Discovery of N-(Naphtho[1,2-b]Furan-5-Yl) Benzenesulfonamides as Novel Selective Inhibitors of Triple-Negative Breast Cancer (TNBC)
Abstract
:1. Introduction
2. Results and Discussion
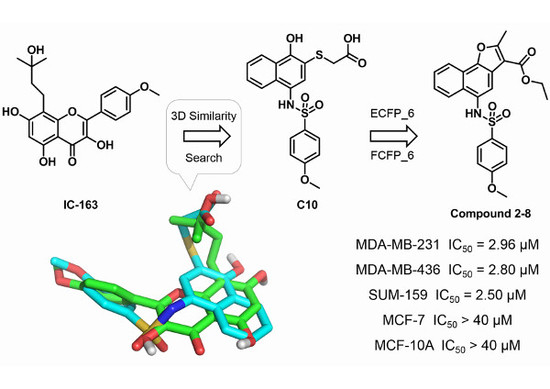
2.1. Three-Dimensional Similarity Search and Bioassays
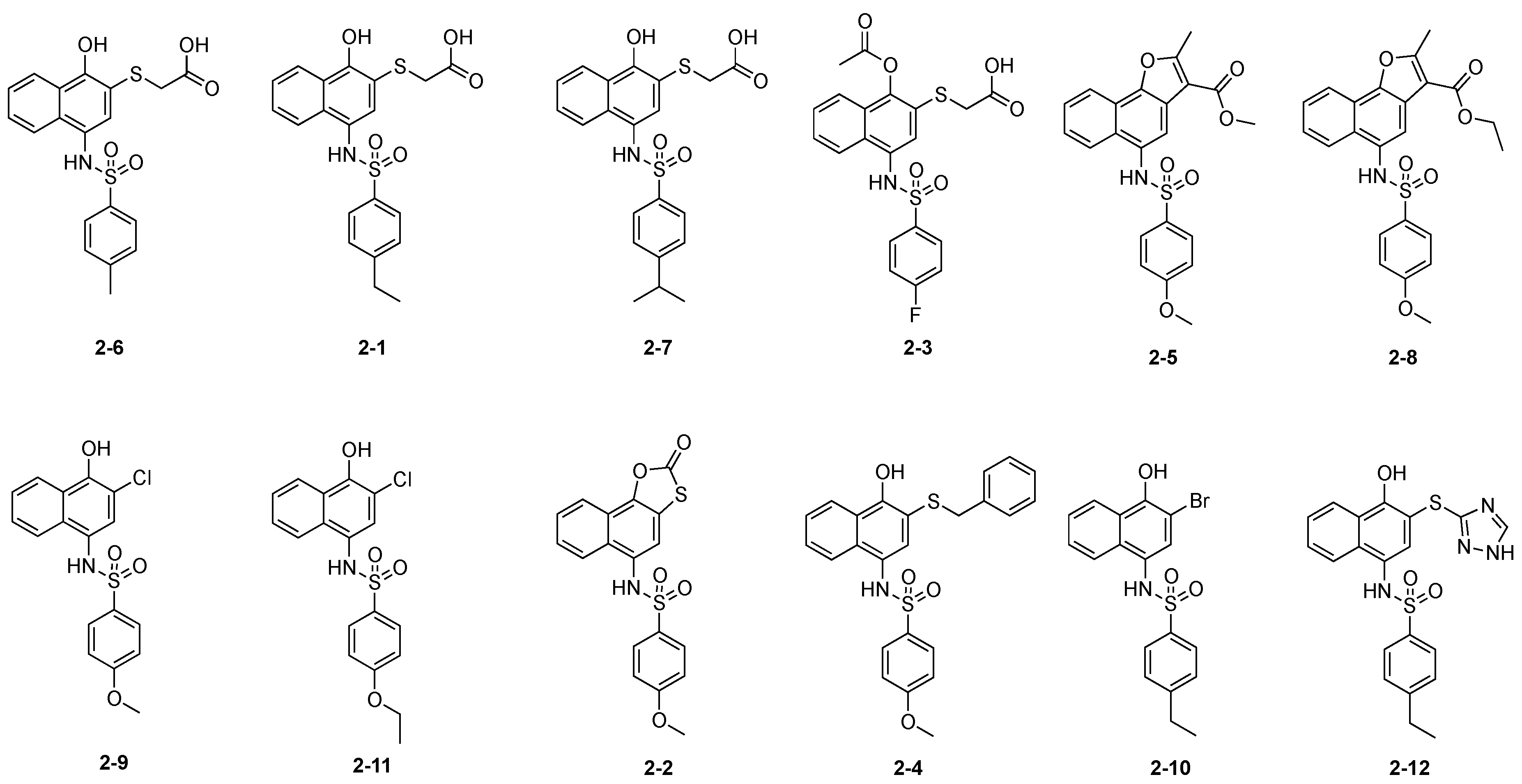
2.2. Hit Follow-up and Expansion
3. Materials and Methods
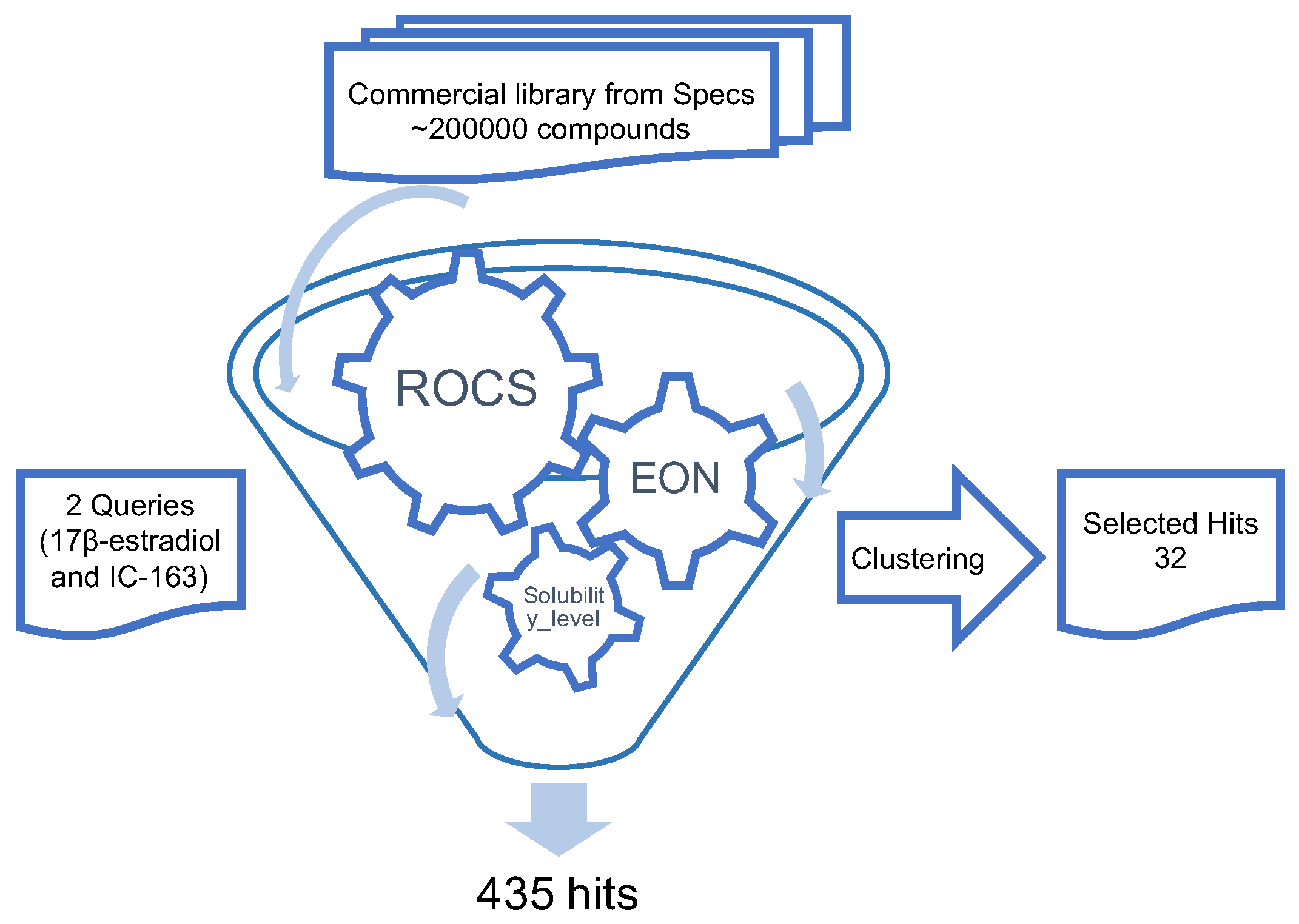
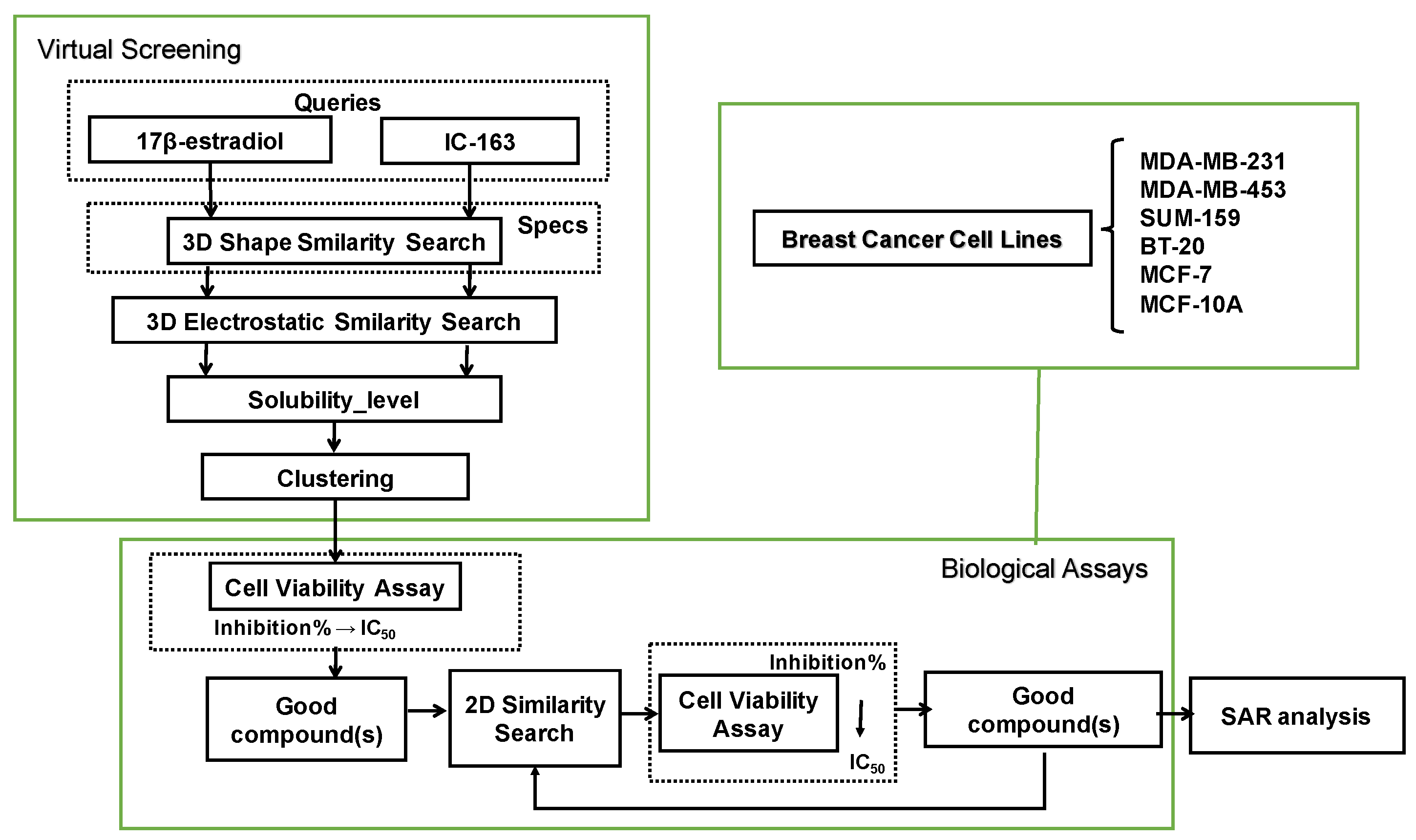
3.1. Overall Protocol
3.2. Three-Dimensional Similarity Search
3.3. Water Solubility Prediction and Structure Cluster Analysis
3.4. Two-Dimensional Similarity Search
3.5. Cell Viability Assays
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TNBC | triple-negative breast cancer |
| ER | estrogen receptor |
| PR | progesterone receptor |
| HER2 | human epidermal growth factor receptor 2 |
| VEGF | the vascular epidermal growth factor |
| 3D | three-dimensional |
| ROCS | Rapid Overlay of Chemical Structures |
| 2D | two-dimensional |
| ECFP_6 | extended connectivity fingerprints of maximum diameter 6 |
| FCFP_6 | function class fingerprints of maximum diameter 6 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Strasser-Weippl, K.; Li, J.J.; St Louis, J.; Finkelstein, D.M.; Yu, K.D.; Chen, W.Q.; Shao, Z.M.; Goss, P.E. Breast cancer in China. Lancet Oncol. 2014, 15, e279–289. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [PubMed]
- Uscanga-Perales, G.I.; Santuario-Facio, S.K.; Ortiz-López, R. Triple negative breast cancer: Deciphering the biology and heterogeneity. Med. Univ. 2016, 18, 105–114. [Google Scholar] [CrossRef]
