A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite
Abstract
1. Introduction
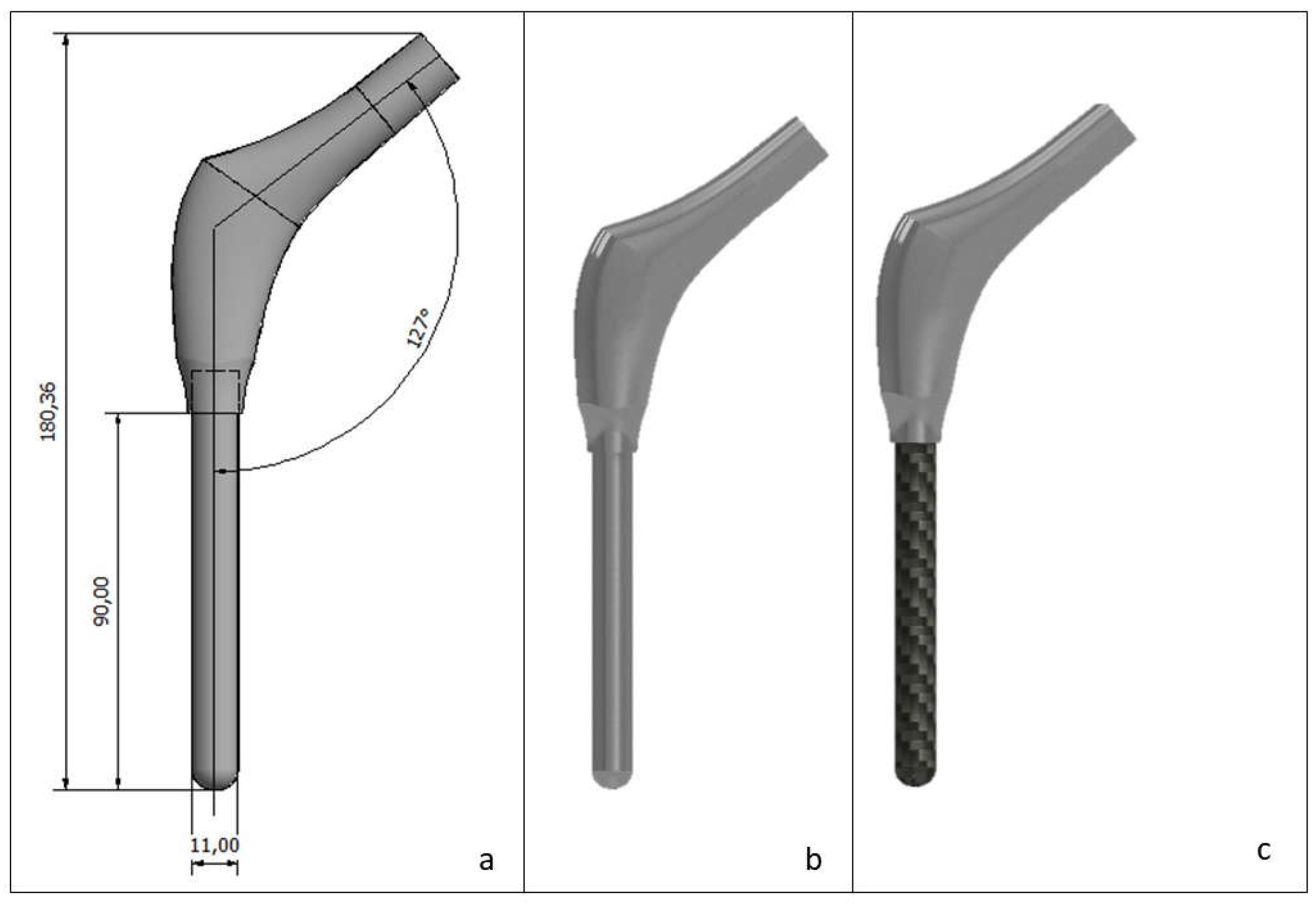
2. Materials and Methods
2.1. Meshing
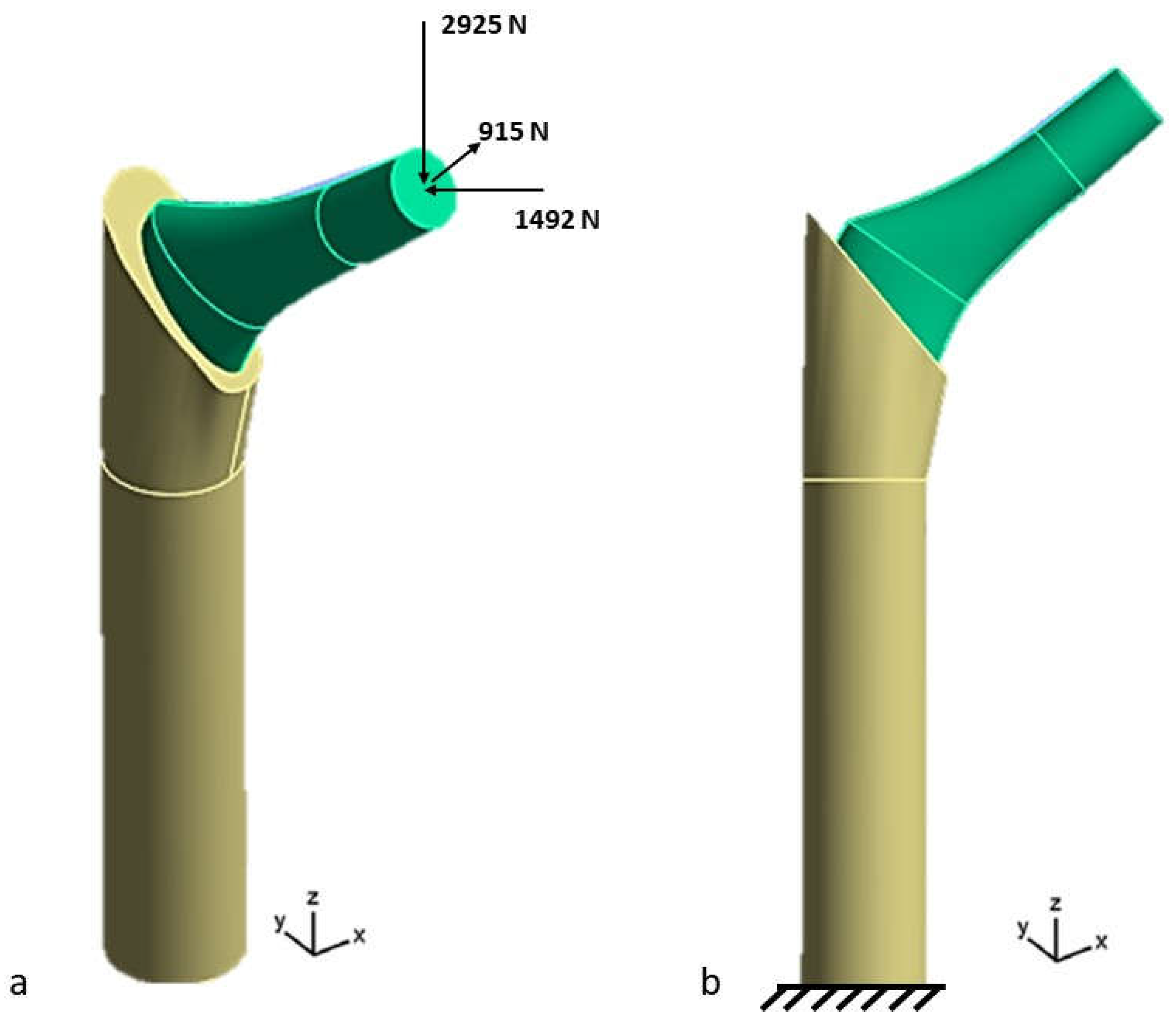
2.2. Loads and Boundary Conditions
Calculation of Stress Shielding
3. Results
Stress Shielding Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty—Over 100 years of operative history. Orthop. Rev. 2011, 3, 16. [Google Scholar]
- Ridzwan, M.I.Z.; Shuib, S.; Hassan, A.Y.; Shokri, A.A.; Mohamad Ibrahim, M.N. Problem of Stress-shielding and Improvement to the Hip Implant Designs: A Review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar] [CrossRef]
- Babaei, N.; Hannani, N.; Dabanloo, N.J.; Bahadori, S. A systematic review of the use of commercial wearable activity trackers for monitoring recovery in individuals undergoing total hip replacement surgery. Cyborg Bionic Syst. 2022, 2022, 9794641. [Google Scholar] [CrossRef]
- Duffy, S.; Gelfer, Y.; Trompeter, A.; Clarke, A.; Monsell, F. The clinical features, management options and complications of paediatric femoral fractures. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, S.; Liu, Z.; Liu, B.; Huo, J.; Li, M.; Han, Y. Clinical and radiologic outcomes in patients undergoing primary total hip arthroplasty with Collum Femoris Preserving stems: A comparison between the direct anterior approach and the posterior approach. BMC Musculoskelet. Disord. 2022, 23, 77. [Google Scholar] [CrossRef]
- Rodrigues, L.; Cornelis, F.H.; Chevret, S. Hip Fracture Prevention in Osteoporotic Elderly and Cancer Patients: An On-Line French Survey Evaluating Current Needs. Medicina 2020, 56, 397. [Google Scholar] [CrossRef] [PubMed]
- Rozell, J.C.; Donegan, D.J. Periprosthetic Femur Fractures Around a Loose Femoral Stem. J. Orthop. Trauma. 2019, 33, S10–S13. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, C.B.; Wanner, R.; Solinger, T.; Gautier, E.; Eijer, H.; Wahl, P. The different failure modes of the connecting elements of the modular hip arthroplasty revision stem Revitan. J. Mech. Behav. Biomed. Mater. 2021, 123, 104778. [Google Scholar]
- Bischoff, P.; Kramer, T.S.; Schröder, C.; Behnke, M.; Schwab, F.; Geffers, C.; Aghdassi, S.J.S. Age as a risk factor for surgical site infections: German surveillance data on total hip replacement and total knee replacement procedures 2009 to 2018. Eurosurveillance 2023, 28, 2200535. [Google Scholar] [CrossRef]
