Molecular Mechanisms of Pathogenic Fungal Virulence Regulation by Cell Membrane Phospholipids
Abstract
1. Introduction
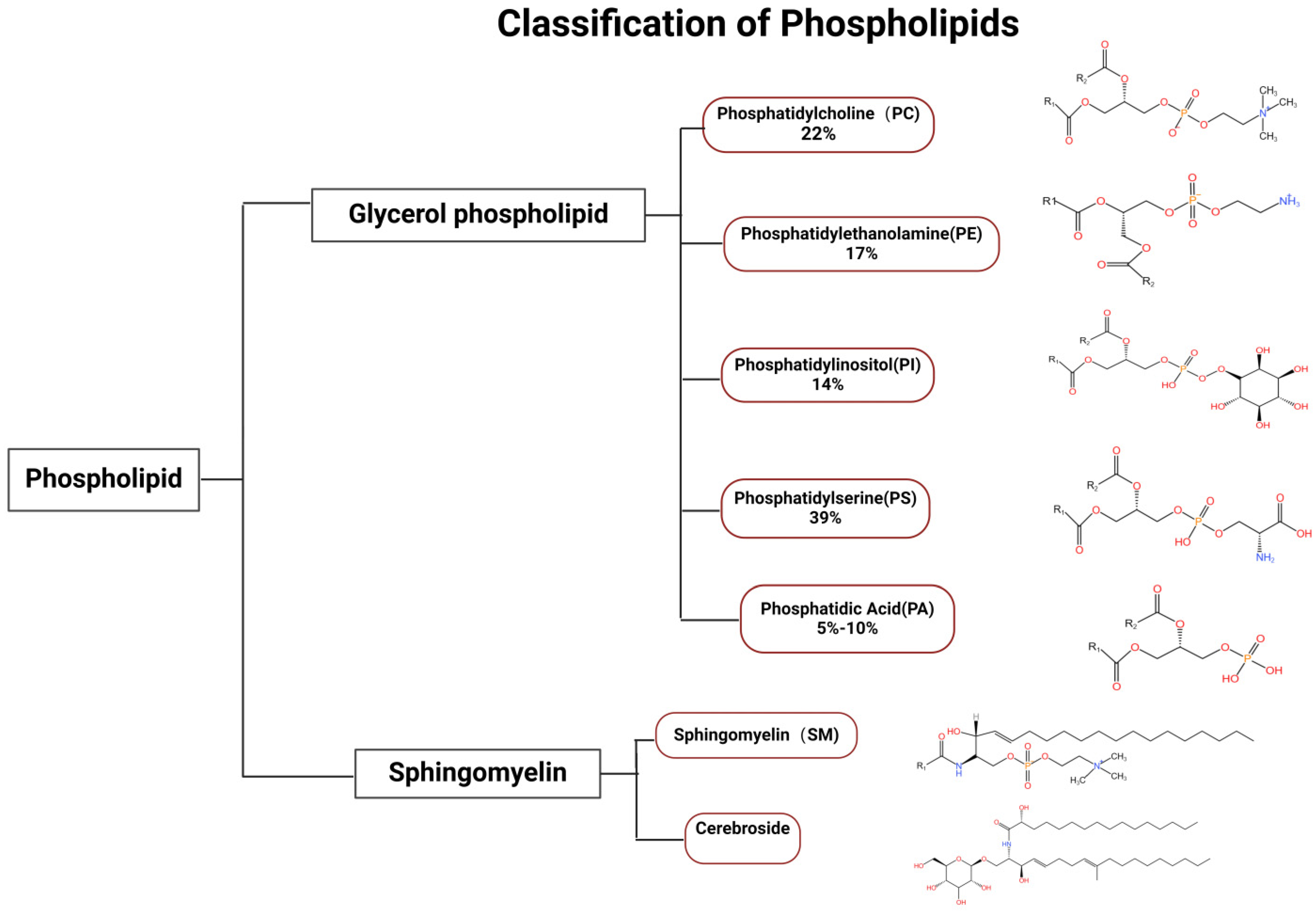
2. Classification and Function of Phospholipid Components
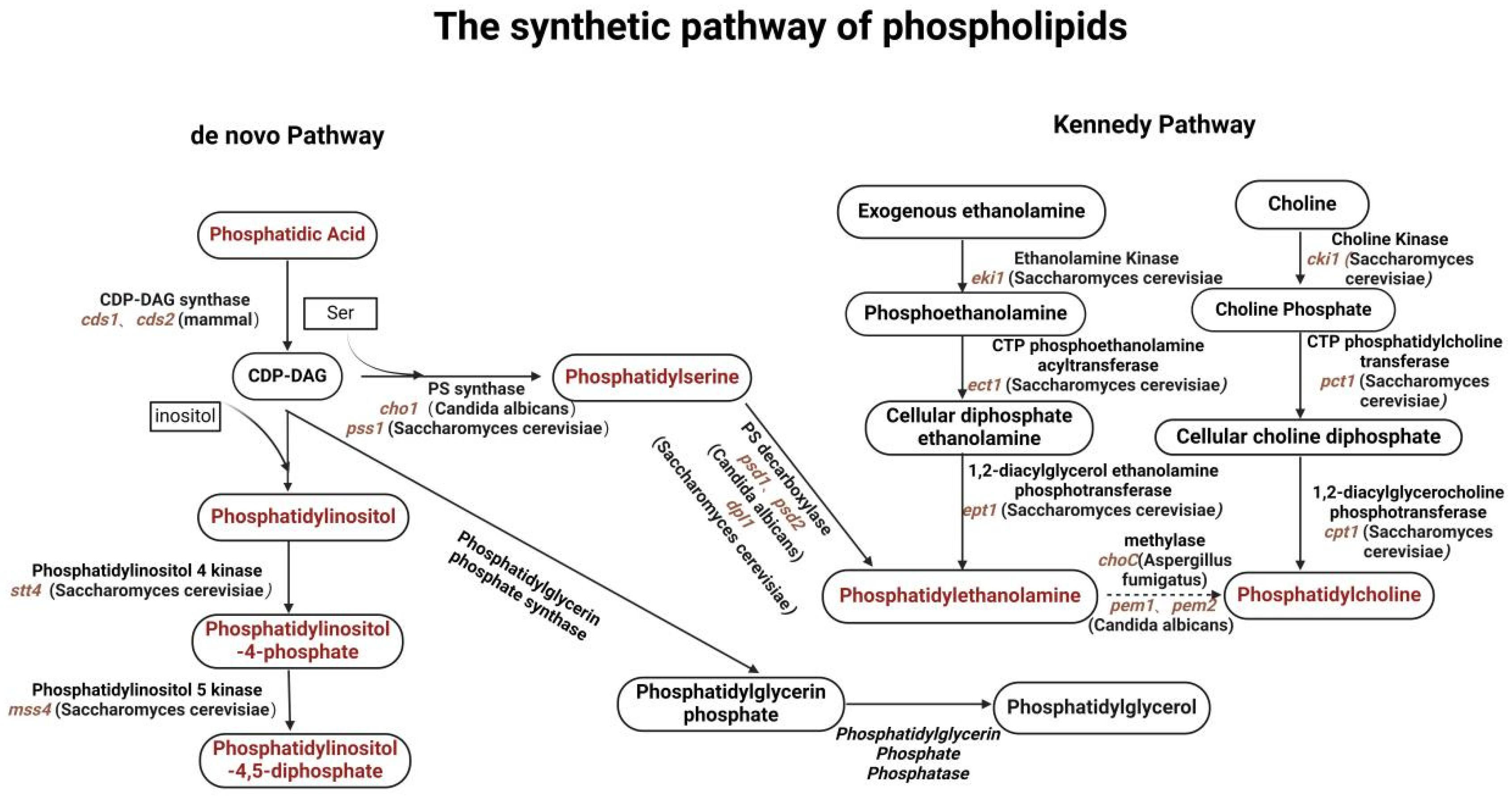
2.1. Biosynthetic Pathways of Glycerophospholipids in Fungal Cells
2.2. Intracellular Transport Mechanisms of Glycerophospholipids
2.3. Cellular Functions of Phospholipids
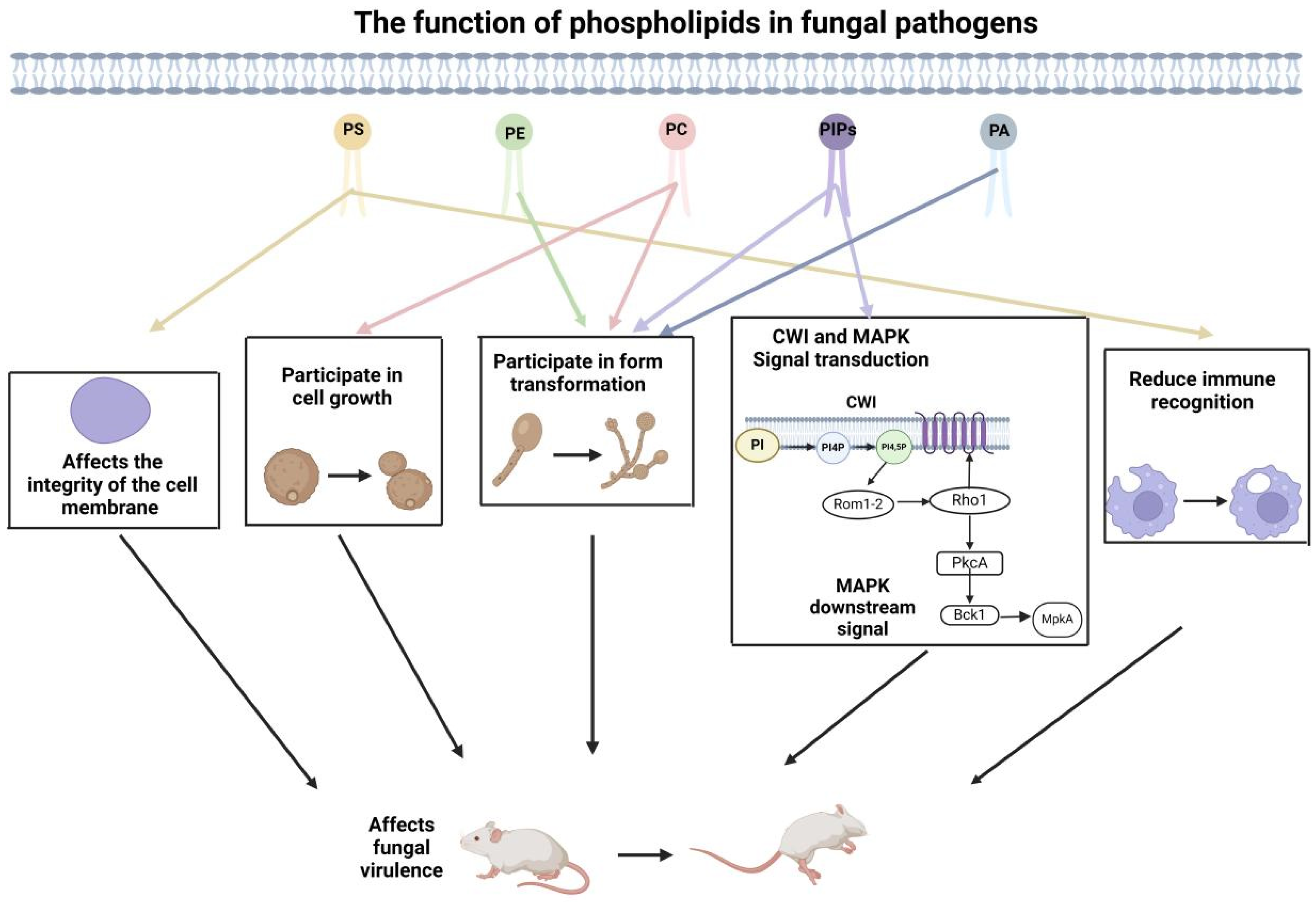
3. The Role of Membrane Phospholipid Composition in Fungal Virulence
3.1. Phosphoinositides (PIPs)
3.2. Phosphatidic Acid (PA)
3.3. Phosphatidylserine (PS) and Phosphatidylethanolamine (PE)
3.4. Phosphatidylcholine (PC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kulbacka, J.; Choromańska, A.; Rossowska, J.; Weżgowiec, J.; Saczko, J.; Rols, M.P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. Adv. Anat. Embryol. Cell Biol. 2017, 227, 39–58. [Google Scholar] [CrossRef]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, D.; Krasowska, A. Alterations in the Level of Ergosterol in Candida albicans’ Plasma Membrane Correspond with Changes in Virulence and Result in Triggering Diversed Inflammatory Response. Int. J. Mol. Sci. 2023, 24, 3966. [Google Scholar] [CrossRef] [PubMed]
- Choy, H.L.; Gaylord, E.A.; Doering, T.L. Ergosterol distribution controls surface structure formation and fungal pathogenicity. mBio 2023, 14, e0135323. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, X.; Wang, N.; Mo, P.; Shen, J.; Liu, M.; Zhang, H.; Wang, P.; Zhang, Z. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol. 2023, 237, 930–943. [Google Scholar] [CrossRef]
- Mishra, P.; Bolard, J.; Prasad, R. Emerging role of lipids of Candida albicans, a pathogenic dimorphic yeast. Biochim. Biophys. Acta 1992, 1127, 1–14. [Google Scholar] [CrossRef]
- Davis, S.E.; Hopke, A.; Minkin, S.C.; Montedonico, A.E.; Wheeler, R.T.; Reynolds, T.B.; Deepe, G.S. Masking of β(1-3)-Glucan in the Cell Wall of Candida albicans from Detection by Innate Immune Cells Depends on Phosphatidylserine. Infect. Immun. 2014, 82, 4405–4413. [Google Scholar] [CrossRef]
- Pereira, R.; Santos Fontenelle, R.O.; Brito, E.H.S.; Morais, S.M. Biofilm of Candida albicans: Formation, regulation and resistance. J. Appl. Microbiol. 2020, 131, 11–22. [Google Scholar] [CrossRef]
- Rhimi, W.; Aneke, C.I.; Annoscia, G.; Camarda, A.; Mosca, A.; Cantacessi, C.; Otranto, D.; Cafarchia, C. Virulence and in vitro antifungal susceptibility of Candida albicans and Candida catenulata from laying hens. Int. Microbiol. 2020, 24, 57–63. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.; Han, X.; Tao, S.; Zheng, D.; Cheng, Y.; Yu, R.; Han, G.; Schmidt, M.; Han, L.; et al. Disruption of the Phospholipase D Gene Attenuates the Virulence of Aspergillus fumigatus. Infect. Immun. 2012, 80, 429–440. [Google Scholar] [CrossRef]
- Chen, Y.L.; Montedonico, A.E.; Kauffman, S.; Dunlap, J.R.; Menn, F.M.; Reynolds, T.B. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol. Microbiol. 2010, 75, 1112–1132. [Google Scholar] [CrossRef] [PubMed]
- Konarzewska, P.; Wang, Y.; Han, G.-S.; Goh, K.J.; Gao, Y.-G.; Carman, G.M.; Xue, C. Phosphatidylserine synthesis is essential for viability of the human fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 2329–2339. [Google Scholar] [CrossRef]
