Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Selection
2.2. Experimental Setup
2.3. Types and Composition of Media Used for Cultivation
2.4. Cultivation of Media Dynamics
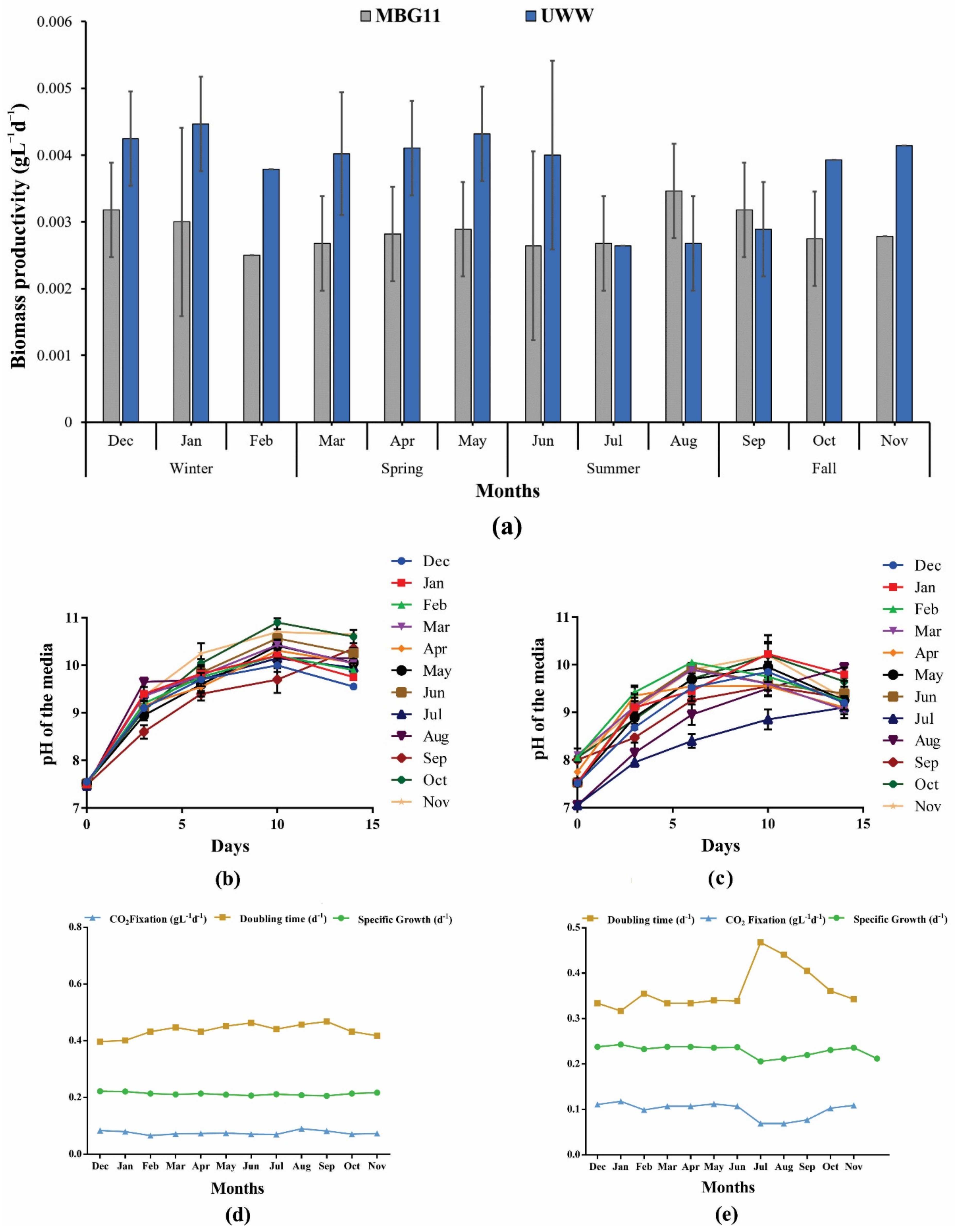
2.5. Growth Kinetics and CO2 Fixation Potential
2.6. Cascading Processing of the Algal Biomass
2.6.1. Extraction and Estimation of Phycobilins from the Wastewater-Cultivated Biomass
2.6.2. Extraction and Estimation of Lipid and Biodiesel Production
2.6.3. Estimation of Carbohydrate Content
2.6.4. Estimation of the Protein Content of the Biomass
2.6.5. Fungal Fermentation of the Pigment-Free De-oiled Residual Biomass
2.6.6. Enzyme Assay for the Quantification of α-Amylase Activity
3. Results and Discussion
3.1. Variation in the Nutrients and Their Impact on the Growth
3.2. Growth-Dependent pH Variation of the Cultivation Media
3.3. Impact of Nutrition Variation on Protein Biosynthesis
3.3.1. Impact of Nutrition Variation on Carbohydrate Biosynthesis
3.3.2. Impact of Nutrition Variation on the Biosynthesis of Lipids
3.3.3. Biodiesel Properties of Wastewater Cultivated Biomass
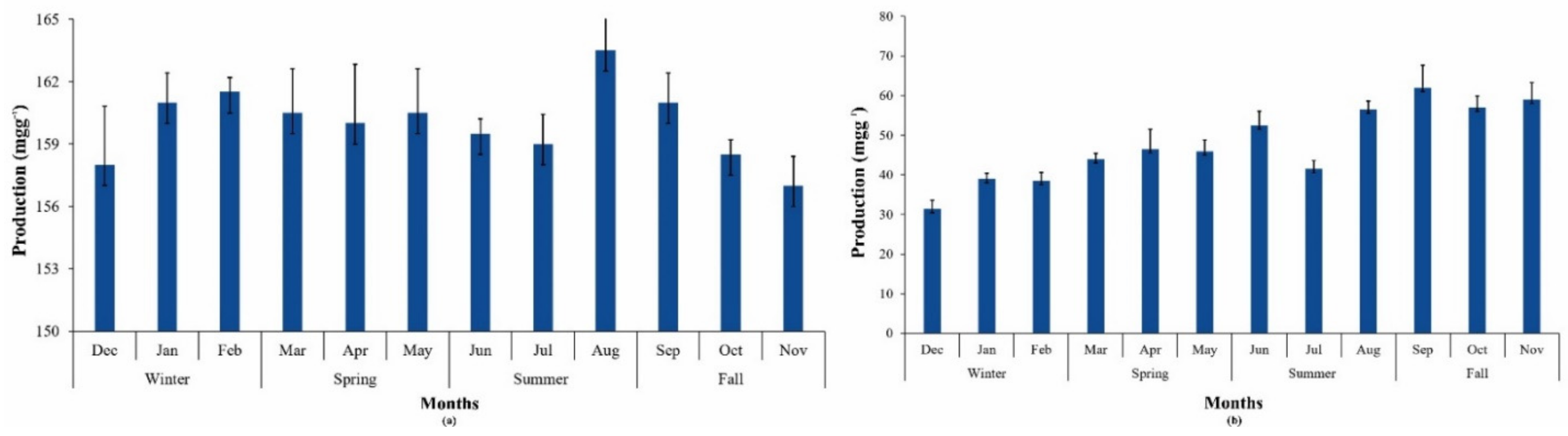
3.3.4. Phycobilin Production during Different Seasons
3.4. Wastewater Treatment Potential of Plectonema terebrans BERC10
3.5. Cascading Processing of the Biomass in Multiproduct Paradigm
3.5.1. Product 1: Phycobilins
3.5.2. Product 2: Lipids Extraction from Pigment-Free Biomass for Biodiesel Production
3.5.3. Product 3: Fermentation of the Pigment-Free Defatted Residual Biomass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, B.R.; Mathimani, T.; Sudhakar, M.; Rajendran, K.; Nizami, A.-S.; Brindhadevi, K.; Pugazhendhi, A. A state of the art review on the cultivation of algae for energy and other valuable products: Application, challenges, and opportunities. Renew. Sust. Energ. Rev. 2021, 138, 110649. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Resource recovery through bioremediation of wastewaters and waste carbon by microalgae: A circular bioeconomy approach. Environ. Sci. Pollut. Res. 2021, 28, 58837–58856. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Shahid, A.; Betenbaugh, M.J.; Liu, C.-G.; Mehmood, M.A. A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers. Manag. 2022, 256, 115360. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; De Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.; Kleinegris, D.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 9, 3036–3043. [Google Scholar] [CrossRef]
- Gifuni, I.; Pollio, A.; Safi, C.; Marzocchella, A.; Olivieri, G. Current bottlenecks and challenges of the microalgal biorefinery. Trends Biotechnol. 2019, 37, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Oswald, W.J.; Gotaas, H.; Ludwig, H.F.; Lynch, V. Algae symbiosis in oxidation ponds: III. Photosynthetic oxygenation. Sewage Ind. Wastes 1953, 25, 692–705. [Google Scholar]