- Hennessy, B.T.; Gonzalez-Angulo, A.-M.; Stemke-Hale, K.; Gilcrease, M.Z.; Krishnamurthy, S.; Lee, J.-S.; Fridlyand, J.; Sahin, A.; Agarwal, R.; Joy, C.; et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009, 69, 4116–4124. [Google Scholar] [CrossRef] [PubMed]
- Herschkowitz, J.I.; Simin, K.; Weigman, V.J.; Mikaelian, I.; Usary, J.; Hu, Z.; Rasmussen, K.E.; Jones, L.P.; Assefnia, S.; Chandrasekharan, S.; et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007, 8, R76. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Adamo, B.; Cheang, M.C.; Anders, C.K.; Carey, L.A.; Perou, C.M. Molecular characterization of basal-like and non-basal-like triple-negative breast cancer. Oncologist 2013, 18, 123–133. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.Z.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Mancini, P.; Angeloni, A.; Risi, E.; Orsi, E.; Mezi, S. Standard of care and promising new agents for triple negative metastatic breast cancer. Cancers 2014, 6, 2187–2223. [Google Scholar] [CrossRef] [PubMed]
- Suba, Z. Triple-negative breast cancer risk in women is defined by the defect of estrogen signaling: Preventive and therapeutic implications. OncoTargets Ther. 2014, 7, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, K. Compounds and Methods for Treating Estrogen Receptor-Related Diseases. U.S. Patent 20080146658 A1, 19 June 2008. [Google Scholar]
