The Effects of Patient-Reported Outcome Screening on the Survival of People with Cancer: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Eligibility
2.3. Population Eligibility
2.4. Selection Process
2.5. Data Extraction
2.6. Risk of Bias Assessment
2.7. Data Synthesis
2.8. Patient and Public Involvement
3. Results
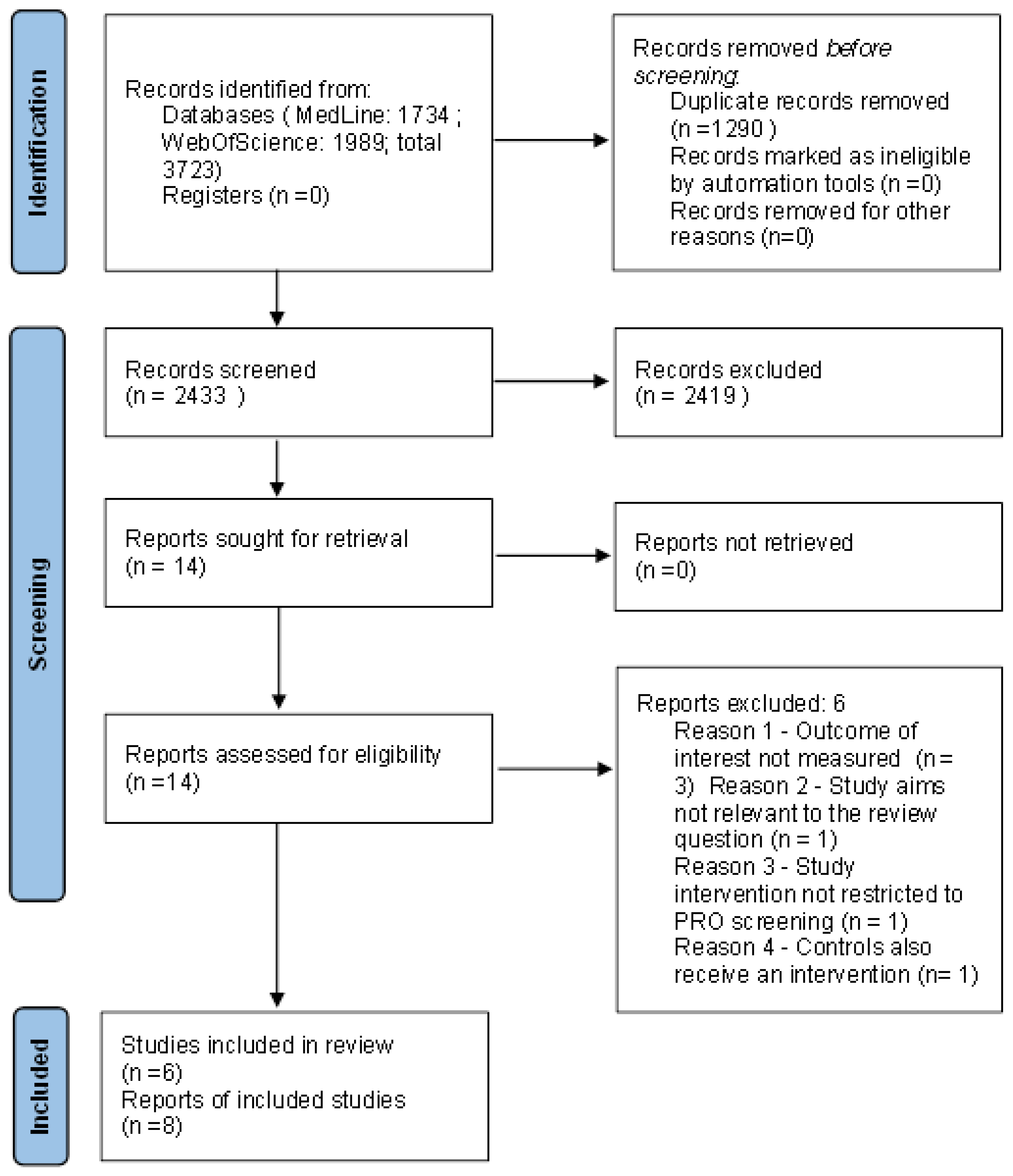