- Heinisch, J.J.; Rodicio, R. Protein kinase C in fungi-more than just cell wall integrity. FEMS Microbiol. Rev. 2018, 42, fux051. [Google Scholar] [CrossRef]
- Hu, G.; Kronstad, J.W. A Putative P-Type ATPase, Apt1, Is Involved in Stress Tolerance and Virulence in Cryptococcus neoformans. Eukaryot. Cell 2010, 9, 74–83. [Google Scholar] [CrossRef]
- Rizzo, J.; Stanchev, L.D.; da Silva, V.K.A.; Nimrichter, L.; Pomorski, T.G.; Rodrigues, M.L. Role of lipid transporters in fungal physiology and pathogenicity. Comput. Struct. Biotechnol. J. 2019, 17, 1278–1289. [Google Scholar] [CrossRef]
- Gangadhar, B.H.; Sajeesh, K.; Venkatesh, J.; Baskar, V.; Abhinandan, K.; Yu, J.W.; Prasad, R.; Mishra, R.K. Enhanced Tolerance of Transgenic Potato Plants Over-Expressing Non-specific Lipid Transfer Protein-1 (StnsLTP1) against Multiple Abiotic Stresses. Front. Plant Sci. 2016, 7, 1228. [Google Scholar] [CrossRef]
- Nagata, S.; Suzuki, J.; Segawa, K.; Fujii, T. Exposure of phosphatidylserine on the cell surface. Cell Death Differ. 2016, 23, 952–961. [Google Scholar] [CrossRef]
- Sivagnanam, U.; Palanirajan, S.K.; Gummadi, S.N. The role of human phospholipid scramblases in apoptosis: An overview. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2261–2271. [Google Scholar] [CrossRef]
- Wright, M.M.; Howe, A.G.; Zaremberg, V. Cell membranes and apoptosis: Role of cardiolipin, phosphatidylcholine, and anticancer lipid analogues. Biochem. Cell Biol. 2004, 82, 18–26. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Mota Fernandes, C.; Del Poeta, M. Fungal sphingolipids: Role in the regulation of virulence and potential as targets for future antifungal therapies. Expert Rev. Anti-Infect. Ther. 2020, 18, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Park, T.S.; Fischl, A.S.; Ye, X.S. Cell cycle progression and cell polarity require sphingolipid biosynthesis in Aspergillus nidulans. Mol. Cell. Biol. 2001, 21, 6198–6209. [Google Scholar] [CrossRef]
- Mago, N.; Khuller, G.K. Lipids of Candida albicans: Subcellular distribution and biosynthesis. Microbiology 1990, 136, 993–996. [Google Scholar]
- Tams, R.N.; Cassilly, C.D.; Anaokar, S.; Brewer, W.T.; Dinsmore, J.T.; Chen, Y.-L.; Patton-Vogt, J.; Reynolds, T.B. Overproduction of Phospholipids by the Kennedy Pathway Leads to Hypervirulence in Candida albicans. Front. Microbiol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Máté, G.; Kovács, D.; Gazdag, Z.; Pesti, M.; Szántó, Á. Linalool-induced oxidative stress processes in the human pathogen Candida albicans. Acta Biol. Hung. 2017, 68, 220–231. [Google Scholar] [CrossRef]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and Regulation of Glycerolipids in the Yeast Saccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef]
- Nocua-Báez, L.C.; Uribe-Jerez, P.; Tarazona-Guaranga, L.; Robles, R.; Cortés, J.A. Azoles of then and now: A review. Rev. Chil. Infectol. 2020, 37, 219–230. [Google Scholar] [CrossRef]
- Dolan, J.W.; Bell, A.C.; Hube, B.; Schaller, M.; Warner, T.F.; Balish, E. Candida albicans PLD1 activity is required for full virulence. Med. Mycol. 2004, 42, 439–447. [Google Scholar] [CrossRef]