- Goodman, S.M.; Mehta, B.Y.; Mandl, L.A.; Szymonifka, J.D.; Finik, J.; Figgie, M.P.; Singh, J.A. Validation of the hip disability and osteoarthritis outcome score and knee injury and osteoarthritis outcome score pain and function subscales for use in total hip replacement and total knee replacement clinical trials. J. Arthroplast. 2020, 35, 1200–1207. [Google Scholar] [CrossRef]
- Perticarini, L.; Rossi, S.M.; Benazzo, F. Unstable total hip replacement: Why? Clinical and radiological aspects. Hip Int. 2020, 30, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ray, G.S.; Ekelund, P.; Nemes, S.; Rolfson, O.; Mohaddes, M. Changes in health-related quality of life are associated with patient satisfaction following total hip replacement: An analysis of 69,083 patients in the Swedish Hip Arthroplasty Register. Acta Orthop. 2020, 91, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Moon, N.H.; Do, M.U.; Jung, S.W.; Suh, K.T.; Shin, W.C. The use of dual mobility acetabular cups in total hip replacement reduces dislocation rates in hip dysplasia patients. Sci. Rep. 2023, 13, 22404. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, L.; Jiang, W.; Wu, W.; Ai, S.; Shen, L.; Dai, K. 3D printing hip prostheses offer accurate reconstruction, stable fixation, and functional recovery for revision total hip arthroplasty with complex acetabular bone defect. Engineering 2020, 6, 1285–1290. [Google Scholar] [CrossRef]
- Hevesi, M.; Wyles, C.C.; Rouzrokh, P.; Erickson, B.J.; Maradit-Kremers, H.; Lewallen, D.G.; Berry, D.J. Redefining the 3D topography of the acetabular safe zone: A multivariable study evaluating prosthetic hip stability. JBJS 2022, 104, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Lakstein, D.; Eliaz, N.; Levi, O.; Backstein, D.; Kosashvili, Y.; Safir, O.; Gross, A.E. Fracture of cementless femoral stems at the mid-stem junction in modular revision hip arthroplasty systems. J. Bone Jt. Surg. 2011, 93, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Cassar-Gheiti, A.J.; McColgan, R.; Kelly, M.; Cassar-Gheiti, T.M.; Kenny, P.; Murphy, C.G. Current concepts and outcomes in cemented femoral stem design and cementation techniques: The argument for a new classification system. EFORT Open Rev. 2020, 5, 241–252. [Google Scholar] [CrossRef]