3.1. Study Selection
3.2. Study Characteristics
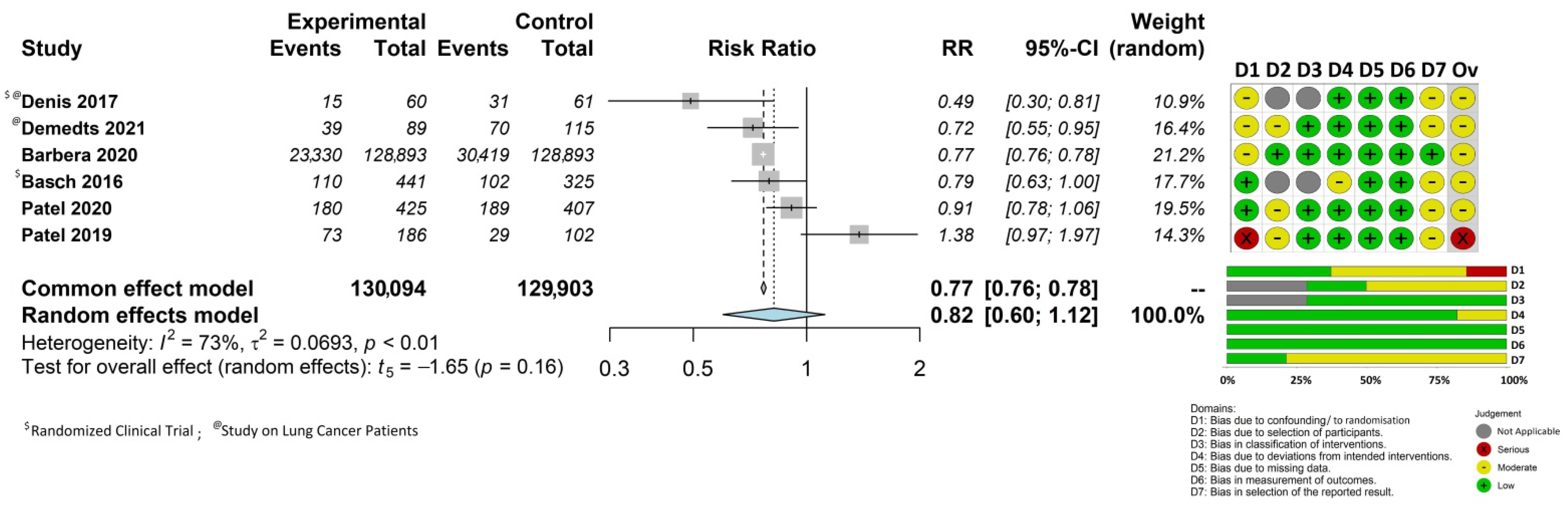
3.3. Impact of Patient-Reported Outcome Monitoring on Overall Survival
3.4. Quality of Included Studies
3.5. Further Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-reported-outcome-measures-use-medical-product-development-support-labeling-claims (accessed on 11 October 2022).
