P.; Camats-Tarruella, N.; García-Cruz, R.; Roig, I.; Cabero, L.; Martínez, F.; Caldés, M.G. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum. Reprod. 2011, 26, 2807–2818. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Hernández, D.G.; Arreola-Mendoza, L.; Santacruz-Márquez, R.; García-Zepeda, S.P.; Parra-Forero, L.Y.; Olivares-Reyes, J.A.; Hernández-Ochoa, I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol. Appl. Pharmacol. 2018, 344, 13–22. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Gao, F.; Peng, Z.; Zhang, J.; Li, S.; Lu, D.; Pan, X. BPA interferes with granulosa cell development and oocyte meiosis in mouse preantral follicles. Exp. Biol. Med. 2023, 248, 1145–1158. [Google Scholar] [CrossRef]
- Lin, T.C.; Wang, K.H.; Chuang, K.H.; Kao, A.P.; Kuo, T.C. Downregulation of gap junctional intercellular communication and connexin 43 expression by bisphenol A in human granulosa cells. Biotechnol. Appl. Biochem. 2021, 68, 676–682. [Google Scholar] [CrossRef]
- Lin, M.; Hua, R.; Ma, J.; Zhou, Y.; Li, P.; Xu, X.; Yu, Z.; Quan, S. Bisphenol A promotes autophagy in ovarian granulosa cells by inducing AMPK/mTOR/ULK1 signalling pathway. Environ. Int. 2021, 147, 106298. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.; Adir, M.; Racowsky, C.; Combelles, C.M.; Landa, N.; Machtinger, R. Susceptibility of human cumulus cells to bisphenol a In vitro. Reprod. Toxicol. 2017, 74, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, W.; Peretz, J.; Flaws, J.A. Bisphenol A exposure inhibits germ cell nest breakdown by reducing apoptosis in cultured neonatal mouse ovaries. Reprod. Toxicol. 2015, 57, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, M.; Li, X.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca(2+)-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol. Environ. Saf. 2021, 208, 111429. [Google Scholar] [CrossRef] [PubMed]
- Urriola-Muñoz, P.; Lagos-Cabré, R.; Patiño-García, D.; Reyes, J.G.; Moreno, R.D. Bisphenol-A and Nonylphenol Induce Apoptosis in Reproductive Tract Cancer Cell Lines by the Activation of ADAM17. Int. J. Mol. Sci. 2018, 19, 2238. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Li, X.; Jia, S.; Liu, S.; Fu, L.; Jiang, X.; Yang, M. Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ. Pollut. 2021, 270, 116051. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Lin, Y.; Wang, A.; Zhu, W.; Liang, Y.; Su, X.; Hong, C.; Wan, J.; Wang, Y.; Tian, H. Cytogenetic evaluation for the genotoxicity of bisphenol-A in Chinese hamster ovary cells. Environ. Toxicol. Pharmacol. 2015, 40, 524–529. [Google Scholar] [CrossRef]
- Brieño-Enríquez, M.A.; Reig-Viader, R.; Cabero, L.; Toran, N.; Martínez, F.; Roig, I.; Garcia Caldés, M. Gene expression is altered after bisphenol A exposure in human fetal oocytes in vitro. Mol. Hum. Reprod. 2012, 18, 171–183. [Google Scholar] [CrossRef]
- Campen, K.A.; Kucharczyk, K.M.; Bogin, B.; Ehrlich, J.M.; Combelles, C.M.H. Spindle abnormalities and chromosome misalignment in bovine oocytes after exposure to low doses of bisphenol A or bisphenol S. Hum. Reprod. 2018, 33, 895–904. [Google Scholar] [CrossRef]
- Qi, J.; Liu, L.; Yang, J.; Gao, X.; Zhang, W. Bisphenol A decreases progesterone synthesis in human ovarian granulosa cells. Birth Defects Res. 2020, 112, 1843–1849. [Google Scholar] [CrossRef]