- Cockcroft, S.; Raghu, P. Phospholipid transport protein function at organelle contact sites. Curr. Opin. Cell Biol. 2018, 53, 52–60. [Google Scholar] [CrossRef]
- Wimley, W.C.; Thompson, T.E. Phosphatidylethanolamine enhances the concentration-dependent exchange of phospholipids between bilayers. Biochemistry 1991, 30, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Kawano, S.; Tamura, Y.; Kojima, R.; Bala, S.; Asai, E.; Michel, A.H.; Kornmann, B.; Riezman, I.; Riezman, H.; Sakae, Y.; et al. Structure–function insights into direct lipid transfer between membranes by Mmm1–Mdm12 of ERMES. J. Cell Biol. 2018, 217, 959–974. [Google Scholar] [CrossRef]
- Posor, Y.; Eichhorn-Grünig, M.; Haucke, V. Phosphoinositides in endocytosis. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Bankaitis, V.A.; Garcia-Mata, R.; Mousley, C.J. Golgi membrane dynamics and lipid metabolism. Curr. Biol. 2012, 22, R414–R424. [Google Scholar] [CrossRef]
- Khandelwal, N.K.; Kaemmer, P.; Förster, T.M.; Singh, A.; Coste, A.T.; Andes, D.R.; Hube, B.; Sanglard, D.; Chauhan, N.; Kaur, R.; et al. Pleiotropic effects of the vacuolar ABC transporter MLT1 of Candida albicans on cell function and virulence. Biochem. J. 2016, 473, 1537–1552. [Google Scholar] [CrossRef]
- Kong, D.; Yu, Y. Prostaglandin D2 signaling and cardiovascular homeostasis. J. Mol. Cell. Cardiol. 2022, 167, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rasul, F.; Zheng, F.; Dong, F.; He, J.; Liu, L.; Liu, W.; Cheema, J.Y.; Wei, W.; Fu, C. Emr1 regulates the number of foci of the endoplasmic reticulum-mitochondria encounter structure complex. Nat. Commun. 2021, 12, 521. [Google Scholar] [CrossRef]
- Lo Vasco, V.R. The Phosphoinositide Signal Transduction Pathway in the Pathogenesis of Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 15, 355–362. [Google Scholar] [CrossRef]
- Csernoch, L.; Jacquemond, V. Phosphoinositides in Ca2+ signaling and excitation-contraction coupling in skeletal muscle: An old player and newcomers. J. Muscle Res. Cell Motil. 2015, 36, 491–499. [Google Scholar] [CrossRef]
- Wei, W.; Zheng, B.; Zheng, S.; Wu, D.; Chu, Y.; Zhang, S.; Wang, D.; Ma, X.; Liu, X.; Yao, X.; et al. The Cdc42 GAP Rga6 promotes monopolar outgrowth of spores. J. Cell Biol. 2023, 222, e202202064. [Google Scholar] [CrossRef]
- Xu, B.Y.; Xu, J.; Yomo, T. A protocell with fusion and division. Biochem. Soc. Trans. 2019, 47, 1909–1919. [Google Scholar] [CrossRef]
- Liang, D.; Minikes, A.M.; Jiang, X. Ferroptosis at the intersection of lipid metabolism and cellular signaling. Mol. Cell 2022, 82, 2215–2227. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Khandelwal, N.K.; Sarkar, P.; Gaur, N.A.; Chattopadhyay, A.; Prasad, R. Phosphatidylserine decarboxylase governs plasma membrane fluidity and impacts drug susceptibilities of Candida albicans cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2308–2319. [Google Scholar] [CrossRef]
- Haupt, A.; Minc, N.; Bassereau, P. Gradients of phosphatidylserine contribute to plasma membrane charge localization and cell polarity in fission yeast. Mol. Biol. Cell 2017, 28, 210–220. [Google Scholar] [CrossRef]