- Al Zoubi, N.F.; Tarlochan, F.; Mehboob, H.; Jarrar, F. Design of titanium alloy femoral stem cellular structure for stress shielding and stem stability: Computational analysis. Appl. Sci. 2022, 12, 1548. [Google Scholar] [CrossRef]
- Zajc, J.; Moličnik, A.; Fokter, S.K. Dual modular titanium alloy femoral stem failure mechanisms and suggested clinical approaches. Materials 2021, 14, 3078. [Google Scholar] [CrossRef]
- Mavčič, B.; Antolič, V. Cementless femoral stem fixation and leg-length discrepancy after total hip arthroplasty in different proximal femoral morphological types. Int. Orthop. 2021, 45, 891–896. [Google Scholar] [CrossRef]
- Radaelli, M.; Buchalter, D.B.; Mont, M.A.; Schwarzkopf, R.; Hepinstall, M.S. A new classification system for cementless femoral stems in total hip arthroplasty. J. Arthroplast. 2023, 38, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Springer, B.D.; Hubble, M.J.; Howell, J.R.; Moskal, J.T. Cemented femoral stem fixation: Back to the future. J. Arthroplast. 2023, 38, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Buks, Y.; Wendelburg, K.L.; Stover, S.M.; Garcia-Nolen, T.C. The effects of interlocking a universal hip cementless stem on implant subsidence and mechanical properties of cadaveric canine femora. Vet. Surg. 2016, 45, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Toci, G.R.; Magnuson, J.A.; DeSimone, C.A.; Stambough, J.B.; Star, A.M.; Saxena, A. A systematic review and meta-analysis of non-database comparative studies on cemented versus uncemented femoral stems in primary elective total hip arthroplasty. J. Arthroplast. 2022, 37, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Jafari Chashmi, M.; Fathi, A.; Shirzad, M.; Jafari-Talookolaei, R.A.; Bodaghi, M.; Rabiee, S.M. Design and analysis of porous functionally graded femoral prostheses with improved stress shielding. Designs 2020, 4, 12. [Google Scholar] [CrossRef]
- López-Subías, J.; Panisello, J.J.; Mateo-Agudo, J.; Lillo-Adán, M.; Bejarano, C.; Rodríguez-Chacón, L.; Martín-Hernández, C. Bone remodelling, around an anatomical hip stem: A one year prospective study using DEXA. Rev. Española Cirugía Ortopédica y Traumatol. (Engl. Ed.) 2021, 65, 31–40. [Google Scholar] [CrossRef]
- Wolff, J. The Classic: On the Inner Architecture of Bones and its Importance for Bone Growth:(Ueber die innere Architectur der Knochen und ihre Bedeutung für die Frage vom Knochenwachsthum). Clin. Orthop. Relat. Res. 2010, 468, 1056–1065. [Google Scholar] [CrossRef]
