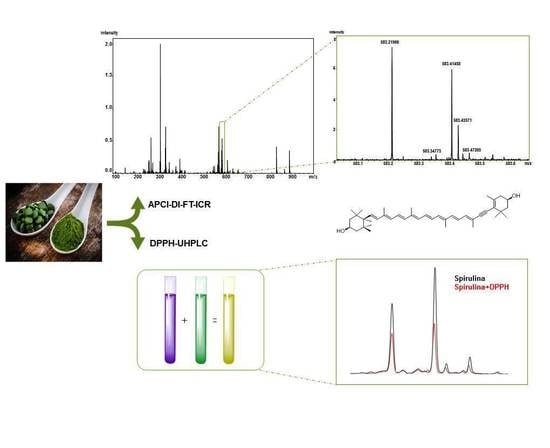
Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay
Abstract
:1. Introduction
2. Results and Discussion
2.1. High-Resolution Mass Spectrometry Profiling of Spirulina Pigments
2.2. Quantitative Profile of Spirulina Pigments by UHPLC-PDA
2.3. Evaluation of Antioxidant Potential of Single Pigments by DPPH-UHPLC-PDA
3. Material and Methods
3.1. Chemicals
3.2. Sample Extraction
3.3. Instrumentation
3.4. Columns
3.5. DIMS and LC-HRMS Parameters
3.6. DPPH-UHPLC-PDA Parameters
3.7. Qualitative and Quantitative Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Siró, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kate, B.N.; Banerjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- De Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.C.; Phuc, A.P.; Dubacq, J.P. Nutritional value of alga Spirulina. World Rev. Nutr. Diet. 1995, 77, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Marín, F.R.; Hernández, S.F.; Arredondo, B.O.; Señoráns, F.J.; Ibañez, E.; Reglero, G. Characterization via liquid chromatography coupled to diode array detector and tandem mass spectrometry of supercritical fluid antioxidant extracts of Spirulina platensis microalga. J. Sep. Sci. 2005, 28, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.b.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Rao, A.R.; Baskaran, V.; Sarada, R.; Ravishankar, G.A. In vivo bioavailability and antioxidant activity of carotenoids from microalgal biomass—A repeated dose study. Food Res. Int. 2013, 54, 711–717. [Google Scholar] [CrossRef]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Montone, C.M.; Piovesana, S.; Samperi, R.; Chiozzi, R.Z.; Laganà, A. Liquid chromatography-high resolution mass spectrometry for the analysis of phytochemicals in vegetal-derived food and beverages. Food Res. Int. 2017, 100, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Forcisi, S.; Moritz, F.; Kanawati, B.; Tziotis, D.; Lehmann, R.; Schmitt-Kopplin, P. Liquid chromatography–mass spectrometry in metabolomics research: Mass analyzers in ultra high pressure liquid chromatography coupling. J. Chromatogr. A 2013, 1292, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.C.; Kruppa, G.; Dasseux, J.L. Metabolomics applications of FT-ICR mass spectrometry. Mass Spectrom. Rev. 2005, 24, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Magnúsdóttir, M.; Brynjólfson, S.; Palsson, B.Ø.; Paglia, G. UPLC-UV-MSE analysis for quantification and identification of major carotenoid and chlorophyll species in algae. Anal. Bioanal. Chem. 2012, 404, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry of Carotenoids. Int. J. Mass Spectrom. 