- Renuka, N.; Ratha, S.K.; Kader, F.; Rawat, I.; Bux, F. Insights into the potential impact of algae-mediated wastewater beneficiation for the circular bioeconomy: A global perspective. J. Environ. Manag. 2021, 297, 113257. [Google Scholar] [CrossRef]
- Batool, M.; Shahzad, L. An analytical study on municipal wastewater to energy generation, current trends, and future prospects in South Asian developing countries (an update on Pakistan scenario). Environ. Sci. Pollut. Res. 2021, 28, 32075–32094. [Google Scholar] [CrossRef]
- Liu, J.; Pemberton, B.; Lewis, J.; Scales, P.J.; Martin, G.J. Wastewater treatment using filamentous algae—A review. Bioresour. Technol. 2020, 298, 122556. [Google Scholar] [CrossRef]
- Pancha, I.; Chokshi, K.; Mishra, S. Enhanced biofuel production potential with nutritional stress amelioration through optimization of carbon source and light intensity in Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2015, 179, 565–572. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Liu, C.-G.; Musharraf, S.G.; Siddiqui, A.J.; Khan, F.; Tarbiah, N.I.; Gull, M.; Rashid, U.; Mehmood, M.A. Characterization of a newly isolated cyanobacterium Plectonema terebrans for biotransformation of the wastewater-derived nutrients to biofuel and high-value bioproducts. J. Water Process. Eng. 2021, 39, 101702. [Google Scholar] [CrossRef]
- Afzal, M.; Rehman, K.; Shabir, G.; Tahseen, R.; Ijaz, A.; Hashmat, A.J.; Brix, H. Large-scale remediation of oil-contaminated water using floating treatment wetlands. NPJ Clean Water 2019, 2, 3. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Rausch, T. The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia 1981, 78, 237–251. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Gill, D. A micro-biuret method for estimating proteins. Anal. Biochem. 1964, 9, 401–410. [Google Scholar] [CrossRef]
- Aleem, B.; Rashid, M.H.; Zeb, N.; Saqib, A.; Ihsan, A.; Iqbal, M.; Ali, H. Random mutagenesis of super Koji (Aspergillus oryzae): Improvement in production and thermal stability of α-amylases for maltose syrup production. BMC Microbiol. 2018, 18, 200. [Google Scholar] [CrossRef]
- Tayyaba, H.; Muhammad, H.R.; Muhammad, R.J.; Asma, A. Gamma ray mediated mutagenesis of Phialocephala humicola: Effect on kinetics and thermodynamics of a-amylase production. Afr. J. Microbiol. Res. 2012, 6, 4639–4646. [Google Scholar] [CrossRef]
- Singh, J.; Thakur, I.S. Evaluation of cyanobacterial endolith Leptolyngbya sp. ISTCY101, for integrated wastewater treatment and biodiesel production: A toxicological perspective. Algal Res. 2015, 11, 294–303. [Google Scholar] [CrossRef]
- Shahid, A.; Usman, M.; Atta, Z.; Musharraf, S.G.; Malik, S.; Elkamel, A.; Shahid, M.; Alkhattabi, N.A.; Gull, M.; Mehmood, M.A. Impact of wastewater cultivation on pollutant removal, biomass production, metabolite biosynthesis, and carbon dioxide fixation of newly isolated cyanobacteria in a multiproduct biorefinery paradigm. Bioresour. Technol. 2021, 333, 125194. [Google Scholar] [CrossRef] [PubMed]
