Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Experimental Setup and Animals
2.3. Experimental Diets
2.4. Production Parameters
2.5. Good Practices for Euthanasia and Sample Collection
2.6. Intestinal Morphometry
2.7. RNA Extraction and Expression of Enzymatic and Barrier Proteins
2.8. Metagenomic Sequencing and Bioinformatic Sequence Analysis
2.9. Statistical Analysis
3. Results
3.1. Production Parameters
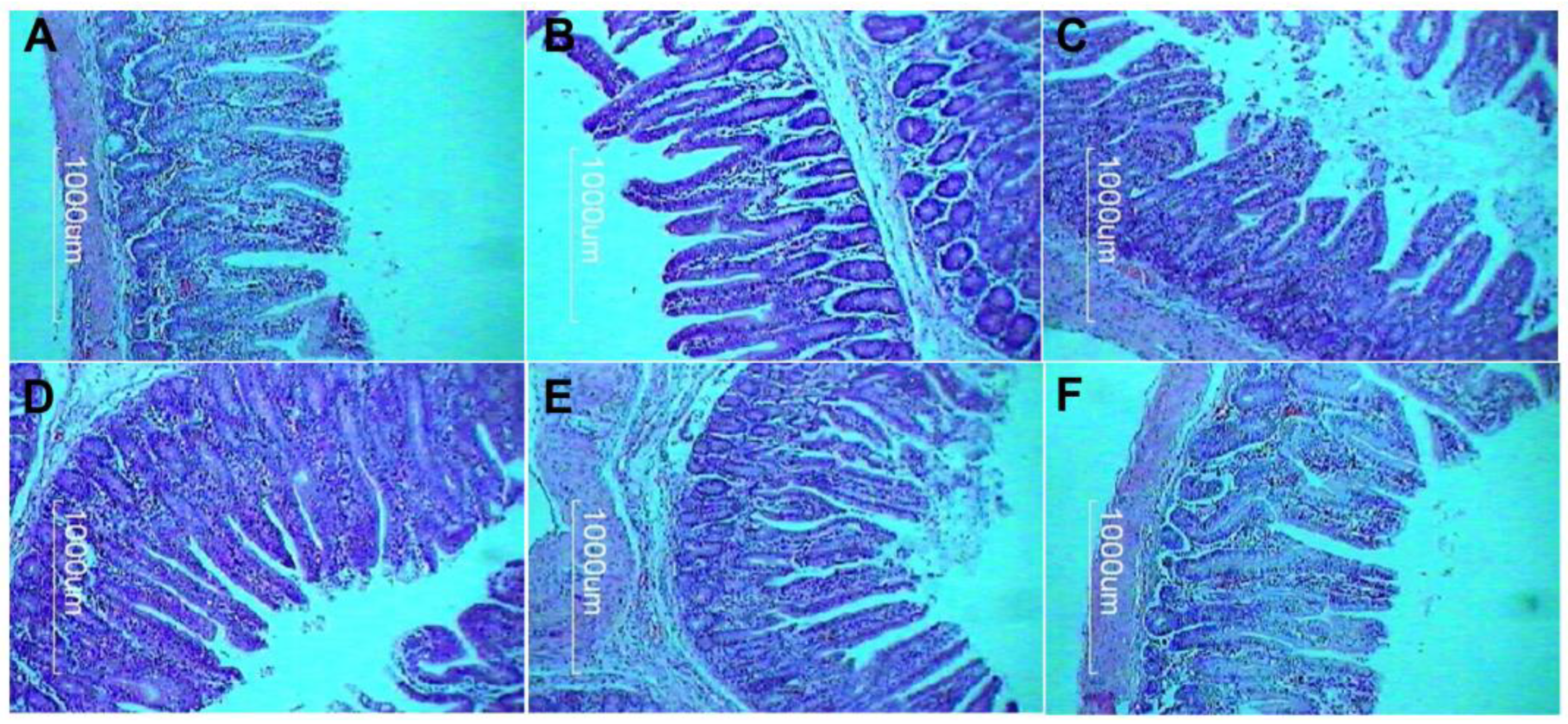
3.2. Intestinal Morphometry
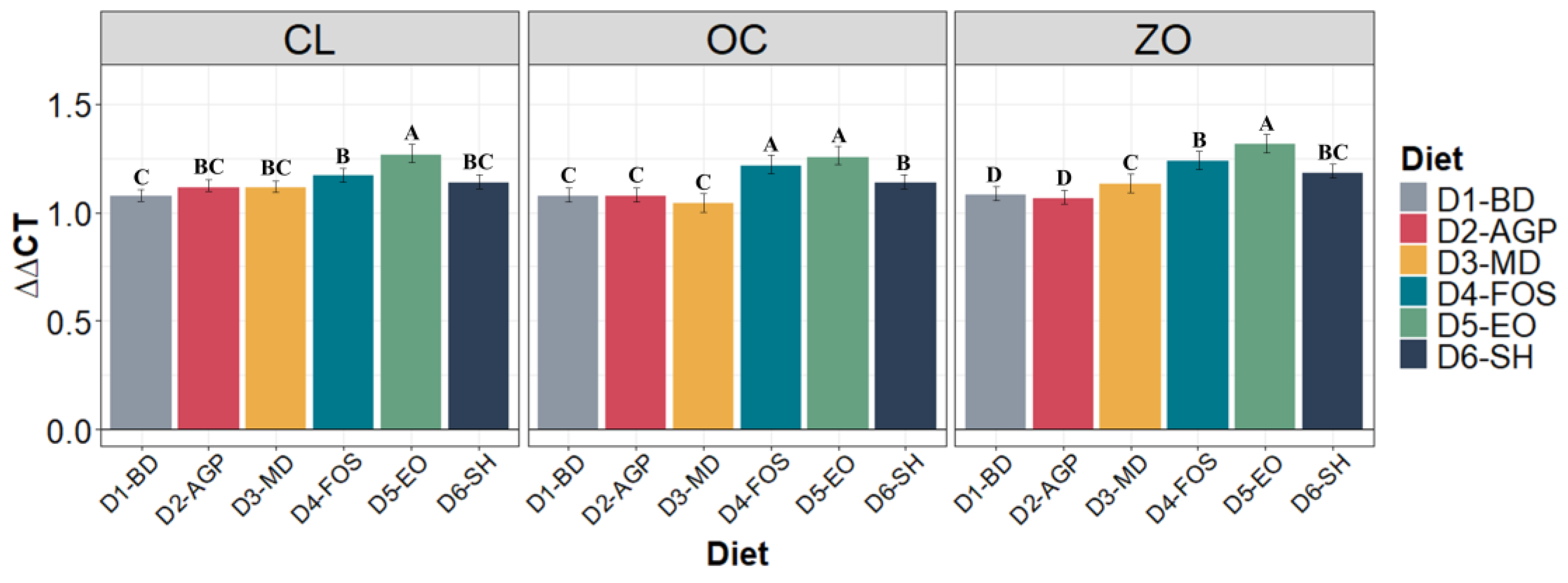
3.3. Barrier Protein Expression
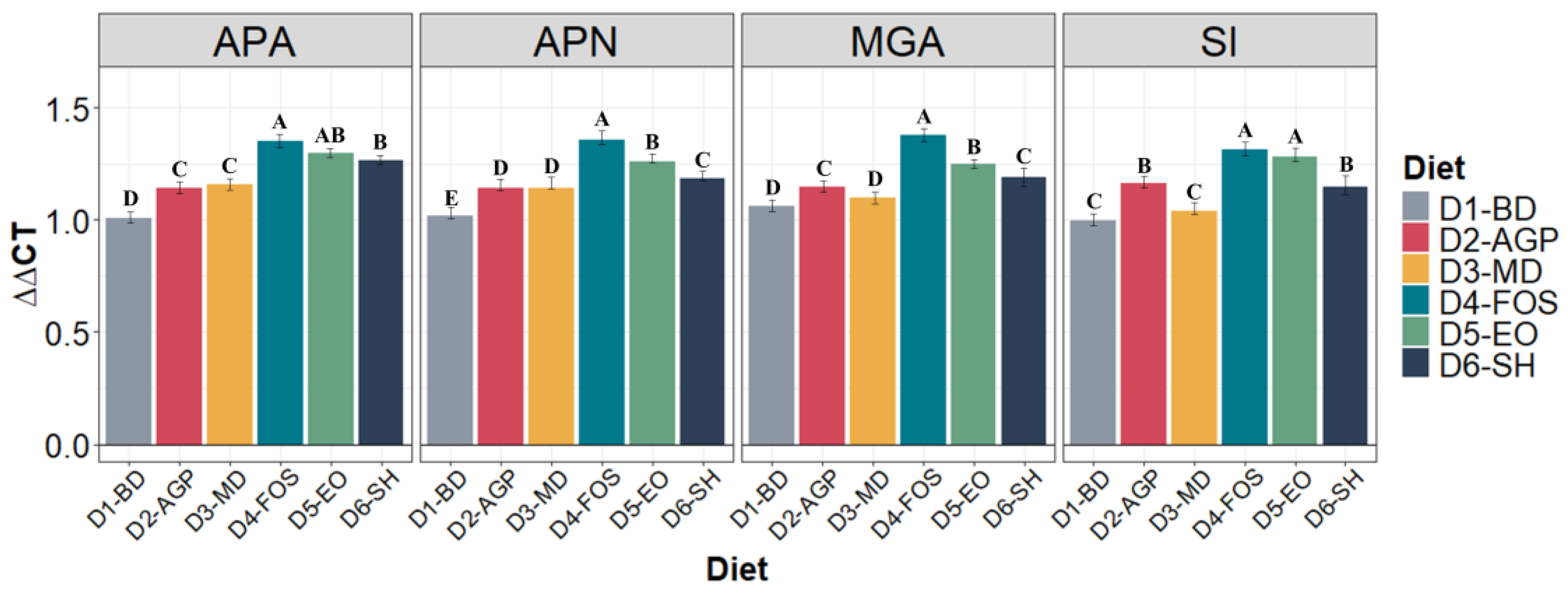
3.4. Expression of Enzymatic Proteins
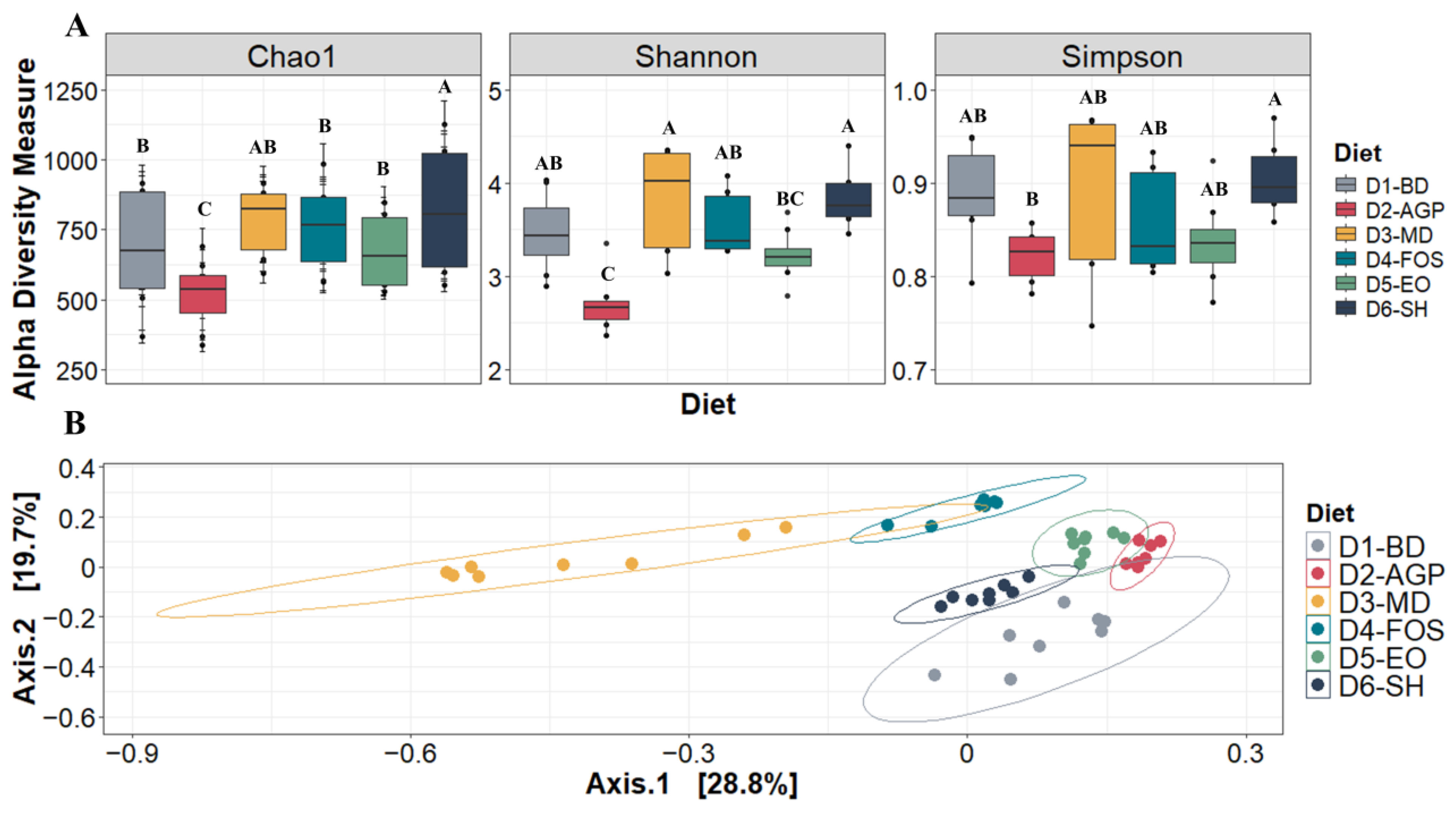
3.5. Gut Microbial Communities
3.5.1. Diversity Analysis of Gut Microbial Communities
3.5.2. Core Microbiota
3.5.3. Taxonomic Analysis
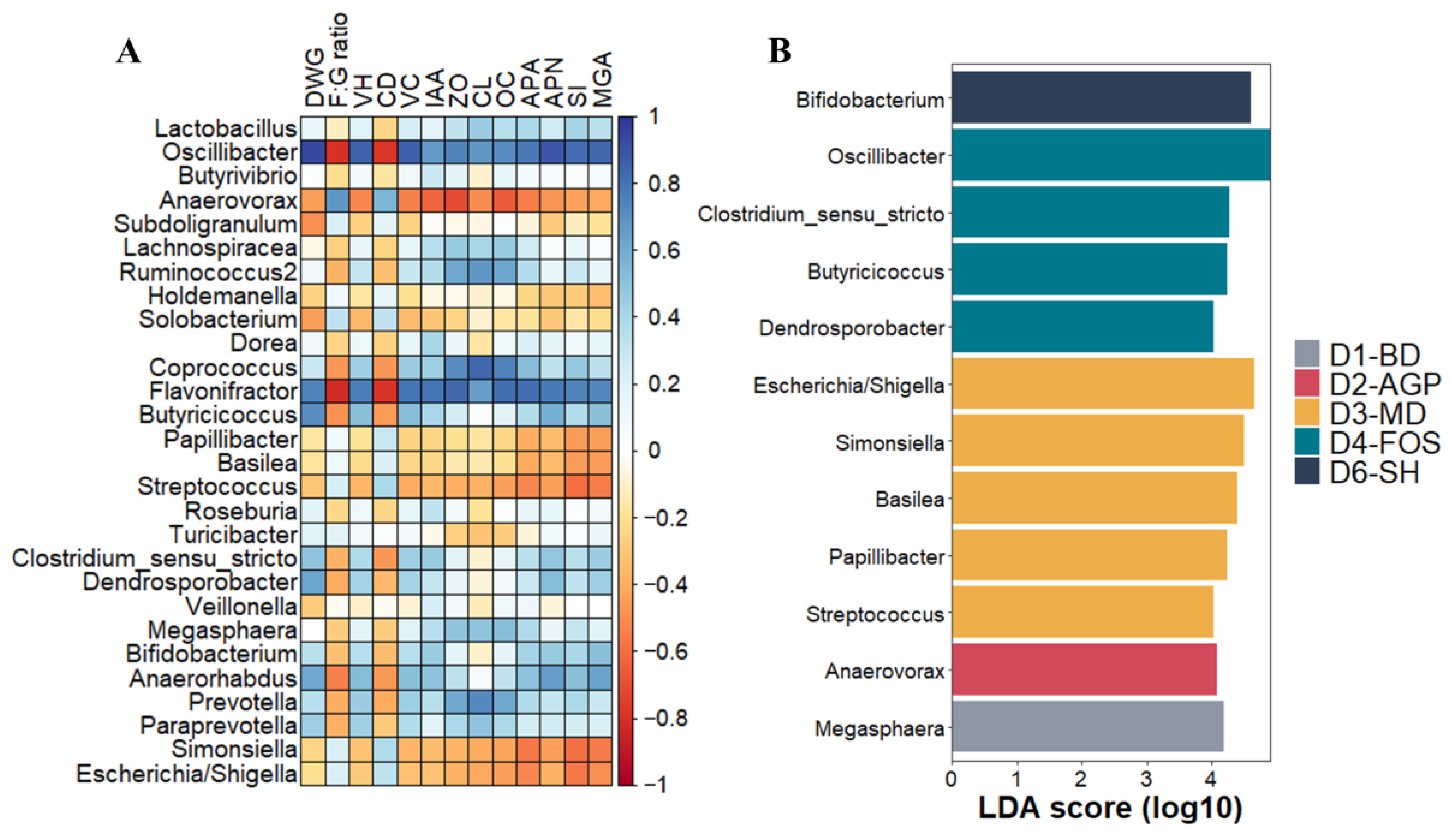
3.5.4. Taxa Associated with Diets and Productive Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herrera, F.V.; Pardo, C.S.; Parra, S.J. Antimicrobials Added to the Feed of Weaned Piglets at Two Ages Improves the Molecular Expression of Intestinal Barrier Proteins. Anim. Prod. Sci. 2022, 62, 511–520. [Google Scholar] [CrossRef]
- Ming, D.; Wang, W.; Huang, C.; Wang, Z.; Shi, C.; Ding, J.; Liu, H.; Wang, F. Effects of Weaning Age at 21 and 28 Days on Growth Performance, Intestinal Morphology and Redox Status in Piglets. Animals 2021, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.-R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The Dynamics of the Piglet Gut Microbiome during the Weaning Transition in Association with Health and Nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Y.; Wen, Z.; Jiang, X.; Ma, X.; Han, X. Weaning Stress Perturbs Gut Microbiome and Its Metabolic Profile in Piglets. Sci. Rep. 2018, 8, 18068. [Google Scholar] [CrossRef] [PubMed]