- Hawkins, P.C.; Skillman, A.G.; Nicholls, A. Comparison of shape-matching and docking as virtual screening tools. J. Med. Chem. 2007, 50, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Rush, T.S.; Grant, J.A.; Mosyak, L.; Nicholls, A. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 2005, 48, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Abulwerdi, F.A.; Liao, C.; Mady, A.S.; Gavin, J.; Shen, C.; Cierpicki, T.; Stuckey, J.A.; Showalter, H.D.; Nikolovska-Coleska, Z. 3-substituted-N-(4-hydroxynaphthalen-1-yl)arylsulfonamides as a novel class of selective Mcl-1 inhibitors: Structure-based design, synthesis, SAR, and biological evaluation. J. Med. Chem. 2014, 57, 4111–4133. [Google Scholar] [CrossRef] [PubMed]
- Rathore, D.; Jani, D.; Nagarkatti, R. HDP (Heme Detoxification Protein) Involved in Hemozoin Formation in Plasmodium and Theileria as an Anti-protozoal Target, and High-throughput Screening for Antimalarial HDP Inhibitors. U.S. Patent 20070148185 A1, 28 June 2007. [Google Scholar]
- Tweardy, D.J.; Huang, X.; Kasembeli, M.M. Stat3 Inhibitors. W.O. Patent 2009149192 A1, 10 December 2009. [Google Scholar]
- Tweardy, D.J.; Kasembeli, M.M.; Xu, M.X.; Eckols, T.K. Methods and Compositions for Treatment of Muscle Wasting, Muscle Weakness, and/or Cachexia Using Inhibitors of Stat3. W.O. Patent 2015010107 A1, 22 January 2015. [Google Scholar]