- Shi, J.; Liu, C.; Chen, M.; Yan, J.; Wang, C.; Zuo, Z.; He, C. The interference effects of bisphenol A on the synthesis of steroid hormones in human ovarian granulosa cells. Environ. Toxicol. 2021, 36, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jin, H.; Kim, G.; Bae, J. A low dose of bisphenol A stimulates estradiol production by regulating β-catenin-FOXL2-CYP19A1 pathway in human ovarian granulosa cells. Biochem. Biophys. Res. Commun. 2021, 583, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Tyner, M.D.W.; Maloney, M.O.; Kelley, B.J.B.; Combelles, C.M.H. Comparing the effects of bisphenol A, C, and F on bovine theca cells in vitro. Reprod. Toxicol. 2022, 111, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pogrmic-Majkic, K.; Samardzija Nenadov, D.; Fa, S.; Stanic, B.; Trninic Pjevic, A.; Andric, N. BPA activates EGFR and ERK1/2 through PPARγ to increase expression of steroidogenic acute regulatory protein in human cumulus granulosa cells. Chemosphere 2019, 229, 60–67. [Google Scholar] [CrossRef]
- Grasselli, F.; Baratta, L.; Baioni, L.; Bussolati, S.; Ramoni, R.; Grolli, S.; Basini, G. Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 2010, 39, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Vignault, C.; Cadoret, V.; Jarrier-Gaillard, P.; Papillier, P.; Téteau, O.; Desmarchais, A.; Uzbekova, S.; Binet, A.; Guérif, F.; Elis, S.; et al. Bisphenol S Impairs Oestradiol Secretion during In Vitro Basal Folliculogenesis in a Mono-Ovulatory Species Model. Toxics 2022, 10, 437. [Google Scholar] [CrossRef]
- Jurewicz, J.; Majewska, J.; Berg, A.; Owczarek, K.; Zajdel, R.; Kaleta, D.; Wasik, A.; Rachoń, D. Serum bisphenol A analogues in women diagnosed with the polycystic ovary syndrome—Is there an association? Environ. Pollut. 2021, 272, 115962. [Google Scholar] [CrossRef]
- Akgül, S.; Sur, Ü.; Düzçeker, Y.; Balcı, A.; Kızılkan, M.P.; Kanbur, N.; Bozdağ, G.; Erkekoğlu, P.; Gümüş, E.; Kocer-Gumusel, B.; et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol. Endocrinol. 2019, 35, 1084–1087. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Q.; Dang, X.; He, Y.; Li, X.; Sun, Y. Local effect of bisphenol A on the estradiol synthesis of ovarian granulosa cells from PCOS. Gynecol. Endocrinol. 2017, 33, 21–25. [Google Scholar] [CrossRef]
- Konieczna, A.; Rachoń, D.; Owczarek, K.; Kubica, P.; Kowalewska, A.; Kudłak, B.; Wasik, A.; Namieśnik, J. Serum bisphenol A concentrations correlate with serum testosterone levels in women with polycystic ovary syndrome. Reprod. Toxicol. 2018, 82, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lazúrová, Z.; Figurová, J.; Hubková, B.; Mašlanková, J.; Lazúrová, I. Urinary bisphenol A in women with polycystic ovary syndrome—A possible suppressive effect on steroidogenesis? Horm. Mol. Biol. Clin. Investig. 2021, 42, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, N.B.; Vasishta, S.; Bhat, S.K.; Joshi, M.B.; Kabekkodu, S.P.; Satyamoorthy, K.; Rai, P.S. Distinct metabolic signatures in blood plasma of bisphenol A-exposed women with polycystic ovarian syndrome. Environ. Sci. Pollut. Res. Int. 2023, 30, 64025–64035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fang, F.; Zhu, W.; Chen, Z.J.; Du, Y.; Zhang, J. Bisphenol A and Ovarian Reserve among Infertile Women with Polycystic Ovarian Syndrome. Int. J. Environ. Res. Public Health 2016, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Cedars, M.I. Managing poor ovarian response in the patient with diminished ovarian reserve. Fertil. Steril. 2022, 117, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Aftabsavad, S.; Noormohammadi, Z.; Moini, A.; Karimipoor, M. Effect of bisphenol A on alterations of ICAM-1 and HLA-G genes expression and DNA methylation profiles in cumulus cells of infertile women with poor response to ovarian stimulation. Sci. Rep. 2021, 11, 9595. [Google Scholar] [CrossRef]
- Souter, I.; Smith, K.W.; Dimitriadis, I.; Ehrlich, S.; Williams, P.L.; Calafat, A.M.; Hauser, R. The association of bisphenol-A urinary concentrations with antral follicle counts and other measures of ovarian reserve in women undergoing infertility treatments. Reprod. Toxicol. 2013, 42, 224–231. [Google Scholar] [CrossRef]