- Londoño, S.; Lallès, J.P.; Parra, J. Effect of Probiotic Strain Addition on Digestive Organ Growth and Nutrient Digestibility in Growing Pigs. Rev. Fac. Nac. Agron. Medellin 2016, 69, 7911–7918. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning Stress and Intestinal Health of Piglets: A Review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef] [PubMed]
- Long, S.F.; Xu, Y.T.; Pan, L.; Wang, Q.Q.; Wang, C.L.; Wu, J.Y.; Wu, Y.Y.; Han, Y.M.; Yun, C.H.; Piao, X.S. Mixed Organic Acids as Antibiotic Substitutes Improve Performance, Serum Immunity, Intestinal Morphology and Microbiota for Weaned Piglets. Anim. Feed. Sci. Technol. 2018, 235, 23–32. [Google Scholar] [CrossRef]
- Kherade, M.; Solanke, S.; Tawar, M.; Wankhede, S. Fructooligosaccharides: A Comprehensive Review. J. Ayurvedic Herbal. Med. 2021, 7, 193–200. [Google Scholar] [CrossRef]
- Vera, C.; Illanes, A.; Guerrero, C. Enzymatic Production of Prebiotic Oligosaccharides. Curr. Opin. Food Sci. 2021, 37, 160–170. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Gao, J.; Ma, J.; Li, T.; Tan, B.; Huang, X.; Yin, J. Opportunities of Prebiotics for the Intestinal Health of Monogastric Animals. Anim. Nutr. 2020, 6, 379–388. [Google Scholar] [CrossRef]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, Bioactivities, Mode of Action and Industrial Applications of Essential Oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Betancourt, L.; Hume, M.; Rodríguez, F.; Nisbet, D.; Sohail, M.U.; Afanador-Tellez, G. Effects of Colombian Oregano Essential Oil (Lippia origanoides Kunth) and Eimeria Species on Broiler Production and Cecal Microbiota. Poult. Sci. 2019, 98, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Madrid Garcés, T.A.; Parra Suescún, J.E.; López Herrera, A. La Ingesta de Aceite Esencial de Orégano (Lippia origanoides) Mejora La Morfología Intestinal En Broilers. Arch. Zootec. 2018, 66, 287–299. [Google Scholar] [CrossRef]