- Brar, A.; Kumar, M.; Pareek, N. Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, Y.; Gao, Y.; Zhao, H. Nutrients removal and recovery from saline wastewater by Spirulina platensis. Bioresour. Technol. 2017, 245, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Kamaraj, B.; Chi, N.T.L.; Cornejo, P. Cultivation of Nostoc sp. LS04 in municipal wastewater for biodiesel production and their deoiled biomass cellular extracts as biostimulants for Lactuca sativa growth improvement. Chemosphere 2021, 280, 130644. [Google Scholar] [CrossRef] [PubMed]
- Pena, A.C.; Agustini, C.B.; Trierweiler, L.F.; Gutterres, M. Influence of period light on cultivation of microalgae consortium for the treatment of tannery wastewaters from leather finishing stage. J. Clean. Prod. 2020, 263, 121618. [Google Scholar] [CrossRef]
- Zayadan, B.K.; Sadvakasova, A.K.; Usserbayeva, A.A.; Bolatkhan, K.; Baizhigitova, A.M.; Akmukhanova, N.R.; Sidorov, R.A.; Sinetova, M.A.; Los, D.A. Waste-free technology of wastewater treatment to obtain microalgal biomass for biodiesel production. Int. J. Hydrog. Energy. 2017, 42, 8586–8591. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, D. Biomass and lipid productivities of cyanobacteria-Leptolyngbya foveolarum HNBGU001. BioEnergy Res. 2021, 14, 278–291. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Dailianis, S.; Charalampous, N.; Stefanidou, N.; Moustaka-Gouni, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Brewery wastewater treatment using cyanobacterial-bacterial settleable aggregates. Algal Res. 2020, 49, 101957. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Tekerlekopoulou, A.G.; Vayenas, D.V. Two-step treatment of brewery wastewater using electrocoagulation and cyanobacteria-based cultivation. J. Environ. Manag. 2020, 265, 110543. [Google Scholar] [CrossRef]
- Larsdotter, K. Wastewater treatment with microalgae—A literature review. Vatten 2006, 62, 31. [Google Scholar]
- Ge, S.; Madill, M.; Champagne, P. Use of freshwater macroalgae Spirogyra sp. for the treatment of municipal wastewaters and biomass production for biofuel applications. Biomass Bioenerg. 2018, 111, 213–223. [Google Scholar] [CrossRef]
- Sutherland, D.L.; Ralph, P.J. Productivity and community response along an ammonia gradient in cultured wild marine microalgae, using wastewater-derived nutrients for cost-effective feedstock production. J. Appl. Phycol. 2021, 33, 2933–2945l. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Ma, Y.; Chen, P.; Zheng, H.; Doan, Y.T.; Liu, H.; Chen, C. Mitigating ammonia nitrogen deficiency in dairy wastewaters for algae cultivation. Bioresour. Technol. 2016, 201, 33–40. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Azimi, Y.; Yu, D.; Wu, Y.-H.; Hu, H.-Y. Effects of nitrogen and phosphorus concentrations on the growth of microalgae Scenedesmus. LX1 in suspended-solid phase photobioreactors (ssPBR). Biomass Bioenerg. 2018, 109, 47–53. [Google Scholar] [CrossRef]