- Frost Harold, M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar]
- Nabudda, K.; Suriyawanakul, J.; Tangchaichit, K.; Pholdee, N.; Kosuwon, W.; Wisanuyotin, T.; Sukhonthamarn, K. Identification of Flexural Modulus and Poisson’s Ratio of Fresh Femoral Bone Based on a Finite Element Model. Int. J. Online Biomed. Eng. 2022, 16. [Google Scholar] [CrossRef]
- Barui, S.; Chatterjee, S.; Mandal, S.; Kumar, A.; Basu, B. Microstructure and compression properties of 3D powder printed Ti-6Al-4V scaffolds with designed porosity: Experimental and computational analysis. Mater. Sci. Engineering. C Mater. Biol. Appl. 2017, 70, 812–823. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Hwang, K.L.; Amanatullah, D.F.; Huddleston, J.I., III; Maloney, W.J.; Goodman, S.B. Revision hip arthroplasty using a modular, cementless femoral stem: Long-term follow-up. J. Arthroplast. 2023, 38, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, C.; Mashadi, M.; Parvizi, J.; Austin, M.S.; Hozack, W.J. Modular femoral stems for revision total hip arthroplasty. Clin. Orthop. Relat. Res. 2011, 469, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Sinico, M.; Vrancken, B.; Denis, K. Investigation of the laser powder bed fusion manufacturing process and quasi-static behaviour of Ti6Al4V Voronoi structures. J. Mater. Process. Technol. 2024, 328, 118410. [Google Scholar] [CrossRef]
- Thomas, J.; Alsaleh, N.A.; Ahmadein, M.; Elfar, A.A.; Farouk, H.A.; Essa, K. Graded cellular structures for enhanced performance of additively manufactured orthopaedic implants. Int. J. Adv. Manuf. Technol. 2024, 130, 1887–1900. [Google Scholar] [CrossRef]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low Stiffness Porous Ti Structures for Load–Bearing Implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Bougherara, H.; Zdero, R.; Dubov, A.; Shah, S.; Khurshid, S.; Schemitsch, E.H. A preliminary biomechanical study of a novel carbon–fibre hip implant versus standard metallic hip implants. Med. Eng. Phys. 2011, 33, 121–128. [Google Scholar] [CrossRef]
- Gok, M.G. Static, fatigue and stress-shielding analysis of the use of different PEEK based materials as hip stem implants. Int. Polym. Process. 2022, 37, 152–163. [Google Scholar] [CrossRef]
- Ortega, P.C.; Medeiros, W.B., Jr.; Moré, A.D.O.; Vasconcelos, R.F.; da Rosa, E.; Roesler, C.R.M. Failure analysis of a modular revision total HIP arthroplasty femoral stem fractured in vivo. Eng. Fail. Anal. 2020, 114, 104591. [Google Scholar] [CrossRef]