- Qiao, L.; Zheng, L.; Cai, D. Study on the levels of the bisphenol A, octylphenol, 4-nonylphenol in serum of precocious girls. Wei Sheng Yan Jiu 2010, 39, 9–12. [Google Scholar]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Pan, X.; Wang, X.; Sun, Y.; Dou, Z.; Li, Z. Inhibitory effects of preimplantation exposure to bisphenol-A on blastocyst development and implantation. Int. J. Clin. Exp. Med. 2015, 8, 8720–8729. [Google Scholar]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.H.; Van Winkle, L.S.; Williams, C.J.; Hunt, P.A.; VandeVoort, C.A. Prenatal Bisphenol A Exposure Alters Epithelial Cell Composition in the Rhesus Macaque Fetal Oviduct. Toxicol. Sci. 2019, 167, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, L. Development of the mammalian female reproductive tract. J. Biochem. 2005, 137, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Cunha, G.R.; Robboy, S.J.; Kurita, T.; Isaacson, D.; Shen, J.; Cao, M.; Baskin, L.S. Development of the human female reproductive tract. Differentiation 2018, 103, 46–65. [Google Scholar] [CrossRef]
- Newbold, R.R. Prenatal exposure to diethylstilbestrol and long-term impact on the breast and reproductive tract in humans and mice. J. Dev. Orig. Health Dis. 2012, 3, 73–82. [Google Scholar] [CrossRef]
- Laronda, M.M.; Unno, K.; Butler, L.M.; Kurita, T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation 2012, 84, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Miyagawa, S.; Katsu, Y.; Watanabe, H.; Mizutani, T.; Sato, T.; Morohashi, K.; Takeuchi, T.; Iguchi, T.; Ohta, Y. Wnt family genes and their modulation in the ovary-independent and persistent vaginal epithelial cell proliferation and keratinization induced by neonatal diethylstilbestrol exposure in mice. Toxicology 2012, 296, 13–19. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyagawa, S.; Katsu, Y.; Mizutani, T.; Sato, T.; Takeuchi, T.; Iguchi, T.; Ohta, Y. p21 and Notch signalings in the persistently altered vagina induced by neonatal diethylstilbestrol exposure in mice. J. Vet. Med. Sci. 2012, 74, 1589–1595. [Google Scholar] [CrossRef]
- Katoh, T.; Hayashi, S.; Iguchi, T.; Sato, T. Epithelial-stromal interactions in the mouse vagina exposed neonatally to diethylstilbestrol. In Vivo 2013, 27, 333–337. [Google Scholar]
- Laronda, M.M.; Unno, K.; Ishi, K.; Serna, V.A.; Butler, L.M.; Mills, A.A.; Orvis, G.D.; Behringer, R.R.; Deng, C.; Sinha, S.; et al. Diethylstilbestrol induces vaginal adenosis by disrupting SMAD/RUNX1-mediated cell fate decision in the Müllerian duct epithelium. Dev. Biol. 2013, 381, 5–16. [Google Scholar] [CrossRef]
- Ahmed, R.A.; ElGhamrawy, T.A.; Salama, E.E. Effect of prenatal exposure to bisphenol a on the vagina of albino rats: Immunohistochemical and ultrastructural study. Folia Morphol. 2014, 73, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Twaddle, N.C.; Doerge, D.R.; Churchwell, M.I.; Bryant, M.S.; et al. A two-year toxicology study of bisphenol A (BPA) in Sprague-Dawley rats: CLARITY-BPA core study results. Food Chem. Toxicol. 2019, 132, 110728. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Baek, S.K.; Kim, W.; Quah, Y.; Kim, S.Y.; Jeong, J.S.; Lee, J.; Yu, W.J. Reproductive and developmental toxicity screening of bisphenol F by oral gavage in rats. Regul. Toxicol. Pharmacol. 2022, 136, 105286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Zhu, Y.; Zhang, Z.; Wang, D.; Wang, H.; Yan, G. Oral Bisphenol-A Regulates The Development and Function of Reproductive System through Differential Expression of METTL3. Research Square, 2020. Available online: https://www.researchsquare.com/article/rs-28387/v1 (accessed on 26 October 2023).