- Yao, C.-H.; Ai, J.-N.; Cao, X.-P.; Xue, S. Characterization of cell growth and starch production in the marine green microalga Tetraselmis subcordiformis under extracellular phosphorus-deprived and sequentially phosphorus-replete conditions. Appl. Microbiol. Biotechnol. 2013, 97, 6099–6110. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García-Galán, M.J.; García, J. Production of polyhydroxybutyrates and carbohydrates in a mixed cyanobacterial culture: Effect of nutrients limitation and photoperiods. New Biotechnol. 2018, 42, 1–11. [Google Scholar] [CrossRef]
- Kushwaha, D.; Upadhyay, S.; Mishra, P.K. Growth of cyanobacteria: Optimization for increased carbohydrate content. Appl. Biochem. Biotechnol. 2018, 184, 1247–1262. [Google Scholar] [CrossRef]
- Arias, D.M.; Uggetti, E.; García, J. Assessing the potential of soil cyanobacteria for simultaneous wastewater treatment and carbohydrate-enriched biomass production. Algal Res. 2020, 51, 102042. [Google Scholar] [CrossRef]
- Cui, H.; Ma, H.; Chen, S.; Yu, J.; Xu, W.; Zhu, X.; Gujar, A.; Ji, C.; Xue, J.; Zhang, C. Mitigating excessive ammonia nitrogen in chicken farm flushing wastewater by mixing strategy for nutrient removal and lipid accumulation in the green alga Chlorella sorokiniana. Bioresour. Technol. 2020, 303, 122940. [Google Scholar] [CrossRef]
- Feng, X.; Walker, T.H.; Bridges, W.C.; Thornton, C.; Gopalakrishnan, K. Biomass and lipid production of Chlorella protothecoides under heterotrophic cultivation on a mixed waste substrate of brewer fermentation and crude glycerol. Bioresour. Technol. 2014, 166, 17–23. [Google Scholar] [CrossRef]
- Bolatkhan, K.; Sadvakasova, A.K.; Zayadan, B.K.; Kakimova, A.B.; Sarsekeyeva, F.K.; Kossalbayev, B.D.; Bozieva, A.M.; Alwasel, S.; Allakhverdiev, S.I. Prospects for the creation of a waste-free technology for wastewater treatment and utilization of carbon dioxide based on cyanobacteria for biodiesel production. J. Biotechnol. 2020, 324, 162–170. [Google Scholar] [CrossRef]
- Cordeiro, R.S.; Vaz, I.C.; Magalhaes, S.; Barbosa, F.A. Effects of nutritional conditions on lipid production by cyanobacteria. An. Acad. Bras. Cienc. 2017, 89, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hong-Ying, H.; Ke, G.; Ying-Xue, S. Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour. Technol. 2010, 101, 5494–5500. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pei, H.; Jiang, L.; Hou, Q.; Nie, C.; Zhang, L. Phytohormone addition coupled with nitrogen depletion almost tripled the lipid productivities in two algae. Bioresour. Technol. 2018, 247, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Pancha, I.; Chokshi, K.; George, B.; Ghosh, T.; Paliwal, C.; Maurya, R.; Mishra, S. Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour. Technol. 2014, 156, 146–154. [Google Scholar] [CrossRef]