- Chethan, K.N.; Bhat, N.S.; Zuber, M.; Shenoy, B.S. Finite element analysis of hip implant with varying in taper neck lengths under static loading conditions. Comput. Methods Programs Biomed. 2021, 208, 106273. [Google Scholar] [CrossRef]
- Alfonzo-González, M.A.; Cerrolaza, M. Simulación y análisis computacional del conducto medular del fémur en presencia de una prótesis de cadera. Rev. Fac. Ing. Univ. Cent. Venez. 2013, 28, 25–34. [Google Scholar]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Trentadue, B. Evaluation of Rotational Stability and Stress Shielding of a Stem Optimized for Hip Replacements—A Finite Element Study. Prosthesis 2023, 5, 678–693. [Google Scholar] [CrossRef]
- Xe, G. Mechanical properties of cortical bone and cancellous bone tissue. Bone Mech. Handb. 2001, 10, 1–23. [Google Scholar]
- Darwich, A.; Nazha, H.; Daoud, M. Effect of coating materials on the fatigue behavior of hip implants: A three-dimensional finite element analysis. J. Appl. Comput. Mech. 2020, 6, 284–295. [Google Scholar]
- Chen, W.-C.; Lai, Y.-S.; Cheng, C.-K.; Chang, T.-K. A cementless, proximally fixed anatomic femoral stem induces high micromotion with nontraumatic femoral avascular necrosis: A finite element study. J. Orthop. Transl. 2014, 2, 149–156. [Google Scholar] [CrossRef]
- Weinans, H.; Sumner, D.R.; Igloria, R.; Natarajan, R.N. Sensitivity of periprosthetic stress-shielding to load and the bone density–modulus relationship in subject-specific finite element models. J. Biomech. 2000, 33, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Trentadue, B.; De Giosa, G.; Solarino, G. Topology optimization of a femoral stem in titanium and carbon to reduce stress shielding with the FEM method. J. Compos. Sci. 2023, 7, 298. [Google Scholar] [CrossRef]
- Stratton-Powell, A.A.; Pasko, K.M.; Brockett, C.L.; Tipper, J.L. The biologic response 423 to polyetheretherketone (PEEK) wear particles in total joint replacement: A systematic review. Clin. Orthop. Relat. Res. 2016, 474, 2394–2404. [Google Scholar] [CrossRef]
- Chua, C.Y.; Liu, H.C.; Di Trani, N.; Susnjar, A.; Ho, J.; Scorrano, G.; Rhudy, J.; Sizovs, A.; Lolli, G.; Hernandez, N.; et al. Carbon fiber reinforced polymers for implantable medical 426 devices. Biomaterials 2021, 271, 120719. [Google Scholar] [CrossRef]