- Cassilly, C.D.; Reynolds, T.B. PS, It’s Complicated: The Roles of Phosphatidylserine and Phosphatidylethanolamine in the Pathogenesis of Candida albicans and Other Microbial Pathogens. J. Fungi 2018, 4, 28. [Google Scholar] [CrossRef]
- Takagi, K.; Kikkawa, A.; Iwama, R.; Fukuda, R.; Horiuchi, H. Type II phosphatidylserine decarboxylase is crucial for the growth and morphogenesis of the filamentous fungus Aspergillus nidulans. J. Biosci. Bioeng. 2021, 131, 139–146. [Google Scholar] [CrossRef]
- Pan, J.; Yang, X.; Hu, C.; Fu, T.; Zhang, X.; Liu, Z.; Wang, Y.; Zhang, F.; He, X.; Yu, J.H. Functional, transcriptomic, and lipidomic studies of the choC gene encoding a phospholipid methyltransferase in Aspergillus fumigatus. Microbiol. Spectr. 2024, 12, e0216823. [Google Scholar] [CrossRef]
- Xue, C.; Morelli, K.A.; Kerkaert, J.D.; Cramer, R.A. Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression. PLoS Pathog. 2021, 17, e1009794. [Google Scholar] [CrossRef]
- Suzawa, T.; Iwama, R.; Fukuda, R.; Horiuchi, H. Phosphatidylcholine levels regulate hyphal elongation and differentiation in the filamentous fungus Aspergillus oryzae. Sci. Rep. 2024, 14, 11729. [Google Scholar] [CrossRef]
- Ganesan, S.; Shabits, B.N.; Zaremberg, V. Tracking Diacylglycerol and Phosphatidic Acid Pools in Budding Yeast. Lipid Insights 2015, 8 (Suppl. S1), 75–85. [Google Scholar] [CrossRef] [PubMed]
- Barman, A.; Gohain, D.; Bora, U.; Tamuli, R. Phospholipases play multiple cellular roles including growth, stress tolerance, sexual development, and virulence in fungi. Microbiol. Res. 2018, 209, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Ma, C.; Feng, Y.; Zhang, B.; Zhu, M.; Ma, T.; Yu, Q.; Li, M. Phosphate Starvation by Energy Metabolism Disturbance in Candida albicansvip1Δ/Δ Induces Lipid Droplet Accumulation and Cell Membrane Damage. Molecules 2022, 27, 686. [Google Scholar] [CrossRef]
- Vernay, A.; Schaub, S.; Guillas, I.; Bassilana, M.; Arkowitz, R.A. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J. Cell Biol. 2012, 198, 711–730. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, H.; Cullen, P.J. Role of Phosphatidylinositol Phosphate Signaling in the Regulation of the Filamentous-Growth Mitogen-Activated Protein Kinase Pathway. Eukaryot. Cell 2015, 14, 427–440. [Google Scholar] [CrossRef]
- Gao, Q.; Lu, Y.; Yao, H.; Xu, Y.J.; Huang, W.; Wang, C. Phospholipid homeostasis maintains cell polarity, development and virulence in metarhizium robertsii. Environ. Microbiol. 2016, 18, 3976–3990. [Google Scholar] [CrossRef]
- Akhberdi, O.; Zhang, Q.; Wang, H.; Li, Y.; Chen, L.; Wang, D.; Yu, X.; Wei, D.; Zhu, X. Roles of phospholipid methyltransferases in pycnidia development, stress tolerance and secondary metabolism in the taxol-producing fungus Pestalotiopsis microspore. Microbiol. Res. 2018, 210, 33–42. [Google Scholar] [CrossRef]
- Ghugtyal, V.; Garcia-Rodas, R.; Seminara, A.; Schaub, S.; Bassilana, M.; Arkowitz, R.A. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc. Natl. Acad. Sci. USA 2015, 112, 8644–8649. [Google Scholar] [CrossRef]
- Li, S.; Feng, X.; Zhang, X.; Xie, S.; Ma, F. Phospholipid and antioxidant responses of oleaginous fungus Cunninghamella echinulata against hydrogen peroxide stress. Arch. Biochem. Biophys. 2022, 731, 109447. [Google Scholar] [CrossRef]