- Chen, J.; Ou, L.; Hollis, S.J. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv. Res. 2013, 13, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nic Giolla Easpaig, B.; Tran, Y.; Bierbaum, M.; Arnolda, G.; Delaney, G.P.; Liauw, W.; Ward, R.L.; Olver, I.; Currow, D.; Girgis, A.; et al. What are the attitudes of health professionals regarding patient reported outcome measures (PROMs) in oncology practice? A mixed-method synthesis of the qualitative evidence. BMC Health Serv. Res. 2020, 20, 102. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.; Sequeira, T.; Gonçalves, J.; Lopes Ferreira, P. Patient reported outcomes in oncology: Changing perspectives-a systematic review. Health Qual. Life Outcomes 2022, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Gallo, C.; Leighl, N.B.; Piccirillo, M.C.; Daniele, G.; Nuzzo, F.; Gridelli, C.; Gebbia, V.; Ciardiello, F.; De Placido, S.; et al. Symptomatic toxicities experienced during anticancer treatment: Agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 2015, 33, 910–915. [Google Scholar] [CrossRef]
- Yang, L.Y.; Manhas, D.S.; Howard, A.F.; Olson, R.A. Patient-reported outcome use in oncology: A systematic review of the impact on patient-clinician communication. Support Care Cancer 2018, 26, 41–60. [Google Scholar] [CrossRef]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef]
- National Cancer Institute, National Institutes of Health. US Department of Health and Human Services: Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://evs.nci.nih.gov/ftp1/CTCAE/About.html (accessed on 11 October 2022).
- Caminiti, C.; Bryce, J.; Riva, S.; Ng, D.; Diodati, F.; Iezzi, E.; Sparavigna, L.; Novello, S.; Porta, C.; Del Mastro, L.; et al. Cultural adaptation of the Italian version of the Patient-Reported Outcomes Common Terminology Criteria for Adverse Event (PRO-CTCAE®). Tumori J. 2022, 2022, 3008916221099558. [Google Scholar] [CrossRef]
- Howell, D.; Molloy, S.; Wilkinson, K.; Green, E.; Orchard, K.; Wang, K.; Liberty, J. Patient-reported outcomes in routine cancer clinical practice: A scoping review of use, impact on health outcomes, and implementation factors. Ann. Oncol. 2015, 26, 1846–1858. [Google Scholar] [CrossRef]
- Graupner, C.; Kimman, M.L.; Mul, S.; Slok, A.H.M.; Claessens, D.; Kleijnen, J.; eDirksen, C.D.; Breukink, S.O. Patient outcomes, patient experiences and process indicators associated with the routine use of patient-reported outcome measures (PROMs) in cancer care: A systematic review. Support Care Cancer 2021, 29, 573–593. [Google Scholar] [CrossRef]
- Lizán, L.; Pérez-Carbonell, L.; Comellas, M. Additional Value of Patient-Reported Symptom Monitoring in Cancer Care: A Systematic Review of the Literature. Cancers 2021, 13, 4615. [Google Scholar] [CrossRef]
- International Prospective Register of Systematic Reviews (PROSPERO). PROSPERO. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 11 October 2022).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Rayyan–Intelligent Systematic Review. Available online: https://www.rayyan.ai/ (accessed on 11 October 2022).
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med Res. 2020, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Veroniki, A.A.; Jackson, D.; Viechtbauer, W.; Bender, R.; Bowden, J.; Knapp, G.; Kuss, O.; Higgins, J.P.; Langan, D.; Salanti, G. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 2016, 7, 55–79. [Google Scholar] [CrossRef] [Green Version]
- Axfors, C.; Janiaud, P.; Schmitt, A.M.; Van’t Hooft, J.; Smith, E.R.; Haber, N.A.; Abayomi, A.; Abduljalil, M.; Abdulrahman, A.; Acosta-Ampudia, Y.; et al. Association between convalescent plasma treatment and mortality in COVID-19: A collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect. Dis. 2021, 21, 1170. [Google Scholar] [CrossRef]