- Patiño, F.; Víctor Herrera, F.; Daniela López, D.; Jaime Parra, S. Blood Metabolites and Zootechnical Parameters in Piglets Weaned at Two Ages and with the Addition of Antimicrobials in the Feed. Rev. Investig. Vet. Peru. 2019, 30, 612–623. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of Dietary Supplementation of Humic Acid Sodium and Zinc Oxide on Growth Performance, Immune Status and Antioxidant Capacity of Weaned Piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef]
- Al-Taey, D.K.; Al-Shareefi, M.J.; Mijwel, A.K.; Razzaq Al-Tawaha, A.; Rahman Al-Tawaha, A. The Benefi Cial Effects of Bio-Fertilizers Combinations and Humic Acid on Growth, Yield Parameters and Nitrogen Content of Broccoli Grown under Drip Irrigation System Abstract. Bulg. J. Agric. Sci. 2019, 25, 959–966. [Google Scholar]
- Wang, D.; Jia, H.; Du, Y.; Liu, Y. Effects of Sodium Humate and Glutamine on Growth Performance, Diarrhoea Incidence, Blood Parameters, and Faecal Microflora of Pre-weaned Calves. J. Anim. Physiol. Anim. Nutr. 2022, 107, 103–112. [Google Scholar] [CrossRef]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef]
- de Lourdes Angeles, M.; Gómez-Rosales, S.; Téllez-Isaias, G. Mechanisms of Action of Humic Substances as Growth Promoters in Animals. In Humus and Humic Substances—Recent Advances; IntechOpen: London, UK, 2022. [Google Scholar]
- Leal, A.; Braga, A.; de Araújo, B.B.; Rodrigues, A.; de Carvalho, T.F. Antimicrobial Action of Essential Oil of Lippia origanoides H.B.K. J. Clin. Microbiol. Biochem. Technol. 2019, 5, 7–12. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.M. Potential of Essential Oils for Poultry and Pigs. Anim. Nutr. 2018, 4, 179–186. [Google Scholar] [CrossRef]