- Murphy, C.T.; He, B.; Xu, J.; Pang, S.; Tang, H. Phosphatidylcholine mediates the crosstalk between LET-607 and DAF-16 stress response pathways. PLoS Genet. 2021, 17, e1009573. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. 2019, 23, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Fortwendel, J.R.; Juvvadi, P.R.; Rogg, L.E.; Asfaw, Y.G.; Burns, K.A.; Randell, S.H.; Steinbach, W.J. Plasma membrane localization is required for RasA-mediated polarized morphogenesis and virulence of Aspergillus fumigatus. Eukaryot. Cell 2012, 11, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A. Extracellular phospholipases as universal virulence factor in pathogenic fungi. Nihon Ishinkin Gakkai Zasshi 1998, 39, 55–59. [Google Scholar] [CrossRef]
- Olivotto, M.; Arcangeli, A.; Carla, M.; Wanke, E. Electric fields at the plasma membrane level: A neglected element in the mechanisms of cell signalling. Bioessays 1996, 18, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Konopka, J.B. Plasma Membrane Phosphatidylinositol 4-Phosphate Is Necessary for Virulence of Candida albicans. mBio 2022, 13, e0036622. [Google Scholar] [CrossRef]
- Martin-Vicente, A.; Souza, A.C.O.; Nywening, A.V.; Ge, W.; Fortwendel, J.R. Overexpression of the Aspergillus fumigatus Small GTPase, RsrA, Promotes Polarity Establishment during Germination. J. Fungi 2020, 6, 285. [Google Scholar] [CrossRef]
- Günther, J.; Nguyen, M.; Härtl, A.; Künkel, W.; Zipfel, P.F.; Eck, R. Generation and functional in vivo characterization of a lipid kinase defective phosphatidylinositol 3-kinase Vps34p of Candida albicans. Microbiology 2005, 151, 81–89. [Google Scholar] [CrossRef]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicansSecreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef]
- Bruckmann, A.; Künkel, W.; Härtl, A.; Wetzker, R.; Eck, R. A phosphatidylinositol 3-kinase of Candida albicans influences adhesion, filamentous growth and virulence. Microbiology 2000, 146 Pt 11, 2755–2764. [Google Scholar] [CrossRef]
- Garcia-Rodas, R.; Labbaoui, H.; Orange, F.; Solis, N.; Zaragoza, O.; Filler, S.G.; Bassilana, M.; Arkowitz, R.A. Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection. mBio 2021, 13, e0387321. [Google Scholar] [CrossRef]
- Hairfield, M.L.; Westwater, C.; Dolan, J.W. Phosphatidylinositol-4-phosphate 5-kinase activity is stimulated during temperature-induced morphogenesis in Candida albicans. Microbiology 2002, 148 Pt 6, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Cappell, S.D.; Dohlman, H.G. Selective regulation of MAP kinase signaling by an endomembrane phosphatidylinositol 4-kinase. J. Biol. Chem. 2011, 286, 14852–14860. [Google Scholar] [CrossRef]
- Garrenton, L.S.; Stefan, C.J.; McMurray, M.A.; Emr, S.D.; Thorner, J. Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 11805–11810. [Google Scholar] [CrossRef]
- Csank, C.; Schröppel, K.; Leberer, E.; Harcus, D.; Mohamed, O.; Meloche, S.; Thomas, D.Y.; Whiteway, M. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 1998, 66, 2713–2721. [Google Scholar] [CrossRef]