- Yalcin, D. Growth, lipid content, and fatty acid profile of freshwater cyanobacteria Dolichospermum affine (Lemmermann) Wacklin, Hoffmann, & Komárek by using modified nutrient media. Aquac. Int. 2020, 28, 1371–1388. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Fact. 2019, 18, 178. [Google Scholar] [CrossRef]
- Nzayisenga, J.C.; Farge, X.; Groll, S.L.; Sellstedt, A. Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnol. Biofuels 2020, 13, 4. [Google Scholar] [CrossRef]
- Tsolcha, O.N.; Tekerlekopoulou, A.G.; Akratos, C.S.; Antonopoulou, G.; Aggelis, G.; Genitsaris, S.; Moustaka-Gouni, M.; Vayenas, D.V. A Leptolyngbya-based microbial consortium for agro-industrial wastewaters treatment and biodiesel production. Environ. Sci. Pollut. Res. 2018, 25, 17957–17966. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Ferrer, I.; Paániker, C.C.; Goómez-Pinchetti, J.L.; Rousseau, D.P.; Van Hulle, S.W.; Garfí, M. Natural pigments and biogas recovery from microalgae grown in wastewater. ACS Sustain. Chem. Eng. 2020, 8, 10691–10701. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef]
- Mogany, T.; Swalaha, F.M.; Kumari, S.; Bux, F. Elucidating the role of nutrients in C-phycocyanin production by the halophilic cyanobacterium Euhalothece sp. J. Appl. Phycol. 2018, 30, 2259–2271. [Google Scholar] [CrossRef]
- Aketo, T.; Hoshikawa, Y.; Nojima, D.; Yabu, Y.; Maeda, Y.; Yoshino, T.; Takano, H.; Tanaka, T. Selection and characterization of microalgae with potential for nutrient removal from municipal wastewater and simultaneous lipid production. J. Biosci. Bioeng. 2020, 129, 565–572. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xu, J.; Ma, L. Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J. Biosci. Bioeng. 2018, 126, 644–648. [Google Scholar] [CrossRef]
- Cole, A.J.; Neveux, N.; Whelan, A.; Morton, J.; Vis, M.; de Nys, R.; Paul, N.A. Adding value to the treatment of municipal wastewater through the intensive production of freshwater macroalgae. Algal res. 2016, 20, 100–109. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Zhang, H.; Ma, X.; Li, L.; Cheng, Y.; Chen, P.; Ruan, R. Growing wastewater-born microalga Auxenochlorella protothecoides UMN280 on concentrated municipal wastewater for simultaneous nutrient removal and energy feedstock production. App. Energy 2012, 98, 433–440. [Google Scholar] [CrossRef]
- Gentili, F.G. Microalgal biomass and lipid production in mixed municipal, dairy, pulp and paper wastewater together with added flue gases. Bioresour. Technol. 2014, 169, 27–32. [Google Scholar] [CrossRef]
- EUR-Lex. Commission directive 98/15/EC of 27 February 1998 amending council directive 91/271/EEC with respect to certain requirements established in annex I thereof. Off. J. Eur. Comm. 1998, 67, 29–30. Available online: http://data.europa.eu/eli/dir/1998/15/oj (accessed on 10 September 2022).