2012, 312, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Juin, C.; Bonnet, A.; Nicolau, E.; Bérard, J.B.; Devillers, R.; Thiéry, V.; Cadoret, J.P.; Picot, L. UPLC-MSE Profiling of Phytoplankton Metabolites: Application to the Identification of Pigments and Structural Analysis of Metabolites in Porphyridium purpureum. Mar. Drugs 2015, 13, 2541–2558. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pagano, F.; Salviati, E.; Chieppa, M.; Bertamino, A.; Manfra, M.; Sala, M.; Novellino, E.; Campiglia, P. Chemical profiling of bioactive constituents in hop cones and pellets extracts by online comprehensive two-dimensional liquid chromatography with tandem mass spectrometry and direct infusion Fourier transform ion cyclotron resonance mass spectrometry. J. Sep. Sci. 2018, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Rivera, S.M.; Canela-Garayoa, R. Analytical tools for the analysis of carotenoids in diverse materials. J. Chromatogr. A 2012, 1224, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Ostacolo, C.; Tenore, G.C.; Russo, M.T.; Novellino, E.; Manfra, M.; Campiglia, P. Detailed polyphenolic profiling of Annurca apple (M. pumila Miller cv Annurca) by a combination of RP-UHPLC and HILIC, both hyphenated to IT-TOF mass spectrometry. Food Res. Int. 2015, 76, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. Chromatographic column evaluation for the untargeted profiling of glucosinolates in cauliflower by means of ultra-high performance liquid chromatography coupled to high resolution mass spectrometry. Talanta 2018, 179, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Bijttebier, S.; Els D’Hondt, E.; Noten, B.; Hermans, N.; Apers, S.; Voorspoels, S. Ultra high performance liquid chromatography versus high performance liquid chromatography: Stationary phase selectivity for generic carotenoid screening. J. Chromatogr. A 2014, 1332, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Zoccali, M.; Giofrè, S.V.; Dugo, P.; Mondello, L. Apocarotenoids determination in Capsicum chinense Jacq. cv. Habanero, by supercritical fluid chromatography-triple-quadrupole/mass spectrometry. Food Chem. 2017, 231, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, D.; Pintea, A.; Dugo, P.; Torre, G.; Raluca, M.P.; Mondello, L. Determination of Carotenoids and their Esters in Fruits of Sea Buckthorn (Hippophae rhamnoides L.) by HPLC-DAD-APCI-MS. Phytochem. Anal. 2012, 23, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Pagano, F.; Conte, G.; Carimi, F.; Tenore, G.C.; Novellino, E.; Manfra, M.; Russo, M.; Campiglia, P. Rapid Screening of Antioxidant Anthocyanins in Autochthonous Nero d’Avola Grape Clones by Pre-column DPPH Reaction Coupled to UHPLC-UV/Vis-IT-TOF: A Strategy to Combine Chemical data and Genetic Diversity. Food Anal. Methods 2016, 10, 2780–2790. [Google Scholar] [CrossRef]