- González, B.; Mirzaei, M.; Basu, S.; Pujari, A.N.; Vandermeulen, M.D.; Prabhakar, A.; Cullen, P.J. Turnover and bypass of p21-activated kinase during Cdc42-dependent MAPK signaling in yeast. J. Biol. Chem. 2023, 299, 105297. [Google Scholar] [CrossRef]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef]
- Walch-Solimena, C.; Novick, P. The yeast phosphatidylinositol-4-OH kinase pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1999, 1, 523–525. [Google Scholar] [CrossRef] [PubMed]
- Hama, H.; Schnieders, E.A.; Thorner, J.; Takemoto, J.Y.; DeWald, D.B. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1999, 274, 34294–34300. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; Sabbagh, W., Jr.; Graham, E.; Irick, M.M.; van Olden, E.K.; Neal, C.; Delrow, J.; Bardwell, L.; Sprague, G.F., Jr. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 2004, 18, 1695–1708. [Google Scholar] [CrossRef]
- O’Rourke, S.M.; Herskowitz, I. A third osmosensing branch in Saccharomyces cerevisiae requires the Msb2 protein and functions in parallel with the Sho1 branch. Mol. Cell. Biol. 2002, 22, 4739–4749. [Google Scholar] [CrossRef]
- Maeda, T.; Takekawa, M.; Saito, H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science 1995, 269, 554–558. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.; Neiman, A.M.; Park, H.O.; van Lohuizen, M.; Herskowitz, I. Functional analysis of the interaction between the small GTP binding protein Cdc42 and the Ste20 protein kinase in yeast. Embo J. 1996, 15, 7046–7059. [Google Scholar] [PubMed]
- Leberer, E.; Wu, C.; Leeuw, T.; Fourest-Lieuvin, A.; Segall, J.E.; Thomas, D.Y. Functional characterization of the Cdc42p binding domain of yeast Ste20p protein kinase. Embo J. 1997, 16, 83–97. [Google Scholar] [CrossRef]
- Yakir-Tamang, L.; Gerst, J.E. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol. Biol. Cell 2009, 20, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Nunez, L.R.; Jesch, S.A.; Gaspar, M.L.; Almaguer, C.; Villa-Garcia, M.; Ruiz-Noriega, M.; Patton-Vogt, J.; Henry, S.A. Cell Wall Integrity MAPK Pathway Is Essential for Lipid Homeostasis. J. Biol. Chem. 2008, 283, 34204–34217. [Google Scholar] [CrossRef]
- Audhya, A.; Emr, S.D. Stt4 PI 4-kinase localizes to the plasma membrane and functions. Dev. Cell 2002, 2, 593–605. [Google Scholar] [CrossRef]
- Bartual, S.G.; Wei, W.; Zhou, Y.; Pravata, V.M.; Fang, W.; Yan, K.; Ferenbach, A.T.; Lockhart, D.E.; van Aalten, D.M. The citron homology domain as a scaffold for Rho1 signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2110298118. [Google Scholar]
- Xie, J.L.; Grahl, N.; Sless, T.; Leach, M.D.; Kim, S.H.; Hogan, D.A.; Robbins, N.; Cowen, L.E. Signaling through Lrg1, Rho1 and Pkc1 Governs Candida albicans Morphogenesis in Response to Diverse Cues. PLoS Genet. 2016, 12, e1006405. [Google Scholar] [CrossRef]
- McLain, N.; Dolan, J.W. Phospholipase D activity is required for dimorphic transition in Candida albicans. Microbiology 1997, 143 Pt 11, 3521–3526. [Google Scholar] [CrossRef]
- Putta, P.; Rankenberg, J.; Korver, R.A.; van Wijk, R.; Munnik, T.; Testerink, C.; Kooijman, E.E. Phosphatidic acid binding proteins display differential binding as a function of membrane curvature stress and chemical properties. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 2709–2716. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase C: Perfectly balanced. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 208–230. [Google Scholar] [CrossRef] [PubMed]