- Zhao, F.; Xia, Z. Application of FOS and CPP in Intestinal Health of Weaned Piglets. E3S Web Conf. 2019, 131, 01077. [Google Scholar] [CrossRef]
- ICLAS. International Guiding Principles for Biomedical Research Involving Animals; ICLAS: Seoul, Republic of Korea, 2012. [Google Scholar]
- Padilla, P.M. Manual de Porcicultura; Ministerio de Agricultura y Ganadería (MAG): San José, Costa Rica, 2007; ISBN 9789968877244. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Swine; National Research Council: Ottawa, ON, Canada, 2012. [Google Scholar]
- Canadian Council on Animal Care (CCAC). Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing Canadian Council on Animal Care in Science; Canadian Council on Animal Care (CCAC): Ottawa, ON, Canada, 2009; Volume 1, ISBN 9780919087507. [Google Scholar]
- Ciro, J.A.; López, A.; Parra, J. The Probiotic Enterococcus Faecium Modifies the Intestinal Morphometric Parameters in Weaning Piglets. Rev. Fac. Nac. Agron. Medellin 2016, 69, 7803–7811. [Google Scholar] [CrossRef]
- Barrera, M.H.; Rodríguez, S.P.; Torres, G. Efectos de La Adición de Ácido Cítrico y Un Probiótico Comercial En El Agua de Bebida, Sobre La Morfometría Del Duodeno y Parámetros Zootécnicos En Pollo de Engorde. Orinoquia 2014, 18, 52–62. [Google Scholar] [CrossRef]
- Santos, T.G.; Fernandes, S.D.; de Oliveira Araújo, S.B.; Felicioni, F.; de Mérici Domingues e Paula, T.; Caldeira-Brant, A.L.; Ferreira, S.V.; de Paula Naves, L.; de Souza, S.P.; Campos, P.H.R.F.; et al. Intrauterine Growth Restriction and Its Impact on Intestinal Morphophysiology throughout Postnatal Development in Pigs. Sci. Rep. 2022, 12, 11810. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, X.; He, J.; Yu, B.; Huang, Z.; Yu, J.; Zheng, P.; Chen, D. Dietary Fibre Affects Intestinal Mucosal Barrier Function and Regulates Intestinal Bacteria in Weaning Piglets. Br. J. Nutr. 2013, 110, 1837–1848. [Google Scholar] [CrossRef] [PubMed]