- Sommella, E.; Pagano, F.; Pepe, G.; Ostacolo, C.; Manfra, M.; Chieppa, M.; Di Sanzo, R.; Carabetta, S.; Campiglia, P.; Russo, M. Flavonoid Composition of Tarocco (Citrus sinensis L. Osbeck) Clone “Lempso” and Fast Antioxidant Activity Screening by DPPH-UHPLC-PDA-IT-TOF. Phytochem. Anal. 2017, 28, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Escrig, A.; Jiménez-Jiménez, I.; Sánchez-Moreno, C.; Saura-Calixto, F. Evaluation of free radical scavenging of dietary carotenoids by the stable radical 2,2-diphenyl-1-picrylhydrazyl. J. Sci. Food Agric. 2000, 80, 1686–1690. [Google Scholar] [CrossRef]
- Frank, H.A.; Young, J.; Britton, G.; Cogdell, R.J. The Photochemistry of Carotenoids; Springer: Dordrecht, The Netherlands, 1999; pp. 203–222. [Google Scholar]
- Ngo, T.C.; Dao, D.Q.; Nguyen, M.T.; Nam, P.C. A DFT analysis on the radical scavenging activity of oxygenated terpenoids present in the extract of the buds of Cleistocalyx operculatus. RSC Adv. 2017, 7, 39686–39698. [Google Scholar] [CrossRef]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Peak | rt | Compound | Molecular Formula | [M + H]+ DIMS-APCI | Error ppm DIMS-APCI | [M + H]+ LC-APCI-FT-ICR MS | MS/MS | Error ppm LC-APCI-FT-ICR MS |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.87 | Apo-12-Violaxanthal 1 | C25H34O3 | 383.25809 | −0.05 | 383.25815 | 365.24754 2, 347.23705 3 | −0.21 |
| 2 | 2.76 | Vaucheriaxanthin 1 | C40H56O5 | 617.42010 | −0.08 | 617.41998 | 599.40933 2, 581.39890 3 | 0.12 |
| 3 | 3.29 | Diadinoxanthin 1 | C40H54O3 | 583.41458 | −0.02 | 583.41456 | 565.40420 2, 221.15364 | 0.01 |
| 4 | 3.33 | Canthaxanthin | C40H52O2 | 565.40402 | −0.01 | 565.40404 | 547.39350 2 | −0.06 |
| 5 | 3.45 | Ethyl β-apo-8′-carotenoate 1 | C32H44O2 | 461.34143 | −0.04 | 461.34155 | −0.32 | |
| 6 | 3.67 | Adonirubin 1 | C40H52O3 | 581.39892 | 0.01 | 581.39891 | 0.02 | |
| 7 | 3.69 | Diatoxanthin 1 | C40H54O2 | 567.41967 | −0.02 | 567.41971 | 221.13248, 549.40979 2 | −0.09 |
| 8 | 3.78 | β-Apo-8′-carotenal 1 | C30H40O | 417.3152 | −0.01 | 417.31542 | 399.30463 2, 293.22642 | −0.34 |
| 9 | 3.85 | Hexadehydro-β,β-caroten-3-ol 1 | C40H50O | 547.39347 | −0.06 | 547.39344 | −0.36 | |
| 10 | 3.88 | Rhodoxanthin 1 | C40H50O2 | 563.38838 | −0.04 | 563.38856 | 545.37778 2 | −0.36 |
| 11 | 3.90 | Astaxanthin | C40H52O4 | 597.39382 | 0.02 | 597.39384 | 0.01 | |
| 12 | 4.04 | Antheraxanthin 1 | C40H56O3 | 585.43023 | −0.02 | 585.43029 | 567.41961 2, 549.40920 3, 493.40407 | −0.12 |
| 13 | 4.20 | Myxoxanthophyll | C46H66O7 | 731.48807 | 0.08 | 731.48917 | −1.42 | |
| 14 | 4.38 | Zeaxanthin | C40H56O2 | 569.43529 | 0.02 | 569.43552 | 551.42497 2, 459.36256 | −0.37 |
| 15 | 5.06 | 10-Apo-β-carotenal 1 | C27H36O | 377.28389 | −0.06 | 377.28403 | −0.37 | |