- Hube, B.; Hess, D.; Baker, C.A.; Schaller, M.; Schäfer, W.; Dolan, J.W. The role and relevance of phospholipase D1 during growth and dimorphism of Candida albicans. Microbiology 2001, 147 Pt 4, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, G.; Williamson, P.; Puts, C.F.; Holthuis, J.C.M. Cdc50p Plays a Vital Role in the ATPase Reaction Cycle of the Putative Aminophospholipid Transporter Drs2p. J. Biol. Chem. 2009, 284, 17956–17967. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimura-Kamada, K.; Yamamoto, T. Functions of phospholipid flippases. J. Biochem. 2010, 149, 131–143. [Google Scholar] [CrossRef]
- Saito, K.; Fujimura-Kamada, K.; Furuta, N.; Kato, U.; Umeda, M.; Tanaka, K. Cdc50p, a Protein Required for Polarized Growth, Associates with the Drs2p P-Type ATPase Implicated in Phospholipid Translocation in Saccharomyces cerevisiae. Mol. Biol. Cell 2004, 15, 3418–3432. [Google Scholar] [CrossRef]
- Cory, S. Phosphatidylserine hide-and-seek. Proc. Natl. Acad. Sci. USA 2018, 115, 12092–12094. [Google Scholar] [CrossRef]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef]
- Huang, W.; Liao, G.; Baker, G.M.; Wang, Y.; Lau, R.; Paderu, P.; Perlin, D.S.; Xue, C. Lipid Flippase Subunit Cdc50 Mediates Drug Resistance and Virulence in Cryptococcus neoformans. mBio 2016, 7, e00478-16. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, Y.; Shadkchan, Y.; Levdansky, E.; Morozov, M.; Romano, J.; Osherov, N. The three Aspergillus fumigatus CFEM-domain GPI-anchored proteins (CfmA-C) affect cell-wall stability but do not play a role in fungal virulence. Fungal Genet. Biol. 2014, 63, 55–64. [Google Scholar] [CrossRef]
- Samalova, M.; Carr, P.; Bromley, M.; Blatzer, M.; Moya-Nilges, M.; Latgé, J.P.; Mouyna, I. GPI Anchored Proteins in Aspergillus fumigatus and Cell Wall Morphogenesis. Curr. Top. Microbiol. Immunol. 2020, 425, 167–186. [Google Scholar] [CrossRef]
- Lagace, T.A.; Ridgway, N.D. The role of phospholipids in the biological activity and structure of the endoplasmic reticulum. Biochim. Biophys. Acta 2013, 1833, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, H.; Luo, Y.; Ouyang, H.; Hu, H.; Jin, C. Glycosylphosphatidylinositol (GPI) anchor is required in Aspergillus fumigatus for morphogenesis and virulence. Mol. Microbiol. 2007, 64, 1014–1027. [Google Scholar] [CrossRef]
- Prasad, R.; Khandelwal, N.K.; Banerjee, A. Yeast ABC transporters in lipid trafficking. Fungal Genet. Biol. 2016, 93, 25–34. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.-B.; Delmas, G.; Park, S.; Perlin, D.; Xue, C. Two Major Inositol Transporters and Their Role in Cryptococcal Virulence. Eukaryot. Cell 2011, 10, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot. Cell 2013, 12, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Barhoom, S.; Kupiec, M.; Zhao, X.; Xu, J.R.; Sharon, A. Functional characterization of CgCTR2, a putative vacuole copper transporter that is involved in germination and pathogenicity in Colletotrichum gloeosporioides. Eukaryot. Cell 2008, 7, 1098–1108. [Google Scholar] [CrossRef]