- Hawkins, P.C.D.; Skillman, A.G.; Warren, G.L.; Ellingson, B.A.; Stahl, M.T. Conformer generation with OMEGA: Algorithm and validation using high quality structures from the protein databank and cambridge structural database. J. Chem. Inf. Model. 2010, 50, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.A.; Gallardo, M.; Pickup, B.T. A fast method of molecular shape comparison: A simple application of a gaussian description of molecular shape. J. Comput. Chem. 1996, 17, 1653–1666. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2-5, 2-8, 3-1 to 3-40 are available from the authors. |




| Compound | IC50 (μM) | ||
|---|---|---|---|
| MDA-MB-231 | SUM-159 | MCF-7 | |
| B09 | ~20 | >40 | 1.45 |
| C10 | 2.32 | 3.45 | 20 |
| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| MDA-MB-231 | MDA-MB-453 | SUM-159 | BT-20 | MCF-7 | |
| Tamoxifen | 2.03 | 3.64 | 13.48 | 8.54 | 9.08 |
| IC-163 | >40 | >40 | >40 | >40 | >40 |
| C10 | 5.34 | 2.30 | 11.13 | 10.63 | 9.38 |
| 2-1 | 4.36 | 3.19 | 30.76 | 13.34 | 13.97 |
| 2-3 | 7.99 | 8.59 | >40 | >40 | >40 |
| 2-5 | 3.12 | 2.95 | 2.91 | >40 | >40 |
| 2-6 | 4.97 | 3.36 | 19.21 | 25.02 | >40 |
| 2-7 | 3.95 | 3.65 | >40 | >40 | >40 |
| 2-8 | 2.96 | 3.09 | 2.50 | 10.10 | >40 |
| 2-9 | 6.22 | 4.61 | 18.06 | >40 | >40 |
| 2-11 | 6.92 | 5.72 | >40 | >40 | >40 |

| Compound | R1 | R2 | R3 | Inhibition% | ||
|---|---|---|---|---|---|---|
| MDA-MB-231 | SUM-159 | MCF-7 | ||||
| 3-17 | -OCH2CH3 | -CH3 | -CH3 | 68.567 | 60.956 | 40.549 |
| 3-8 | -CH2CH3 | -OH | -CH3 | −1.131 | 0.221 | −6.706 |
| 3-32 | -CH(CH3)2 | -OH | -CH3 | 24.718 | −0.964 | −7.568 |
| 3-1 | -Cl | -OH | -CH3 | 31.450 | −0.754 | −12.178 |
| 3-9 | -OCH2CH3 | -OCH3 | -CH3 | 63.008 | 60.778 | 24.251 |