- IntHout, J.; Ioannidis, J.P.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Brabaharan, S.; Veettil, S.K.; Kaiser, J.E.; Raja Rao, V.R.; Wattanayingcharoenchai, R.; Maharajan, M.; Insin, P.; Talungchit, P.; Anothaisintawee, T.; Thakkinstian, A.; et al. Association of Hormonal Contraceptive Use With Adverse Health Outcomes: An Umbrella Review of Meta-analyses of Randomized Clinical Trials and Cohort Studies. JAMA Netw. Open 2022, 5, e2143730. [Google Scholar] [CrossRef]
- Partlett, C.; Riley, R.D. Random effects meta-analysis: Coverage performance of 95% confidence and prediction intervals following REML estimation. Stat. Med. 2017, 36, 301–317. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Schwarzer, G.; Carpenter, J.R.; Rücker, G. Metaanalysis with R; Springer: Berlin/Heidelberg, Germany, 2015; Volume 4784. [Google Scholar]
- De Rooij, B.H.; Ezendam, N.P.M.; Mols, F.; Vissers, P.A.J.; Thong, M.S.Y.; Vlooswijk, C.C.P.; Oerlemans, S.; Husson, O.; Horevoorts, N.J.E.; van de Poll-Franse, L.V. Cancer survivors not participating in observational patient-reported outcome studies have a lower survival compared to participants: The population-based PROFILES registry. Qual. Life Res. 2018, 27, 3313–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geerse, O.P.; Hoekstra-Weebers, J.E.; Stokroos, M.H.; Burgerhof, J.G.; Groen, H.J.; Kerstjens, H.A.; Hiltermann, T.J. Structural distress screening and supportive care for patients with lung cancer on systemic therapy: A randomised controlled trial. Eur. J. Cancer 2017, 72, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.M.; Dunn, R.L.; Wittmann, D.; Montgomery, J.S.; Hollingsworth, J.M.; Miller, D.C.; Hollenbeck, B.K.; Wei, J.T.; Montie, J.E. Quality of life and satisfaction among prostate cancer patients followed in a dedicated survivorship clinic. Cancer 2015, 121, 1484–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschel, L.; Richter, S.; Kopp, H.G.; Kasper, B.; Kunitz, A.; Grünwald, V.; Kessler, T.; Chemnitz, J.M.; Pelzer, U.; Schuler, U.; et al. Quality of life and added value of a tailored palliative care intervention in patients with soft tissue sarcoma undergoing treatment with trabectedin: A multicentre, cluster-randomised trial within the German Interdisciplinary Sarcoma Group (GISG). BMJ Open 2020, 10, e035546. [Google Scholar] [CrossRef]
- Oerlemans, S.; Arts, L.P.J.; Kieffer, J.M.; Prins, J.; Hoogendoorn, M.; van der Poel, M.; Koster, A.; Lensen, C.; Stevens, W.B.C.; Issa, D.; et al. Web-Based Return of Individual Patient-Reported Outcome Results Among Patients With Lymphoma: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e27886. [Google Scholar] [CrossRef]
- Skovlund, P.C.; Vind Thaysen, H.; Schmidt, H.; Alsner, J.; Hjollund, N.H.; Lomborg, K.; Nielsen, B.K. Effect of patient-reported outcomes as a dialogue-based tool in cancer consultations on patient self-management and health-related quality of life: A clinical, controlled trial. Acta Oncol. 2021, 60, 1668–1677. [Google Scholar] [CrossRef]
- Barbera, L.; Sutradhar, R.; Seow, H.; Mittmann, N.; Howell, D.; Earle, C.C.; Li, Q.; Thiruchelvam, D. The impact of routine Edmonton Symptom Assessment System (ESAS) use on overall survival in cancer patients: Results of a population-based retrospective matched cohort analysis. Cancer Med. 2020, 9, 7107–7115. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef]
- Demedts, I.; Himpe, U.; Bossuyt, J.; Anthoons, G.; Bode, H.; Bouckaert, B.; Carron, K.; Dobbelaere, S.; Mariën, H.; Van Haecke, P.; et al. Clinical implementation of value based healthcare: Impact on outcomes for lung cancer patients. Lung Cancer 2021, 162, 90–95. [Google Scholar] [CrossRef]
- Denis, F.; Lethrosne, C.; Pourel, N.; Molinier, O.; Pointreau, Y.; Domont, J.; Bourgeois, H.; Senellart, H.; Trémolières, P.; Lizée, T.; et al. Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients. J. Natl. Cancer Inst. 2017, 109, djx029. [Google Scholar] [CrossRef]