- Nourian, A.; Soleimanzadeh, A.; Shalizar Jalali, A.; Najafi, G. Bisphenol-A analogue (bisphenol-S) exposure alters female reproductive tract and apoptosis/oxidative gene expression in blastocyst-derived cells. Iran. J. Basic Med. Sci. 2020, 23, 576–585. [Google Scholar] [PubMed]
- EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP); Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, S.M.; Barupal, D.K.; Wishart, D.; Vineis, P.; Scalbert, A. The blood exposome and its role in discovering causes of disease. Environ. Health Perspect. 2014, 122, 769–774. [Google Scholar] [CrossRef]
- Liu, M.; Jia, S.; Dong, T.; Zhao, F.; Xu, T.; Yang, Q.; Gong, J.; Fang, M. Metabolomic and Transcriptomic Analysis of MCF-7 Cells Exposed to 23 Chemicals at Human-Relevant Levels: Estimation of Individual Chemical Contribution to Effects. Environ. Health Perspect. 2020, 128, 127008. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- Li, Y.; Perera, L.; Coons, L.A.; Burns, K.A.; Tyler Ramsey, J.; Pelch, K.E.; Houtman, R.; van Beuningen, R.; Teng, C.T.; Korach, K.S. Differential in Vitro Biological Action, Coregulator Interactions, and Molecular Dynamic Analysis of Bisphenol A (BPA), BPAF, and BPS Ligand-ERα Complexes. Environ. Health Perspect. 2018, 126, 017012. [Google Scholar] [CrossRef]
- Iwamoto, M.; Masuya, T.; Hosose, M.; Tagawa, K.; Ishibashi, T.; Suyama, K.; Nose, T.; Yoshihara, E.; Downes, M.; Evans, R.M.; et al. Bisphenol A derivatives act as novel coactivator-binding inhibitors for estrogen receptor β. J. Biol. Chem. 2021, 297, 101173. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.Y.; Ren, X.M.; Li, C.H.; Zhang, J.; Qin, W.P.; Yang, Y.; Wan, B.; Guo, L.H. Bisphenol AF and Bisphenol B Exert Higher Estrogenic Effects than Bisphenol A via G Protein-Coupled Estrogen Receptor Pathway. Environ. Sci. Technol. 2017, 51, 11423–11430. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yang, X.; Geng, X.; Ji, X.; Zhang, X.; Yue, H.; Li, G.; Sang, N. Bisphenol A Analogs Induce Cellular Dysfunction in Human Trophoblast Cells in a Thyroid Hormone Receptor-Dependent Manner: In Silico and In Vitro Analyses. Environ. Sci. Technol. 2022, 56, 8384–8394. [Google Scholar] [CrossRef] [PubMed]