| 3-37 | -OCH2CH3 | -OCH2CH3 | -CH3 | 59.687 | 66.789 | 42.503 |
| 3-23 | -OCH2CH3 | -OCH2CH2OCH3 | -CH3 | 62.014 | 49.127 | 14.707 |
| 3-20 | -CH2CH3 | -OCH3 | -CH3 | 53.486 | 65.439 | 31.221 |
| 3-11 | -CH2CH3 | -OCH2CH3 | -CH3 | 41.115 | 0.175 | 13.005 |
| 3-22 | -CH2CH3 | -OCH2CH3 | -Ph | 53.616 | −0.556 | 2.238 |
| 3-4 | -CH(CH3)2 | -OCH3 | -CH3 | 37.770 | −0.319 | 14.222 |
| 3-31 | -CH(CH3)2 | -OCH2CH3 | -CH3 | 32.074 | −1.330 | 2.952 |
| 3-30 | -H | -OCH3 | -CH3 | 50.872 | 0.862 | 29.311 |
| 3-14 | -CH3 | -OCH3 | -CH3 | 62.843 | 48.214 | 10.194 |
| 2-5 | -OCH3 | -OCH3 | -CH3 | 31.005 | 56.793 | - |
| 3-10 | -F | -OCH3 | -CH3 | 64.477 | 35.466 | 34.480 |
| 3-33 | -Cl | -OCH3 | -CH3 | 32.822 | −0.507 | 2.819 |
| 3-29 | -Br | -OCH3 | -CH3 | 45.039 | 1.3243 | 14.480 |
| 3-34 | -COOH | -OCH3 | -CH3 | 10.991 | −0.042 | 2.848 |
| 3-26 | -H | -OCH2Ph | -CH3 | 51.036 | 1.581 | 25.556 |
| 3-36 | -CH3 | -OCH2CH3 | -CH3 | 16.752 | −0.123 | −4.385 |
| 2-8 | -OCH3 | -OCH2CH3 | -CH3 | 62.936 | 61.771 | - |
| 3-3 | -OCH3 | -OCH2CH2CH2CH3 | -CH3 | 51.145 | 42.064 | 29.745 |
| 3-25 | -OCH3 | -OCH2CH2CH2CH2CH3 | -CH3 | 39.505 | 61.902 | 48.321 |
| 3-28 | -OCH3 | -OCH2CH3 | -CH2CH2CH3 | 61.911 | 46.000 | 33.448 |
| 3-12 | -OCH3 | -OCH3 | -C(CH3)3 | 71.378 | 90.386 | 76.319 |
| 3-39 | -F | -OCH(CH3)2 | -CH3 | 42.330 | −0.193 | 4.948 |
| 3-13 | -COOH | -OCH2CH3 | -CH3 | 19.635 | −0.635 | 5.137 |
| 3-40 | -COOH | -OCH2CH3 | -CH2CH2CH3 | 12.487 | 0.000 | 2.525 |
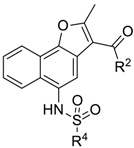