| 16 | 6.10 | α-tocopherol 1 | C29H50O2 | 431.38835 | 0.01 | 431.37971 | −0.91 | |
| 17 | 8.73 | Chlorophyll a isomer | C55H72MgN4O5 | 893.54226 | −0.03 | 893.54274 | 555.22547, 481.18779, 614.23848 | −0.17 |
| 18 | 8.74 | Echinenone 1 | C40H54O | 551.42473 | 0.02 | 551.42473 | 203.17531 | 0.03 |
| 19 | 9.00 | Pyrochlorophyll b 1 | C53H68MgN4O4 | 849.51640 | −0.03 | 849.51620 | 0.21 | |
| 20 | 9.00 | Pheophytin a derivate 1 | C55H72N4O5 | 869.55755 | 0.01 | 869.55795 | −0.13 | |
| 21 | 9.10 | Chlorophyllide b 1 | C35H32MgN4O6 | 629.24050 | 0.01 | 629.22498 | −0.76 | |
| 22 | 9.12 | Chlorophyll b 1 | C55H70MgN4O6 | 907.55824 | 0.01 | 907.55841 | −0.19 | |
| 23 | 9.15 | Pyrochlorophyll a 1 | C53H70MgN4O3 | 835.53711 | 0.01 | 835.53746 | −0.42 | |
| 24 | 9.18 | Pyrochlorophyllide a 1 | C33H32MgN4O3 | 557.23978 | −0.04 | 557.23992 | −0.29 | |
| 25 | 9.26 | Pyrochlorophyllide b | C33H30MgN4O4 | 571.21901 | 0.02 | 571.21902 | 0.01 | |
| 26 | 9.28 | OH-Chlorophyll a 1 | C55H72MgN4O6 | 909.53746 | 0.05 | 909.53786 | 525.21366, 553.20861 | −0.95 |
| 27 | 9.32 | Protochlorophyllide a 1 | C35H32MgN4O5 | 613.22959 | −0.01 | 613.22998 | −0.64 | |
| 28 | 9.32 | 13-OH-Chlorophyllide a 1 | C35H34MgN4O6 | 631.24015 | 0.01 | 631.24054 | −0.62 | |
| 29 | 9.32 | Divinyl Chlorophyll a 1 | C55H70MgN4O5 | 891.52691 | 0.04 | 891.52705 | 555.22506, 614.23423 | 0.21 |
| 30 | 9.56 | Chlorophyll a | C55H72MgN4O5 | 893.54262 | −0.03 | 893.54274 | 555.22547, 481.18779, 614.23848 | −0.17 |
| 31 | 10.03 | Cryptoxanthin 1 | C40H56O | 553.44040 | 0.01 | 553.44047 | 535.430052, 461.37769 | −0.15 |
| 32 | 10.04 | Chlorophyll a isomer | C55H72MgN4O5 | 893.54262 | −0.03 | 893.54274 | 555.22547, 481.18779, 614.23848 | −0.17 |
| 33 | 10.10 | Chlorophyllide a 1 | C35H34MgN4O5 | 615.24526 | −0.04 | 615.2461 | −0.34 | |
| 34 | 10.23 | Pheophytin b 1 | C55H72N4O6 | 885.55233 | 0.14 | 885.53330 | −0.09 | |
| 35 | 10.30 | 15-OH-Lactone-Chlorophyll a 1 | C55H73MgN4O7 | 925.53199 | 0.47 | 925.53324 | 0.89 | |
| 36 | 10.47 | Pyropheophorbide b 1 | C33H32N4O4 | 549.24967 | −0.08 | 549.24980 | −0.31 | |
| 37 | 10.48 | 15-OH-Lactone-Pheophytin a 1 | C55H73N4O7 | 903.56328 | −0.28 | 903.56341 | 537.24965, 547.23401, 607.25553 | −0.09 |
| 38 | 10.79 | Chlorobactene 1 | C40H52 | 533.41416 | 0.03 | 533.41406 | 0.21 | |
| 39 | 11.03 | Chlorophyll a derivate I 1 | C55H68MgN4O5 | 889.51122 | 0.08 | 889.51165 | −0.41 | |
| 40 | 11.06 | Phytoene 1 | C40H64 | 545.50810 | −0.03 | 545.50829 | −0.39 | |
| 41 | 11.12 | 13-OH-Pheophorbide a 1 | C35H36N4O6 | 609.27078 | −0.02 | 609.27091 | −0.24 | |
| 42 | 11.12 | OH-Pheophytin a | C55H73N4O6 | 887.56810 | 0.01 | 887.56826 | 531.23918, 559.23402, 591.26022 | −0.17 |
| 43 | 11.16 | β-carotene | C40H56 | 537.44547 | 0.01 | 537.44562 | 413.32058, 445.38298 | −0.27 |