- Petersen, Y.M.; Burrin, D.G.; Sangild, P.T. GLP-2 Has Differential Effects on Small Intestine Growth and Function in Fetal and Neonatal Pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1986–R1993. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.N.; Chuang, Y.S.; Chiou, H.Y.; Wu, F.Y.; Yen, H.T.; Weng, C.F. Oral Administration Recombinant Porcine Epidermal Growth Factor Enhances the Jejunal Digestive Enzyme Genes Expression and Activity of Early-Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2008, 92, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Zhao, Y.; Xiang, L.; Jia, Y.; Yuan, J.; Dai, X.; Chen, H. 16S RRNA Gene Sequencing Analysis of Gut Microbiome in a Mini-pig Diabetes Model. Anim. Model. Exp. Med. 2022, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to Analyze 16S RRNA Gene Sequences from Microbial Communities. Curr. Protoc. Bioinform. 2011, 36, 10.7.1–10.7.20. [Google Scholar] [CrossRef]
- Mancabelli, L.; Ferrario, C.; Milani, C.; Mangifesta, M.; Turroni, F.; Duranti, S.; Lugli, G.A.; Viappiani, A.; Ossiprandi, M.C.; van Sinderen, D.; et al. Insights into the Biodiversity of the Gut Microbiota of Broiler Chickens. Environ. Microbiol. 2016, 18, 4727–4738. [Google Scholar] [CrossRef]
- Ballou, A.L.; Ali, R.A.; Mendoza, M.A.; Ellis, J.C.; Hassan, H.M.; Croom, W.J.; Koci, M.D. Development of the Chick Microbiome: How Early Exposure Influences Future Microbial Diversity. Front. Vet. Sci. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Lahti, L.; Shetty, S. Microbiome R Package. Bioconductor; Volume 10, B9, 2012. Available online: https://bioconductor.org/packages/release/bioc/html/microbiome.html (accessed on 10 January 2024).