- Kamaraj, M.; Subramaniam, D. Amylase production by Aspergillus niger in submerged cultivation using cassava. J. Appl. Biol. 2020, 8, 82–87. [Google Scholar] [CrossRef]
- Hernández, M.S.; Rodríguez, M.R.; Guerra, N.P.; Rosés, R.P. Amylase production by Aspergillus niger in submerged cultivation on two wastes from food industries. J. Food Eng. 2006, 73, 93–100. [Google Scholar] [CrossRef]
- Hemalatha, M.; Sravan, J.S.; Min, B.; Mohan, S.V. Microalgae-biorefinery with cascading resource recovery design associated to dairy wastewater treatment. Bioresour. Technol. 2019, 284, 424–429. [Google Scholar] [CrossRef] [PubMed]


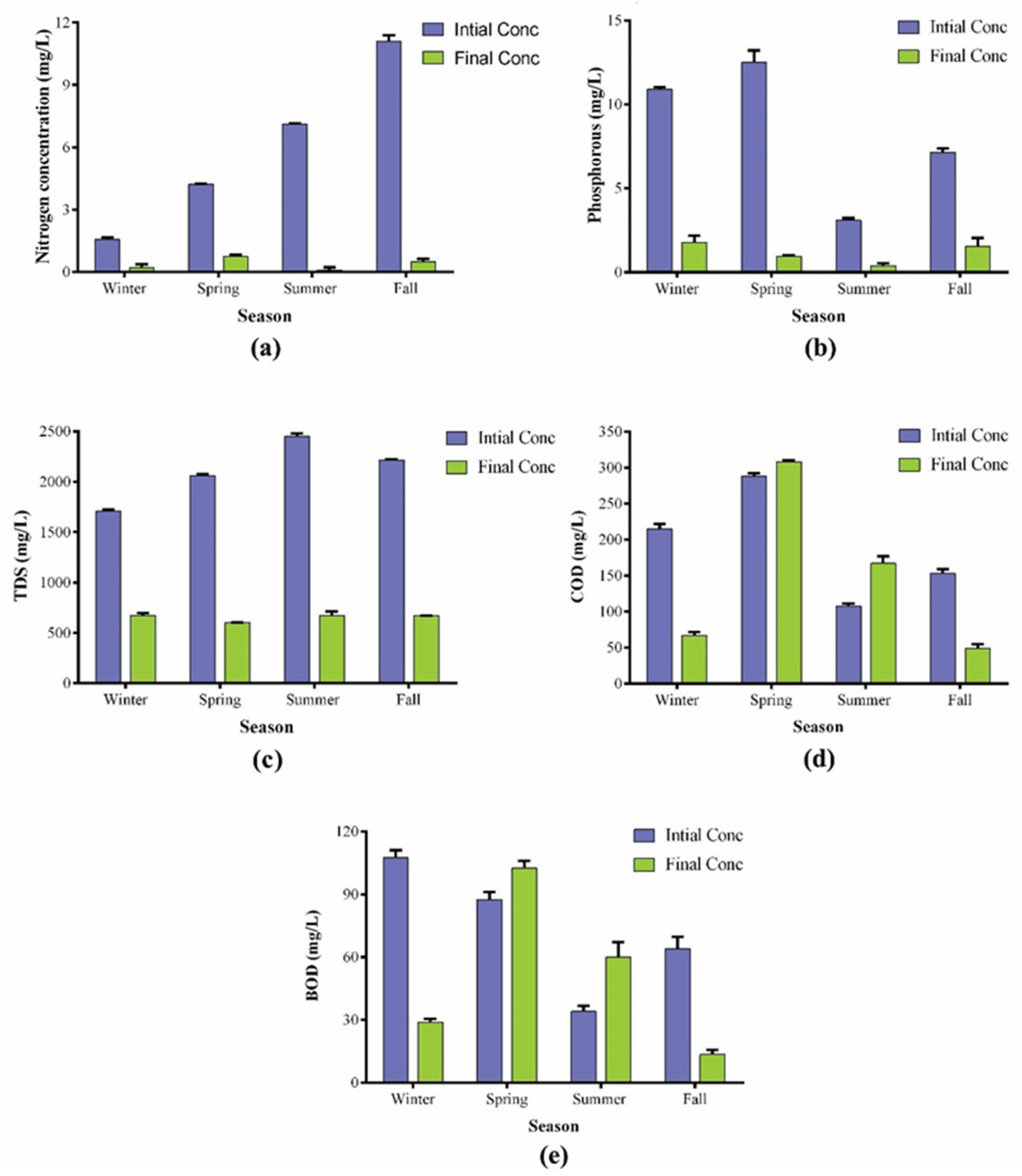

| Wastewater | pH | TDS | COD | BOD | Total Nitrogen | Total Phosphorus | NO3N | NH3-N |
|---|---|---|---|---|---|---|---|---|
| Winter | 8.6 | 1720 | 210 | 110 | 1.5 | 11 | - | - |
| Spring | 9.1 | 2070 | 291 | 90 | 4.2 | 12 | 1.7 | 0.8 |
| Summer | 8.8 | 2470 | 105 | 32 | 7.11 | 3 | 1.7 | 0.2 |
| Fall | 6.6 | 2210 | 149 | 39 | 10.9 | 7 | 4.8 | 1.8 |
| MBG11 | UWW | ASTM D6751 | European 14,214 | |
|---|---|---|---|---|
| Degree of saturation (DU) | 48.9 | 66.7 | - | - |
| Sponification value (SV) | 140.17 | 124.84 | - | - |
| Iodine value (IV) | 45 | 73.678 | - | ≤120 |
| Cetane number (CN) | 78.569 | 68.998 | >47 | >51 |
| Long chain saturated factor (LCSF) | 2.50 | 1.98 | - | - |
| Cold filter plugging point (CFPP) | −7.657 | −9.732 | - | - |
| Cloud point (CP) | −4.16 | −4.0 | - | - |
| Pour point (PP) | −10.897 | −9.987 | 3 | 3–15 |