- Yu, T.J.; Zhou, Z.H.; Liu, S.J.; Li, C.J.; Zhang, Z.W.; Zhang, Y.; Jin, W.; Liu, K.Q.; Mao, S.Y.; Zhu, L.; et al. The role of phosphatidylcholine 34:1 in the occurrence, development and treatment of ulcerative colitis. Acta Pharm. Sin. B 2023, 13, 1231–1245. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wang, J.L.; Li, Y.; Wang, B.W.; Tao, G.J.; Wang, X.Y. Structure characterization of phospholipids and lipid A of Pseudomonas putida KT2442. Eur. J. Mass. Spectrom. 2015, 21, 739–746. [Google Scholar] [CrossRef]
| Phospholipid | The Function of Phospholipids | Fungus | |
|---|---|---|---|
| Phosphatidylserine (PS) | Membrane transport | Candida albicans [44] | |
| Regulating membrane fluidity | Candida albicans [44] | ||
| Polarized growth | Schizosaccharomyces pombe [45] | ||
| Affects fungal virulence | Reduce immune recognition | Candida albicans [7] | |
| Affect membrane integrity | Cryptococcus neoformans [46] Candida albicans [11] | ||
| Decreased enzyme secretion ability | Candida albicans [46] | ||
| Phosphatidylethanolamine (PE) | Membrane transport | Candida albicans [6] | |
| Regulating membrane fluidity | Candida albicans [6,11] | ||
| Affects fungal virulence | Cell wall integrity | ||
| Morphogenesis | Candida albicans [6] Aspergillus fumigatus [47] | ||
| Phosphatidylcholine (PC) | Participates in cell growth and metabolism | Aspergillus fumigatus [48] | |
| Maintain the integrity of cell membrane and cell wall | Aspergillus fumigatus [48] | ||
| Regulating membrane fluidity | Aspergillus fumigatus [49] | ||
| Affects fungal virulence | Morphogenesis | Aspergillus fumigatus [48] Aspergillus oryzae (non-fungal pathogen) [50] | |
| Phosphatidic Acid (PA) | Key signaling molecules | Saccharomyces cerevisiae [51] | |
| Synthesis of secondary metabolites | Ganoderma lucidum [10] | ||
| Affects fungal virulence | Regulating fungal internalization ability | Aspergillus fumigatus [10] | |
| Morphogenesis | Candida albicans [52] | ||
| Phosphatidylinositol family (PIPs) | Energy metabolism | Candida albicans [53] | |
| Affects fungal virulence | Morphogenesis | Candida albicans [54] | |
| CWI and MAPK signal transduction | Candida albicans [55] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, H.; Wang, H.; Wang, T.; Wu, D.; Wei, W. Molecular Mechanisms of Pathogenic Fungal Virulence Regulation by Cell Membrane Phospholipids. J. Fungi 2025, 11, 256. https://doi.org/10.3390/jof11040256
Li Y, Wang H, Wang H, Wang T, Wu D, Wei W. Molecular Mechanisms of Pathogenic Fungal Virulence Regulation by Cell Membrane Phospholipids. Journal of Fungi. 2025; 11(4):256. https://doi.org/10.3390/jof11040256
Chicago/Turabian StyleLi, Yitong, Hongchen Wang, Hengxiu Wang, Tianming Wang, Daqiang Wu, and Wenfan Wei. 2025. "Molecular Mechanisms of Pathogenic Fungal Virulence Regulation by Cell Membrane Phospholipids" Journal of Fungi 11, no. 4: 256. https://doi.org/10.3390/jof11040256
APA StyleLi, Y., Wang, H., Wang, H., Wang, T., Wu, D., & Wei, W. (2025). Molecular Mechanisms of Pathogenic Fungal Virulence Regulation by Cell Membrane Phospholipids. Journal of Fungi, 11(4), 256. https://doi.org/10.3390/jof11040256