- Mendiburu, F.D. Gricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-3. 2015. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 10 January 2024).
- Costa, M.C.; Bessegatto, J.A.; Alfieri, A.A.; Weese, J.S.; Filho, J.A.B.; Oba, A. Different Antibiotic Growth Promoters Induce Specific Changes in the Cecal Microbiota Membership of Broiler Chicken. PLoS ONE 2017, 12, e0171642. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Roeland Kindt Legendre, P.; Minchin, P.R.; O’Hara, B.; Simpson, G.L.; Solymos, P.; Stevens, H.; Wagner, H.H. Vegan: Community Ecology Package. R Package Version 2.6-4. 2015. Available online: https://vegan.r-forge.r-project.org/ (accessed on 10 January 2024).
- Fey, V.; Jambulingam, D.; Sara, H.; Heron, S.; Sipeky, C.; Schleutker, J. BioCPR–A Tool for Correlation Plots. Data 2021, 6, 97. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix. 2021, (Version 0.92). Available online: https://github.com/taiyun/corrplot (accessed on 10 January 2024).
- Cao, Y.; Dong, Q.; Wang, D.; Zhang, P.; Liu, Y.; Niu, C. MicrobiomeMarker: An R/Bioconductor Package for Microbiome Marker Identification and Visualization. Bioinformatics 2022, 38, 4027–4029. [Google Scholar] [CrossRef] [PubMed]
- Dieguez, S.N.; Decundo, J.M.; Martínez, G.; Amanto, F.A.; Bianchi, C.P.; Pérez Gaudio, D.S.; Soraci, A.L. Effect of Dietary Oregano (Lippia origanoides) and Clover (Eugenia caryophillata) Essential Oils’ Formulations on Intestinal Health and Performance of Pigs. Planta Medica 2022, 88, 324–335. [Google Scholar] [PubMed]
- Maya, F.C.A.; Ángel Isaza, J.A.; Martínez Morales, B.C.; Parra Suescún, J.E. Aceite Esencial de Orégano (Lippia origanoides) Mejora Parámetros Productivos y Metabólitos Sanguíneos En Lechones. Biotecnol. En El Sect. Agropecu. Y Agroindustrial 2021, 19, 82–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, G.; Zhang, S.; Zhao, J. Fructooligosaccharide Reduces Weanling Pig Diarrhea in Conjunction with Improving Intestinal Antioxidase Activity and Tight Junction Protein Expression. Nutrients 2022, 14, 512. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, D.; Yu, B.; Yin, H.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Luo, J.; et al. Fructooligosaccharides Improve Growth Performance and Intestinal Epithelium Function in Weaned Pigs Exposed to Enterotoxigenic Escherichia Coli. Food Funct. 2020, 11, 9599–9612. [Google Scholar] [CrossRef]
- Zou, Y.; Xiang, Q.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Improves Intestinal Morphology and Expression of Tight Junction Proteins Associated with Modulation of Selected Intestinal Bacteria and Immune Status in a Pig Model. Biomed. Res. Int. 2016, 2016, 5436738. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Huang, S.; Huang, Y.; Shi, H.; Bai, X. Effects of Dietary Essential Oil Supplementation on Growth Performance, Carcass Yield, Meat Quality, and Intestinal Tight Junctions of Broilers with or without Eimeria Challenge. Poult. Sci. 2023, 102, 102874. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, G.; Wang, R.-J.; Peng, J. Oregano Essential Oil Decreased Susceptibility to Oxidative Stress-Induced Dysfunction of Intestinal Epithelial Barrier in Rats. J. Funct. Foods 2015, 18, 1191–1199. [Google Scholar] [CrossRef]
- Wang, M.; Yang, C.; Wang, Q.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Yang, H.; Yin, Y. The Relationship between Villous Height and Growth Performance, Small Intestinal Mucosal Enzymes Activities and Nutrient Transporters Expression in Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 606–615. [Google Scholar] [CrossRef]