| Allylic position equivalent (APE) | 45.987 | 65.789 | - | - |
| Bis allylic position equivalent (BAPE) | 32.955 | 38.978 | - | - |
| Oxidation stability (OS) | 6.784 | 7.986 | - | - |
| High heating value (HHV) | 24.343 | 22.103 | - | - |
| Viscosity | 2.7342 | 2.31785 | 1.9–6 | 3.5–5 |
| Density | 0.614 | 0.574 | - | 0.86–0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haider, M.N.; Liu, C.-G.; Tabish, T.A.; Balakrishnan, D.; Show, P.-L.; Qattan, S.Y.A.; Gull, M.; Mehmood, M.A. Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm. Fermentation 2022, 8, 650. https://doi.org/10.3390/fermentation8110650
Haider MN, Liu C-G, Tabish TA, Balakrishnan D, Show P-L, Qattan SYA, Gull M, Mehmood MA. Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm. Fermentation. 2022; 8(11):650. https://doi.org/10.3390/fermentation8110650
Chicago/Turabian StyleHaider, Muhammad Nabeel, Chen-Guang Liu, Tanveer A. Tabish, Deepanraj Balakrishnan, Pau-Loke Show, Shaza Yehya Abdulhamed Qattan, Munazza Gull, and Muhammad Aamer Mehmood. 2022. "Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm" Fermentation 8, no. 11: 650. https://doi.org/10.3390/fermentation8110650
APA StyleHaider, M. N., Liu, C.-G., Tabish, T. A., Balakrishnan, D., Show, P.-L., Qattan, S. Y. A., Gull, M., & Mehmood, M. A. (2022). Resource Recovery of the Wastewater-Derived Nutrients into Algal Biomass Followed by Its Cascading Processing to Multiple Products in a Circular Bioeconomy Paradigm. Fermentation, 8(11), 650. https://doi.org/10.3390/fermentation8110650