- Nakahara, I.; Takao, M.; Bandoh, S.; Bertollo, N.; Walsh, W.R.; Sugano, N. In vivo implant fixation of carbon fiber-reinforced PEEK hip prostheses in an ovine model. J. Orthop. Res. 2013, 31, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Hacking, S.A.; Pauyo, T.; Lim, L.; Legoux, J.G.; Bureau, M.N. Tissue response to the components of a hydroxyapatite coated composite femoral implant. J. Biomed. Mater. Res. 2010, 94, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Scotchford, C.A.; Garle, M.J.; Batchelor, J.; Bradley, J.; Grant, D.M. Use of a novel carbon fibre composite material for the femoral stem component of a THR system: In vitro biological assessment. Biomaterials 2003, 24, 4871–4879. [Google Scholar] [PubMed]
- Darwich, A.; Nazha, H.; Abbas, W. Numerical study of stress shielding evaluation of hip implant stems coated with composite (carbon/PEEK) and polymeric (PEEK) coating materials. Biomed. Res. 2019, 30, 169–174. [Google Scholar] [CrossRef]
- Akay, M.; Aslan, N. Numerical and experimental stress analysis of a polymeric composite hip joint prosthesis. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1996, 31, 167–182. [Google Scholar] [CrossRef]
- Caouette, C.; Yahia, L.; Bureau, M. Reduced stress shielding with limited micromotions using a carbon fibre composite biomimetic hip stem: A finite element model. Proc. Inst. Mech. Eng. H 2011, 225, 907–919. [Google Scholar] [CrossRef] [PubMed]
- Allcock, S.; Ali, M.A. Early failure of a carbon-fiber composite femoral component. J. Arthroplast. 1997, 12, 356–358. [Google Scholar] [CrossRef]
- Palmisano, A.C.; Nathani, A.; Weber, A.E.; Blaha, J.D. Femoral Neck Modularity: A Bridge Too Far-Affirms. Semin. Arthroplast. 2014, 25, 93–98. [Google Scholar] [CrossRef]
- Blakey, C.M.; Eswaramoorthy, V.K.; Hamilton, L.C.; Biant, L.C.; Field, R.E. Mid-Term Results of the Modular ANCA-Fit Femoral Component in Total Hip Replacement. J. Bone Jt. Surg. Ser. B 2009, 91, 1561–1565. [Google Scholar] [CrossRef]
- Atwood, S.A.; Patten, E.W.; Bozic, K.J.; Pruitt, L.A.; Ries, M.D. Corrosion-Induced Fracture of a Double-Modular Hip Prosthesis: A Case Report. J. Bone Jt. Surg. Ser. A 2010, 92, 1522–1525. [Google Scholar] [CrossRef]