- Saladrigas-García, M.; D’Angelo, M.; Ko, H.L.; Nolis, P.; Ramayo-Caldas, Y.; Folch, J.M.; Llonch, P.; Solà-Oriol, D.; Pérez, J.F.; Martín-Orúe, S.M. Understanding Host-Microbiota Interactions in the Commercial Piglet around Weaning. Sci. Rep. 2021, 11, 23488. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Thippareddi, H.; Kim, W.K. Effect of Dietary Fructooligosaccharide (FOS) Supplementation on Ileal Microbiota in Broiler Chickens. Poult. Sci. 2018, 97, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhou, P.; Zhang, Y.; Zhang, Z.; Liu, J.; Zhang, H. Short-Chain Fructo-Oligosaccharides Alleviates Oxidized Oil-Induced Intestinal Dysfunction in Piglets Associated with the Modulation of Gut Microbiota. J. Funct. Foods 2020, 64, 103661. [Google Scholar] [CrossRef]
- Ayuso, M.; Michiels, J.; Wuyts, S.; Yan, H.; Degroote, J.; Lebeer, S.; Le Bourgot, C.; Apper, E.; Majdeddin, M.; Van Noten, N.; et al. Short-Chain Fructo-Oligosaccharides Supplementation to Suckling Piglets: Assessment of Pre- and Post-Weaning Performance and Gut Health. PLoS ONE 2020, 15, e0233910. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, R.; Basson, A.R.; Wearsh, P.; Cominelli, F.; Rodriguez-Palacios, A. Validity of Food Additive Maltodextrin as Placebo and Effects on Human Gut Physiology: Systematic Review of Placebo-Controlled Clinical Trials. Eur. J. Nutr. 2022, 61, 2853–2871. [Google Scholar] [CrossRef] [PubMed]
- Holman, D.B.; Chénier, M.R. Temporal Changes and the Effect of Subtherapeutic Concentrations of Antibiotics in the Gut Microbiota of Swine. FEMS Microbiol. Ecol. 2014, 90, 599–608. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Kim, I.H. Maintenance of Gut Microbiome Stability for Optimum Intestinal Health in Pigs—A Review. J. Anim. Sci. Biotechnol. 2022, 13, 140. [Google Scholar] [CrossRef]
- Zhao, W.; Yuan, M.; Li, P.; Yan, H.; Zhang, H.; Liu, J. Short-Chain Fructo-Oligosaccharides Enhances Intestinal Barrier Function by Attenuating Mucosa Inflammation and Altering Colonic Microbiota Composition of Weaning Piglets. Ital. J. Anim. Sci. 2019, 18, 976–986. [Google Scholar] [CrossRef]
- Csernus, B.; Czeglédi, L. Physiological, Antimicrobial, Intestine Morphological, and Immunological Effects of Fructooligosaccharides in Pigs. Arch. Anim. Breed. 2020, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Mo, K.; Li, J.; Liu, F.; Xu, Y.; Huang, X.; Ni, H. Superiority of Microencapsulated Essential Oils Compared with Common Essential Oils and Antibiotics: Effects on the Intestinal Health and Gut Microbiota of Weaning Piglet. Front. Nutr. 2022, 8, 808106. [Google Scholar] [CrossRef] [PubMed]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F.; Magowan, E.; Metzler-Zebeli, B.U.; Berry, D.; et al. Exploring a Possible Link between the Intestinal Microbiota and Feed Efficiency in Pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef] [PubMed]
- Badaras, S.; Ruzauskas, M.; Gruzauskas, R.; Zokaityte, E.; Starkute, V.; Klupsaite, D.; Mockus, E.; Klementaviciute, J.; Vadopalas, L.; Zokaityte, G.; et al. Different Creep Compound Feed Formulations for New Born Piglets: Influence on Growth Performance and Health Parameters. Front. Vet. Sci. 2022, 9, 971783. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.; Tiezzi, F.; Howard, J.; Huang, Y.J.; Gray, K.A.; Schillebeeckx, C.; McNulty, N.P.; Maltecca, C. Gut Microbiome Composition Differences among Breeds Impact Feed Efficiency in Swine. Microbiome 2020, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics Modulated Gut Microbiota Suppresses Hepatocellular Carcinoma Growth in Mice. Proc. Natl. Acad. Sci. USA 2016, 113, 201518189–E1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Jia, Z.; Xiao, S.; Long, C.; Wang, L. Effects of Enterotoxigenic Escherichia Coli Challenge on Jejunal Morphology and Microbial Community Profiles in Weaned Crossbred Piglets. Microorganisms 2023, 11, 2646. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal Dietary Resveratrol Alleviates Weaning-Associated Diarrhea and Intestinal Inflammation in Pig Offspring by Changing Intestinal Gene Expression and Microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Hridayanka, K.S.N.; Duttaroy, A.K. Bioactives and Their Roles in Bone Metabolism of Osteoarthritis: Evidence and Mechanisms on Gut-Bone Axis. Front. Immunol. 2024, 14, 1323233. [Google Scholar] [CrossRef] [PubMed]