- Patel, M.I.; Ramirez, D.; Agajanian, R.; Agajanian, H.; Bhattacharya, J.; Bundorf, K.M. Lay Health Worker-Led Cancer Symptom Screening Intervention and the Effect on Patient-Reported Satisfaction, Health Status, Health Care Use, and Total Costs: Results From a Tri-Part Collaboration. JCO Oncol. Pract. 2020, 16, e19–e28. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.I.; Ramirez, D.; Agajanian, R.; Agajanian, H.; Coker, T. Association of a Lay Health Worker Intervention With Symptom Burden, Survival, Health Care Use, and Total Costs Among Medicare Enrollees With Cancer. JAMA Netw. Open 2020, 3, e201023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017, 318, 197–198. [Google Scholar] [CrossRef] [Green Version]
- Denis, F.; Basch, E.; Septans, A.L.; Bennouna, J.; Urban, T.; Dueck, A.C.; Letellier, C. Two-Year Survival Comparing Web-Based Symptom Monitoring vs. Routine Surveillance Following Treatment for Lung Cancer. JAMA 2019, 321, 306–307. [Google Scholar] [CrossRef] [Green Version]
- Takvorian, S.U.; Anderson, R.T.; Gabriel, P.E.; Poznyak, D.; Lee, S.; Simon, S.; Barrett, K.; Shulman, L.N. Real-World Adherence to Patient-Reported Outcome Monitoring as a Cancer Care Quality Metric. JCO Oncol. Pract. 2022, 18, e1454–e1465. [Google Scholar] [CrossRef]
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Hassett, M.J.; Wong, S.; Osarogiagbon, R.U.; Bian, J.; Dizon, D.S.; Jenkins, H.H.; Uno, H.; Cronin, C.; Schrag, D.; SIMPRO Co-Investigators. Implementation of patient-reported outcomes for symptom management in oncology practice through the SIMPRO research consortium: A protocol for a pragmatic type II hybrid effectiveness-implementation multi-center cluster-randomized stepped wedge trial. Trials 2022, 23, 506. [Google Scholar] [CrossRef]
- Basch, E.; Schrag, D.; Henson, S.; Jansen, J.; Ginos, B.; Stover, A.M.; Carr, P.; Spears, P.A.; Jonsson, M.; Deal, A.M.; et al. Effect of Electronic Symptom Monitoring on Patient-Reported Outcomes Among Patients With Metastatic Cancer: A Randomized Clinical Trial. JAMA 2022, 327, 2413–2422. [Google Scholar] [CrossRef]
| First Author | Year | Study Design | Country | Single Center or Multicenter | No. of Patients | Age (Years) | Sex (% Female) | Cancer Type | Phase of Care (Active Treatment vs. Follow-Up) | Intervention vs. Control | PRO Instrument Used for Intervention | Mode of Administration | Follow-up Length for Survival | Survival as Primary or Secondary Outcome? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barbera | 2020 | Population-based retrospectively matched cohort analysis | Canada | Multicenter | 257,786 (128,893 patients with ESAS exposure matched to 128,893 patients without ESAS exposure) | Mean: 64 SD: 13 | 47.8% | Various | Any | 12-month telephonic screening: high-risk patients were called weekly and low-risk patients were called monthly. Historical controls received usual cancer care without standardized symptom screening or management. | Edmonton Symptom Assessment System (ESAS) | Electronic (Touch screen at the center) | 5 years (median: 1.4) | Primary |
| Basch | 2016 | Randomized controlled trial | USA | Single center | 766 (441 intervention vs. 325 control) |
Median: 61 Range: 26–91 | 58% | Advanced solid tumors: metastatic breast, genitourinary, gynecologic or lung cancers | Active treatment | Patients were randomly assigned to either report 12 common symptoms via tablet computers or receive usual care consisting of symptom monitoring at the discretion of clinicians. Those with home computers received weekly email prompts to report symptoms between visits. Treating physicians received symptom printouts at visits and nurses received email alerts when participants reported severe or worsening symptoms. | Symptom Tracking and Reporting (STAR) | Electronic (web-based) | Median follow-up of 7 years (interquartile range 6.5–7.8) $ | Secondary |