| 44 | 11.21 | Octadehydro-β,β-carotene 1 | C40H48 | 529.38288 | 0.03 | 529.38303 | −0.29 | |
| 45 | 11.41 | Pheophytin a | C55H74N4O5 | 871.57318 | 0.02 | 871.57254 | 593.27615, 533.25473, 519.23921 | 0.75 |
| 46 | 11.68 | Pheophorbide a 1 | C35H36N4O5 | 593.27583 | 0.02 | 593.27601 | −0.27 | |
| 47 | 12.30 | Pyropheophorbide a 1 | C33H34N4O3 | 535.27037 | 0.01 | 535.27058 | 0.39 | |
| 48 | 12.30 | Pyropheophytin a 1 | C53H72N4O3 | 813.56769 | 0.04 | 813.56787 | 535.27058, 507.27549, 461.23369 | −0.18 |
| 49 | δ-tocopherol 1 | C27H46O2 | 403.35706 | 0.01 | ||||
| 50 | γ-tocopherol 1 | C28H48O2 | 417.37270 | 0.02 | ||||
| 51 | Phytofluene 1 | C40H62 | 543.49242 | 0.01 |
| Dietary Supplement Powder | Lab Made Powder | Dietary Supplement Tablet | ||||
|---|---|---|---|---|---|---|
| Quantitative | ||||||
| Peak | Compounds | μg/g | μg/g | μg/g | RSA % | |
| 1 | Diadinoxanthin | 28.01 ± 0.11 | 55.27 ± 0.16 | 30.79 ± 0.05 | 15.07 ± 0.17 | |
| 2 | Alloxanthin/Canthaxanthin | 22.76 ± 0.04 | 26.38 ± 0.17 | 25.79 ± 0.03 | 6.66 ± 0.27 | |
| 3 | Diatoxanthin | 100.11 ± 0.22 | 363.96 ± 1.03 | 78.33 ± 0.29 | 14.45 ± 0.23 | |
| 4 | Antheraxanthin | 27.20 ± 0.02 | 31.60 ± 0.15 | 28.45 ± 0.11 | 5.99 ± 0.11 | |
| 5 | Zeaxanthin | 113.76 ± 0.15 | 362.51 ± 0.61 | 91.95 ± 0.32 | 10.02 ± 0.05 | |
| 6 | Echinenone | 24.95 ± 0.16 | 25.05 ± 0.09 | 32.57 ± 0.15 | 4.54 ± 0.15 | |
| 7 | β-carotene | 1226.99 ± 7.67 | 1544.36 ± 4.06 | 988.47 ± 6.10 | 16.23 ± 0.30 | |
| Dietary Supplement Powder (a) | Lab Made Powder (b) | Dietary Supplement Tablets | Ascorbic Acid | BHT | ||
| IC50 (mg/mL) | 2.99 ± 0.05 | 1.21 ± 0.02 | 2.68 ± 0.03 | 0.03 ± 0.002 | 0.02 ± 0.001 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Prete, F.D.; Aquino, R.P.; Campiglia, P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules 2018, 23, 1152. https://doi.org/10.3390/molecules23051152
Sommella E, Conte GM, Salviati E, Pepe G, Bertamino A, Ostacolo C, Sansone F, Prete FD, Aquino RP, Campiglia P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules. 2018; 23(5):1152. https://doi.org/10.3390/molecules23051152
Chicago/Turabian StyleSommella, Eduardo, Giulio Maria Conte, Emanuela Salviati, Giacomo Pepe, Alessia Bertamino, Carmine Ostacolo, Francesca Sansone, Francesco Del Prete, Rita Patrizia Aquino, and Pietro Campiglia. 2018. "Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay" Molecules 23, no. 5: 1152. https://doi.org/10.3390/molecules23051152
APA StyleSommella, E., Conte, G. M., Salviati, E., Pepe, G., Bertamino, A., Ostacolo, C., Sansone, F., Prete, F. D., Aquino, R. P., & Campiglia, P. (2018). Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules, 23(5), 1152. https://doi.org/10.3390/molecules23051152