Chimeric Antigen Receptor (CAR) Cell Therapy for Cancers
A special issue of Cancers (ISSN 2072-6694). This special issue belongs to the section "Cancer Therapy".
Deadline for manuscript submissions: closed (5 September 2023) | Viewed by 2177
Special Issue Editor
Special Issue Information
Dear Colleagues,
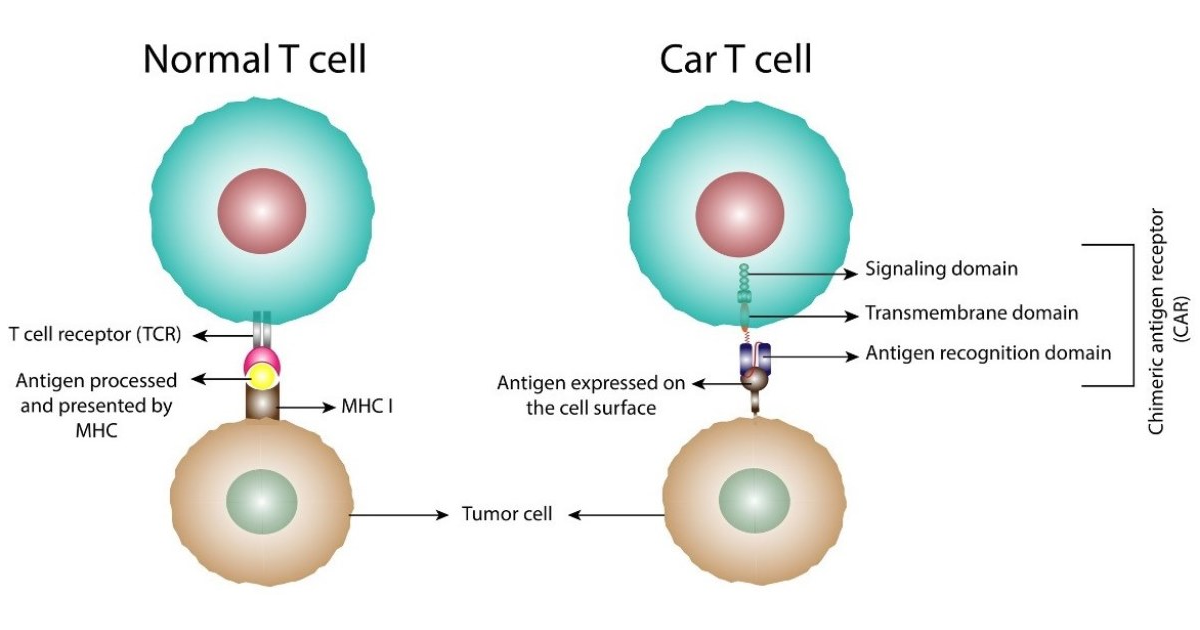
In the last decade, chimeric antigen receptor (CAR)-T cells, engineered from patients’ (autologous) T cells, have become established as an effective therapy in several haematological cancers, including B-cell precursor acute lymphoblastic leukemia (ALL), diffuse large B-cell lymphoma (DLBCL), B-cell lymphoma, mantle cell lymphoma, and multiple myeloma. More recently, investigations are ongoing to broaden the indications for CAR-T cells, to include solid tumors. In addition, similar technology is now being applied to engineer CAR NK cells and CAR macrophages for potential use, especially in solid tumors. As these technologies mature and the possibilities of allogeneic (off-the-shelf) CAR cells emerge, the prospects of a true revolution in cancer treatment with cell therapies look good indeed. Nevertheless, there are many obstacles to overcome before this type of treatment can fulfil its potential. These include complexities in manufacturing (with high expense and potential mishaps) and management of serious adverse effects such as cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS). Strategies are being developed to overcome the efficacy bottlenecks (including poor tissue penetration, manufacturing and costs issues, and mortality caused by serious adverse effects. This Special Issue will capture all developments in the broad field of CARs as significant cell therapies for cancers.
Prof. Dr. Bams Abila
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- chimeric antigen receptor (CAR)
- CAR-T
- CAR-NK
- CAR-macrophages
- hematological and solid cancers