| Demedts | 2021 | Non-randomized controlled study | Belgium | Single center | 204 stage IV non-small cell lung cancer patients (89 intervention vs. 115 control) | Median: 66 Range: 32–88 | 24% | Lung cancer | Active treatment | Patients were invited by email every week to report on the side effects of their treatment. Feedback loops were created with automatically triggered electronic alerts to the care team when a predefined threshold of symptoms was reached. When patients refused, usual care was offered. | Items from the Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) library | Electronic (email reminders and digital reporting) | 4 years | Secondary |
| Denis | 2017 | Randomized controlled trial | France | Multicenter | 133 patients enrolled: 12 deemed ineligible after randomization and 121 retained in the intent-to-treat analysis (60 intervention vs. 61 control) | Median: 64.5 Range: 35.7–88.1 | 33% | Advanced-stage lung cancer | Active treatment | Personalized follow-up strategy based on 12 symptoms that were self-scored weekly and transmitted to the oncologist. Clinical follow-ups in both arms included oncology visits at least every 3 months. | e-Follow-up Application (e-FAP) | Electronic (web-based) | 2 years * | Primary |
| Patel | 2019 | Non-randomized study with historical control | USA | Single center | 288 (186 intervention vs. 102 control) | Mean: 79 SD: 8 | 55% | Advanced cancers | Any | 12-month telephonic program in which a lay health worker (LHW), supervised by a physician assistant (PA), assessed patient symptoms after diagnosis, with the frequency of symptom screening varying on the basis of patient risk (once a week for high-risk patients and at least once a month for low-risk patients). All participants received usual cancer care. | Edmonton Symptom Assessment System (ESAS) | Telephone | 1 year | Secondary |
| Patel | 2020 | Non-randomized study with historical control | USA | Multicenter | 832 (425 intervention vs. 407 control) |
Mean: 79 SD: 8.3 | 41.5% | Various new diagnoses of solid or hematologic malignant neoplasms | Any | 12-month telephonic program in which a lay health worker (LHW), supervised by a physician assistant (PA), assessed patient symptoms after diagnosis, with the frequency of symptom screening varying on the basis of patient risk (once a week for high-risk patients and at least once a month for low-risk patients). All participants received usual cancer care. | For symptoms: Edmonton Symptom Assessment System (ESAS). For depression: the 9-item Patient Health Questionnaire (PHQ-9) | Telephone | 1 year | Secondary |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminiti, C.; Maglietta, G.; Diodati, F.; Puntoni, M.; Marcomini, B.; Lazzarelli, S.; Pinto, C.; Perrone, F. The Effects of Patient-Reported Outcome Screening on the Survival of People with Cancer: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5470. https://doi.org/10.3390/cancers14215470
Caminiti C, Maglietta G, Diodati F, Puntoni M, Marcomini B, Lazzarelli S, Pinto C, Perrone F. The Effects of Patient-Reported Outcome Screening on the Survival of People with Cancer: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(21):5470. https://doi.org/10.3390/cancers14215470
Chicago/Turabian StyleCaminiti, Caterina, Giuseppe Maglietta, Francesca Diodati, Matteo Puntoni, Barbara Marcomini, Silvia Lazzarelli, Carmine Pinto, and Francesco Perrone. 2022. "The Effects of Patient-Reported Outcome Screening on the Survival of People with Cancer: A Systematic Review and Meta-Analysis" Cancers 14, no. 21: 5470. https://doi.org/10.3390/cancers14215470
APA StyleCaminiti, C., Maglietta, G., Diodati, F., Puntoni, M., Marcomini, B., Lazzarelli, S., Pinto, C., & Perrone, F. (2022). The Effects of Patient-Reported Outcome Screening on the Survival of People with Cancer: A Systematic Review and Meta-Analysis. Cancers, 14(21), 5470. https://doi.org/10.3390/cancers14215470





