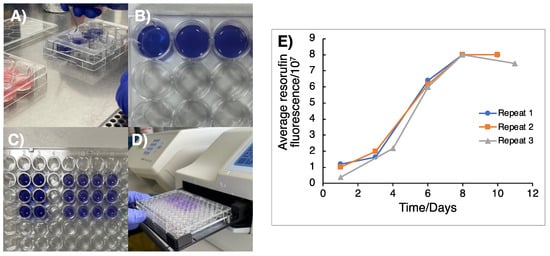
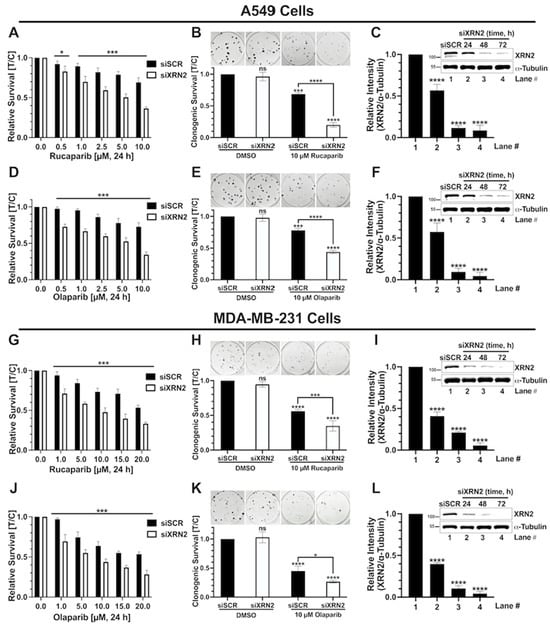
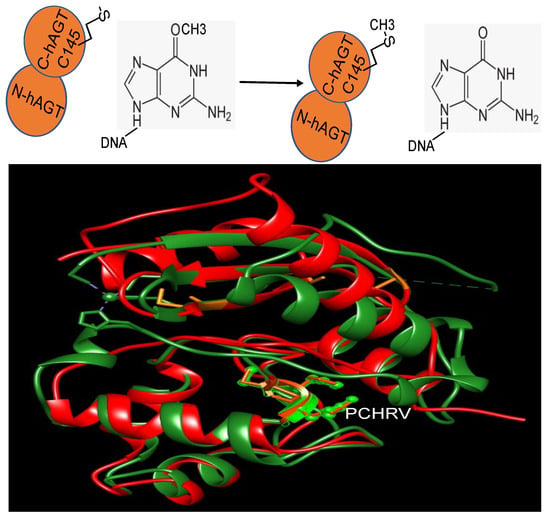
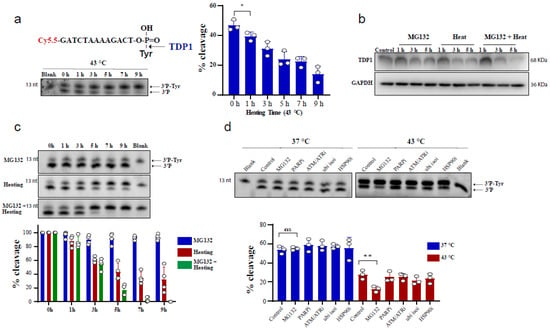
Genome Maintenance in Cancer Biology and Therapy (Closed)
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Molecular Cancer Biology".
Viewed by 16188Editor
Interests: genome stability; DNA damage and repair; DNA replication; cell cycle checkpoints; cancer biology and therapy
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Genome instability is a defined hallmark of cancer, and is an important mechanism in tumour development, progression, heterogeneity and drug resistance; the latter two being two common barriers to effective treatment. Genome instability in tumour cells often arises through heightened DNA damage and/or replication stress; a respective consequence of increased metabolic processes that produce DNA damaging by-products, and an increased proliferative state due to the action of activated oncogenes.
To preserve genomic integrity, human cells have developed interconnected genome maintenance mechanisms that facilitate an orchestration of DNA damage response pathways, DNA repair mechanisms, cell cycle checkpoints and cell division processes. The many cancer predisposing human diseases that are associated with underlying mutations in/dysregulation of key proteins within these pathways highlights the importance of genome stability mechanisms in the suppression of tumour development and progression.
In addition to a deeper understanding of cancer biology, the study of genome maintenance mechanisms and their co-ordinated regulation has revealed new therapeutic targets to improve the effectiveness of existing radio-/chemotherapy regimes, as well as identifying exploitable tumour-selective vulnerabilities around which both novel or repurposed therapeutic strategies can be developed. Furthering our understanding of genome maintenance mechanisms is therefore vital to help understand how such processes become dysfunctional in cancer development/progression, and to identify new targets and/or biomarkers to develop new therapeutic strategies to improve the clinical management of these disease and improve patient survival.
This new Topic section of Cancers therefore aims to publish new research articles and timely reviews on aspects of genome maintenance mechanisms that either play a role in cancer development and progression or reveal new therapeutic opportunities to better treat this disease.
Dr. Spencer Collis
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.