| Compound | R4 | R2 | Inhibition% | ||
|---|---|---|---|---|---|
| MDA-MB-231 | SUM-159 | MCF-7 | |||
| 3-27 | 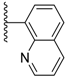 | -OCH3 | 48.153 | 10.205 | 35.546 |
| 3-7 | 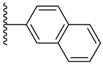 | -OCH3 | 0.431 | 0.085 | −4.733 |
| 3-6 | 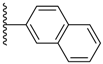 | -OCH2CH3 | 43.365 | 0.292 | 7.724 |
| 3-5 | 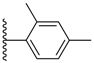 | -OCH3 | 41.065 | 0.757 | 21.98 |
| 3-15 | 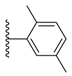 | -OCH3 | 45.584 | 0.037 | 18.546 |
| 3-38 | 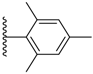 | -OCH3 | 50.558 | 1.346 | 14.827 |
| 3-24 | 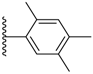 | -OCH3 | 46.563 | 0.233 | 34.910 |
| 3-16 | 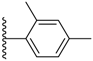 | -OCH2CH3 | 48.171 | 0.732 | 43.439 |
| 3-35 | 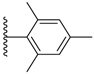 | -OCH2CH3 | 33.853 | 0.352 | 24.499 |
| 3-2 | 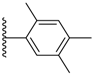 | -OCH2CH3 | 24.255 | −0.884 | 17.700 |
| 3-19 | 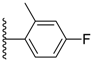 | -OH | 19.306 | 0.380 | 16.340 |
| 3-18 | 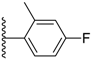 | -OCH3 | 59.421 | 51.425 | 33.244 |
| Compound | IC50 (μM) | |
|---|---|---|
| MDA-MB-231 | SUM-159 | |
| 2-5 | 3.12 | 2.91 |
| 2-8 | 2.96 | 2.50 |
| 3-3 | 3.25 | 8.66 |
| 3-9 | 2.68 | 2.66 |
| 3-12 | 11.91 | 11.53 |
| 3-17 | 1.77 | 1.94 |
| 3-18 | 5.20 | 24.15 |
| 3-20 | 10.04 | 16.97 |
| 3-28 | >40 | 14.41 |
| 3-37 | 5.49 | 4.09 |
| 2-5-COOH | ~24 | 24.25 |
| Compound | IC50 (μM) | Calculated Molecular Properties | ||||||