| Ingredient | Inclusion Rate (%) |
| Corn | 47.66 |
| Sugar | 2.00 |
| Soy Protein Concentrate | 0.48 |
| Soybean Meal | 20.71 |
| Spray-Dried Animal Plasma | 5.00 |
| Fish Meal | 3.50 |
| Whey Permeate | 8.59 |
| Sweet Whey | 4.50 |
| Soybean Oil | 3.16 |
| Calcium Carbonate | 0.30 |
| Monodicalcium Phosphate | 1.34 |
| Salt | 0.20 |
| Mineral–vitamin Premix | 2.56 |
| Analyzed nutrient composition | |
| Nutrient | Value |
| Moisture (%) | 10.90 |
| Crude protein (%) | 21.89 |
| Fat (%) | 5.64 |
| Crude fiber (%) | 1.99 |
| Nitrogen-Free Extract (%) | 54.39 |
| Metabolizable Energy (Kcal/Kg) | 3430 |
| Net Energy (Kcal/Kg) | 2568 |
| Ash (%) | 5.96 |
| Available Phosphorus (%) | 0.55 |
| Calcium (%) | 1.07 |
| Lysine (%) | 1.45 |
| Methionine (%) | 0.41 |
| Met + Cist (%) | 0.81 |
| Threonine (%) | 0.97 |
| Tryptophan | 0.28 |
| Gen | Sequence | Annealing Temperature °C | GenBank/Reference | |
|---|---|---|---|---|
| Claudin-1 (CL) | Forward | 5′-GCCACAGCAAGGTATGGTAAC-3′ | 60 | [30] |
| Reverse | 5′-AGTAGGGCACCTCCCAGAAG-3′ | |||
| Occludin (OC) | Forward | 5′-GGAGGAAGACTGGATCAGGGA-3′ | 62 | [30] |
| Reverse | 5′-AGCAGCAGCCATGTACTCTT-3′ | |||
| Zonula occludens-1 (ZO | Forward | 5′-TGGCATTATTCGCCTTCATAC-3′ | 59 | [30] |
| Reverse | 5′-AGCCTCATTCGCATTGTTT-3′ | |||
| Maltase–glucoamylase (MGA) | Forward | 5′-CCAGAGCTTGTCACTCAGCA-3′ | 56 | [31] |
| Reverse | 5′-GCACGTCATAGGGGATCTGG-3′ | |||
| Sucrase–isomaltase (SI) | Forward | 5′-TGGCCATCCAGTCATGCC-3′ | 56 | [31] |
| Reverse | 5′-CCACCACTCTGCTGTGGA-3′ | |||
| Aminopeptidase A (APA) | Forward | 5′-CCTCCGGCGTCTGTGTTA-3′ | 56 | [31] |
| Reverse | 5′-TGGATTCAGCTCACAGCT-3′ | |||
| Aminopeptidase N (APN) | Forward | 5′-ACATCACTCTCATCCACCCT-3′ | 58 | [31,32] |
| Reverse | 5′-GCAATCACAGTGACAACTCG-3′ | |||
| β-actin | Forward | 5′-CCAGCACGATGAAGATCAAGA-3′ | 60 | AY550069.1 |
| Reverse | 5′-AATGCAACTAACAGTCCGCCTA-3′ | |||
| Variable | Mean | D1-BD | D2-AGP | D3-MD | D4-FOS | D5-EO | D6-SH | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Initial Weight | 6.61 | 6.64 | 6.60 | 6.64 | 6.61 | 6.56 | 6.57 | 0.015 | 0.851 |
| Final Weigh | 20.99 | 19.60 E | 20.33 D | 20.856 C | 22.54 A | 21.76 B | 20.84 C | 0.179 | <0.001 |
| DWG (kg) | 0.479 | 0.432 E | 0.458 D | 0.474 C | 0.531 A | 0.506 B | 0.476 C | 0.006 | <0.001 |
| DFI (kg) | 0.658 | 0.624 D | 0.643 CD | 0.652 C | 0.706 A | 0.677 B | 0.646 C | 0.005 | <0.001 |
| F:G ratio | 1.374 | 1.445 D | 1.404 C | 1.376 BC | 1.329 A | 1.338 A | 1.358 B | 0.007 | <0.001 |
| Variable | Mean | D1-BD | D2-AGP | D3-MD | D4-FOS | D5-EO | D6-SH | SEM | p-value |
|---|---|---|---|---|---|---|---|---|---|
| VH (µm) | 586.21 | 524.73 C | 547.46 C | 561.36 BC | 655.64 A | 640.26 A | 587.80 B | 9.59 | <0.001 |
| CD (µm) | 116.13 | 127.23 A | 124.71 A | 121.35 A | 103.72 C | 107.07 BC | 112.70 B | 0.26 | <0.001 |
| V:C | 5.05 | 4.12 C | 4.39 C | 4.63 BC | 6.32 A | 5.98 A | 5.22 B | 0.16 | <0.001 |
| IAA (mm2) | 6.02 | 5.57 C | 5.69 BC | 5.84 B | 6.44 A | 6.31 A | 6.28 A | 0.40 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ángel-Isaza, J.A.; Herrera Franco, V.; López-Herrera, A.; Parra-Suescun, J.E. Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Vet. Sci. 2024, 11, 332. https://doi.org/10.3390/vetsci11080332
Ángel-Isaza JA, Herrera Franco V, López-Herrera A, Parra-Suescun JE. Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Veterinary Sciences. 2024; 11(8):332. https://doi.org/10.3390/vetsci11080332
Chicago/Turabian StyleÁngel-Isaza, Jaime A., Víctor Herrera Franco, Albeiro López-Herrera, and Jaime E. Parra-Suescun. 2024. "Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets" Veterinary Sciences 11, no. 8: 332. https://doi.org/10.3390/vetsci11080332
APA StyleÁngel-Isaza, J. A., Herrera Franco, V., López-Herrera, A., & Parra-Suescun, J. E. (2024). Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Veterinary Sciences, 11(8), 332. https://doi.org/10.3390/vetsci11080332