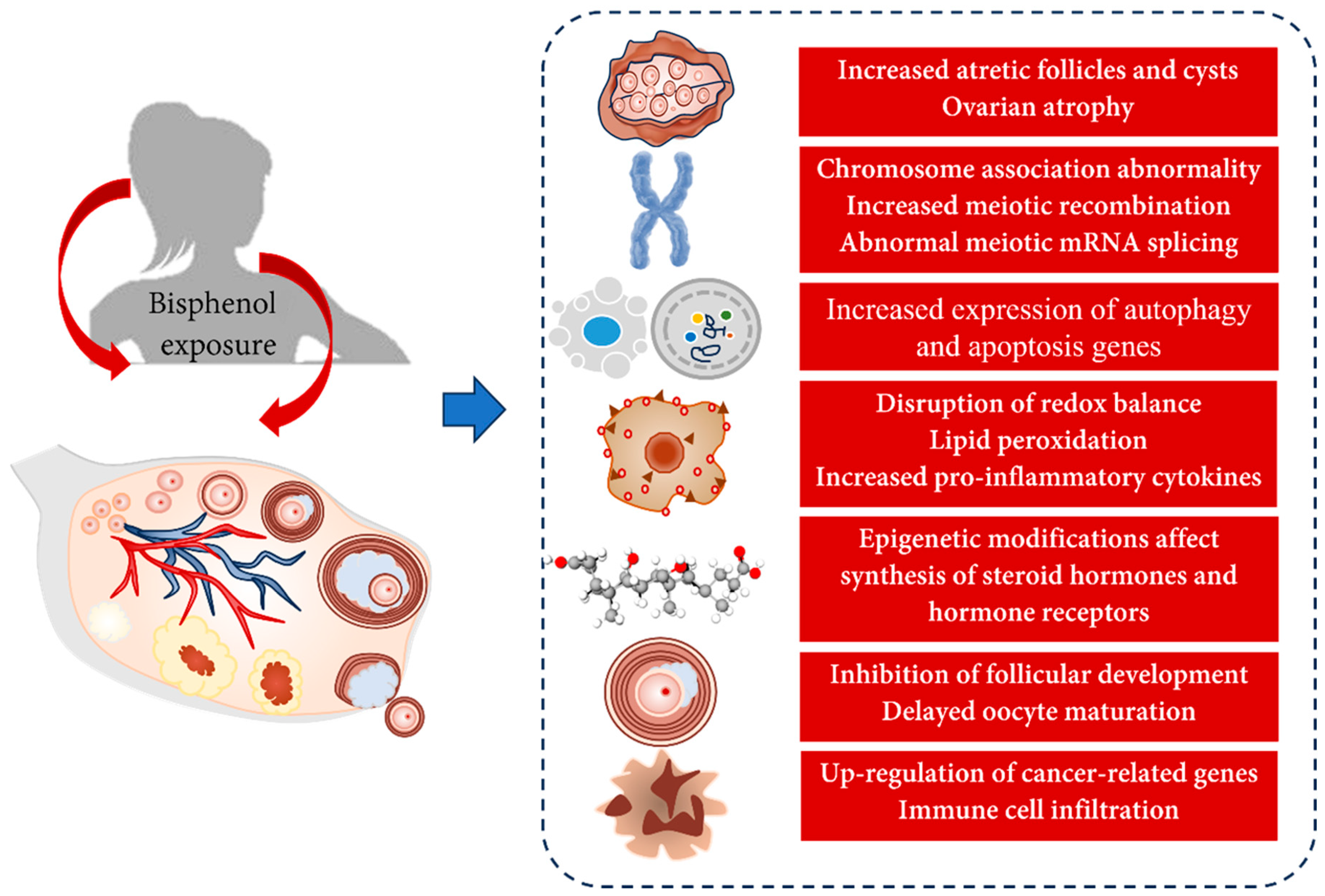
| Study Population | Country | Sample Size | Measurement Time | Sample Sources | Detection Period | BPA | BPS | BPF | BPB | TBBPA | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (Mean/Median) | DR. (%) | Conc. (Mean/Median) | DR. (%) | Conc. (Mean/Median) | DR. (%) | Conc. (Mean/Median) | DR. (%) | Conc. (Mean/Median) | DR. (%) | |||||||
| Pregnant women | Netherlands | 1213 | 2016 | Urine | Early pregnancy | 1.67 ng/mL | 78.8 | 0.35 ng/mL | 68.1 | 0.58 ng/mL | 40.4 | Philips EM et al., 2019 [59] | ||||
| Mid-pregnancy | 1.46 ng/mL | 93.3 | 0.24 ng/mL | 29.1 | ||||||||||||
| Pregnant women | South Korea | 196 | 2017–2019 | Urine | Pregnancy | 2.10 ng/mL | 96.2 | 0.10 ng/mL | 62.1 | 0.20 ng/mL | 84.4 | Kim S et al., 2021 [61] | ||||
| Pregnant women | China | 2023 | 2015–2018 | Serum | Pregnancy | 2.03 ng/mL | 99 | 0.09 ng/mL | 86.9 | 0.44 ng/mL | 61.8 | 0.24 ng/mL | 89 | 0.52 ng/mL | 66.1 | Liang J et al., 2020 [67] |
| Pregnant women | South Africa | 60 | NF | Serum | Pregnancy | 1.16 ng/mL | Gounden V et al., 2021 [65] | |||||||||
| Infants | Infancy | 0.75 ng/mL | ||||||||||||||
| Pregnant women | America | 635 | 2010 | Urine | Early pregnancy | 0.68 ng/mL | 74 | 0.11 ng/mL | 52 | Gaylord A et al., 2023 [66] | ||||||
| Mid-pregnancy | 0.43 ng/mL | 58 | 0.13 ng/mL | 57 | ||||||||||||
| Late pregnancy | 0.73 ng/mL | 73 | 0.09 ng/mL | 51 | ||||||||||||
| Children | South Korea | 619 | 2012–2018 | Urine | 4 years old | 3.29 μg/g creatinine | Kim JI et al., 2022 [68] | |||||||||
| 6 years old | 2.36 μg/g creatinine | 0.10 μg/g creatinine | 0.20 μg/g creatinine | |||||||||||||
| 8 years old | 1.96 μg/g creatinine | 0.16 μg/g creatinine | 0.40 μg/g creatinine | |||||||||||||
| Children | Netherlands | 471 | NF | Urine | 6 years old | 2.50 nmol/L | 73.8 | 0.13 nmol/L | 25.2 | Silva CCV et al., 2021 [69] | ||||||
| Children | Slovenian | 246 | 2016–2019 | Urine | 6–9 years old | 2.10 ng/mL | 0.30 ng/mL | 0.11 ng/mL | Tkalec Ž et al., 2020 [70] | |||||||
| Adolescents | 11–15 years old | 1.90 ng/mL | 96.2 | 0.36 ng/mL | 0.17 ng/mL | |||||||||||