|---|---|---|---|---|---|---|---|---|
| MDA-MB-231 | MDA-MB-436 | SUM-159 | MCF-7 | MCF-10A | Molecular_Weight | AlogP | ADMET_Solubility_level | |
| Tamoxifen | 2.03 | 10.02 | 13.48 | 9.08 | >40 | 371.5 | 6.319 | 1 |
| 2-5 | 3.12 | 1.99 | 2.91 | >40 | >40 | 425.5 | 3.514 | 2 |
| 2-8 | 2.96 | 2.80 | 2.50 | >40 | >40 | 439.5 | 3.863 | 2 |
| 3-3 | 3.25 | 3.36 | 8.66 | ND a | >40 | 467.5 | 4.842 | 1 |
| 3-9 | 2.68 | 2.08 | 2.66 | ND | >40 | 439.5 | 3.863 | 2 |
| 3-12 | 11.91 | 7.13 | 11.53 | ND | >40 | 467.5 | 5.063 | 1 |
| 3-17 | 1.77 | ~0.01 | 1.94 | ND | 0.66 | 423.5 | 3.747 | 2 |
| 3-37 | 5.49 | 1.41 | 4.09 | ND | > 40 | 453.5 | 4.211 | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Tang, Y.; Mao, B.; Li, W.; Jin, H.; Zhang, L.; Liu, Z. Discovery of N-(Naphtho[1,2-b]Furan-5-Yl) Benzenesulfonamides as Novel Selective Inhibitors of Triple-Negative Breast Cancer (TNBC). Molecules 2018, 23, 678. https://doi.org/10.3390/molecules23030678
Chen Y, Tang Y, Mao B, Li W, Jin H, Zhang L, Liu Z. Discovery of N-(Naphtho[1,2-b]Furan-5-Yl) Benzenesulfonamides as Novel Selective Inhibitors of Triple-Negative Breast Cancer (TNBC). Molecules. 2018; 23(3):678. https://doi.org/10.3390/molecules23030678
Chicago/Turabian StyleChen, Ya, Yong Tang, Beibei Mao, Wenchao Li, Hongwei Jin, Liangren Zhang, and Zhenming Liu. 2018. "Discovery of N-(Naphtho[1,2-b]Furan-5-Yl) Benzenesulfonamides as Novel Selective Inhibitors of Triple-Negative Breast Cancer (TNBC)" Molecules 23, no. 3: 678. https://doi.org/10.3390/molecules23030678