- Bennett, D.B.; Hill, J.C.; Dennison, J.; O’Brien, S.; Mantel, J.L.; Isaac, G.H.; Beverland, D.E. Metal-carbon fiber composite femoral stems in hip replacements: A randomized controlled parallel-group study with mean ten-year follow-up. JBJS 2014, 96, 2062–2069. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Mali, S.; Urban, R.M.; Silverton, C.D.; Jacobs, J.J. In Vivo Oxide-Induced Stress Corrosion Cracking of Ti-6Al-4V in a Neck-Stem Modular Taper: Emergent Behavior in a New Mechanism of in Vivo Corrosion. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2012, 100B, 584–594. [Google Scholar] [CrossRef]
- Lanzutti, A.; Andreatta, F.; Rossi, L.; Di Benedetto, P.; Causero, A.; Magnan, M.; Fedrizzi, L. Corrosion Fatigue Failure of a High Carbon CoCrMo Modular Hip Prosthesis: Failure Analysis and Electrochemical Study. Eng. Fail. Anal. 2019, 105, 856–868. [Google Scholar] [CrossRef]







| Material | Modulus of Elasticity E (GPa) | Poisson’s Ratio |
|---|---|---|
| Cortical bone | 16.7 | 0.3 |
| Trabecular bone | 0.155 | 0.3 |
| Ti-6Al-4V | 110 | 0.3 |
| Plane | Elastic Modulus E (GPa) | Shear Modulus G (GPa) | Poisson’s Ratio |
|---|---|---|---|
| xx | 4 | - | |
| yy | 9.8 | - | |
| zz | 9.8 | - | |
| xy | - | 3.5 | 0.3 |
| yz | - | 3 | 0.3 |
| xz | - | 3.5 | 0.3 |
 | Gruen Zone | Prosthesis with CFRPC Distal Part: Von Mises Stress (MPa) | Prosthesis with Ti-6Al-4V Distal Part: Von Mises Stress (MPa) | Stress Shielding Factor |
| 1 | 4.32 | 3.21 | 1.34 | |
| 2 | 25.14 | 31.41 | 0.80 | |
| 3 | 38.31 | 45.21 | 0.84 | |
| 4 | 67.25 | 78.25 | 0.85 | |
| 5 | 42.31 | 48.32 | 0.87 | |
| 6 | 26.75 | 73.25 | 0.36 | |
| 7 | 17.15 | 12.52 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Solarino, G.; Giannini, G.; De Giosa, G.; Tucci, M.; Trentadue, B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. J. Compos. Sci. 2024, 8, 254. https://doi.org/10.3390/jcs8070254
Ceddia M, Solarino G, Giannini G, De Giosa G, Tucci M, Trentadue B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. Journal of Composites Science. 2024; 8(7):254. https://doi.org/10.3390/jcs8070254
Chicago/Turabian StyleCeddia, Mario, Giuseppe Solarino, Giorgio Giannini, Giuseppe De Giosa, Maria Tucci, and Bartolomeo Trentadue. 2024. "A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite" Journal of Composites Science 8, no. 7: 254. https://doi.org/10.3390/jcs8070254
APA StyleCeddia, M., Solarino, G., Giannini, G., De Giosa, G., Tucci, M., & Trentadue, B. (2024). A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. Journal of Composites Science, 8(7), 254. https://doi.org/10.3390/jcs8070254