| Children | Egypt | 97 | NF | Urine | 3–8 years old | 1.16 ng/mL | Youssef MM et al., 2018 [71] | |||||||||
| Children | China | 345 | 2018–2019 | Serum | 6–11 years old | 1.60 ng/mL | 63 | 0.043 ng/mL | 43 | 0.075 ng/ml | 68 | Guo C et al., 2021 [72] | ||||
| Adolescents | China | 1317 | 2013–2016 | Serum | 6–19 years old | 1.2 ng/mL | 97.8 | 0.3 ng/mL | 88.4 | 0.2 ng/mL | 54.8 | Wang Y et al., 2021 [73] | ||||
| Adolescents | Belgium | 423 | 2016–2020 | Urine | 14–15 years old | 1.05 ng/mL | 86 | 0.12 ng/mL | 83 | 0.14 ng/mL | 97 | Gys C et al., 2021 [74] | ||||
| Adults | America | 1046 | 2013–2016 | Urine | >12 years old | 1.23 ng/mL | 96.8 | 0.58 ng/mL | 92.3 | 0.41 ng/mL | 56.5 | Choi JY et al., 2021 [75] | ||||
| South Korea | 3268 | 2015–2017 | >19 years old | 1.27 ng/mL | 98.1 | 0.03 ng/mL | 54.8 | 0.10 ng/mL | 40.7 | |||||||
| Adults | Norway | 144 | 2016–2017 | Urine | 24–72 years old | 3.0 ng/mL | 96 | 0.5 ng/mL | 29 | 0.09 ng/mL | 4 | Husøy T et al., 2019 [76] | ||||
| Adults | China | 160 | 2018 | Urine | 19–25 years old | 4.89 ng/mL | 99 | 0.15 ng/mL | 88 | 0.13 ng/mL | 80 | Zhang H et al., 2020 [14] | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Tian, Y.; Zhu, H.; Xu, P.; Zhang, J.; Hu, Y.; Ji, X.; Yan, R.; Yue, H.; Sang, N. Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives. Toxics 2023, 11, 1000. https://doi.org/10.3390/toxics11121000
Wu X, Tian Y, Zhu H, Xu P, Zhang J, Hu Y, Ji X, Yan R, Yue H, Sang N. Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives. Toxics. 2023; 11(12):1000. https://doi.org/10.3390/toxics11121000
Chicago/Turabian StyleWu, Xiaoyun, Yuchai Tian, Huizhen Zhu, Pengchong Xu, Jiyue Zhang, Yangcheng Hu, Xiaotong Ji, Ruifeng Yan, Huifeng Yue, and Nan Sang. 2023. "Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives" Toxics 11, no. 12: 1000. https://doi.org/10.3390/toxics11121000
APA StyleWu, X., Tian, Y., Zhu, H., Xu, P., Zhang, J., Hu, Y., Ji, X., Yan, R., Yue, H., & Sang, N. (2023). Invisible Hand behind Female Reproductive Disorders: Bisphenols, Recent Evidence and Future Perspectives. Toxics, 11(12), 1000. https://doi.org/10.3390/toxics11121000