Drought Impact on Eco-Physiological Responses and Growth Performance of Healthy and Declining Pinus sylvestris L. Trees Growing in a Dry Area of Southern Poland
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Dendrochronological Analyses
2.3. Resistance, Resilience, and Recovery Indices
2.4. Isotopes Analysis and iWUE
3. Results
3.1. Tree Growth Analysis
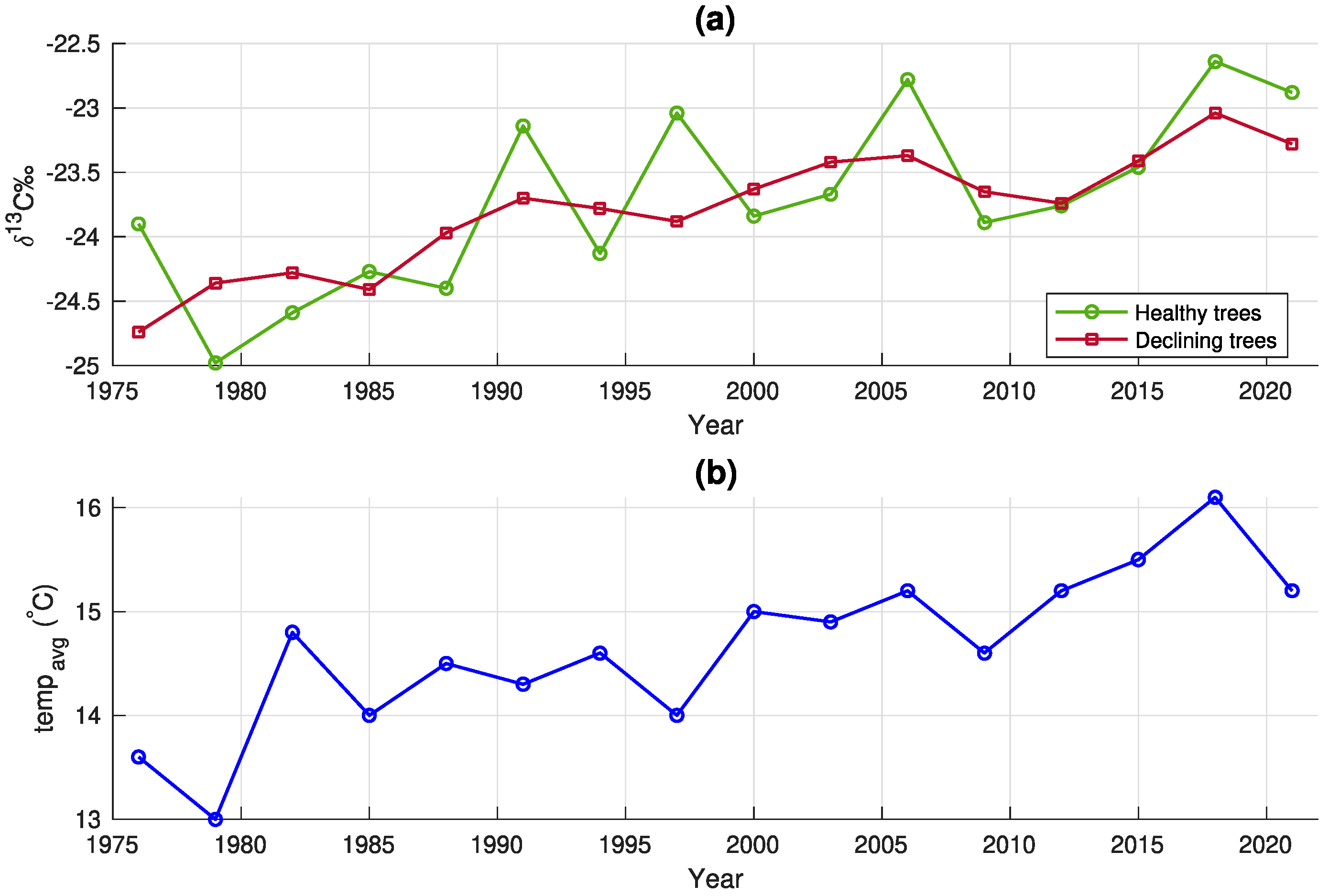
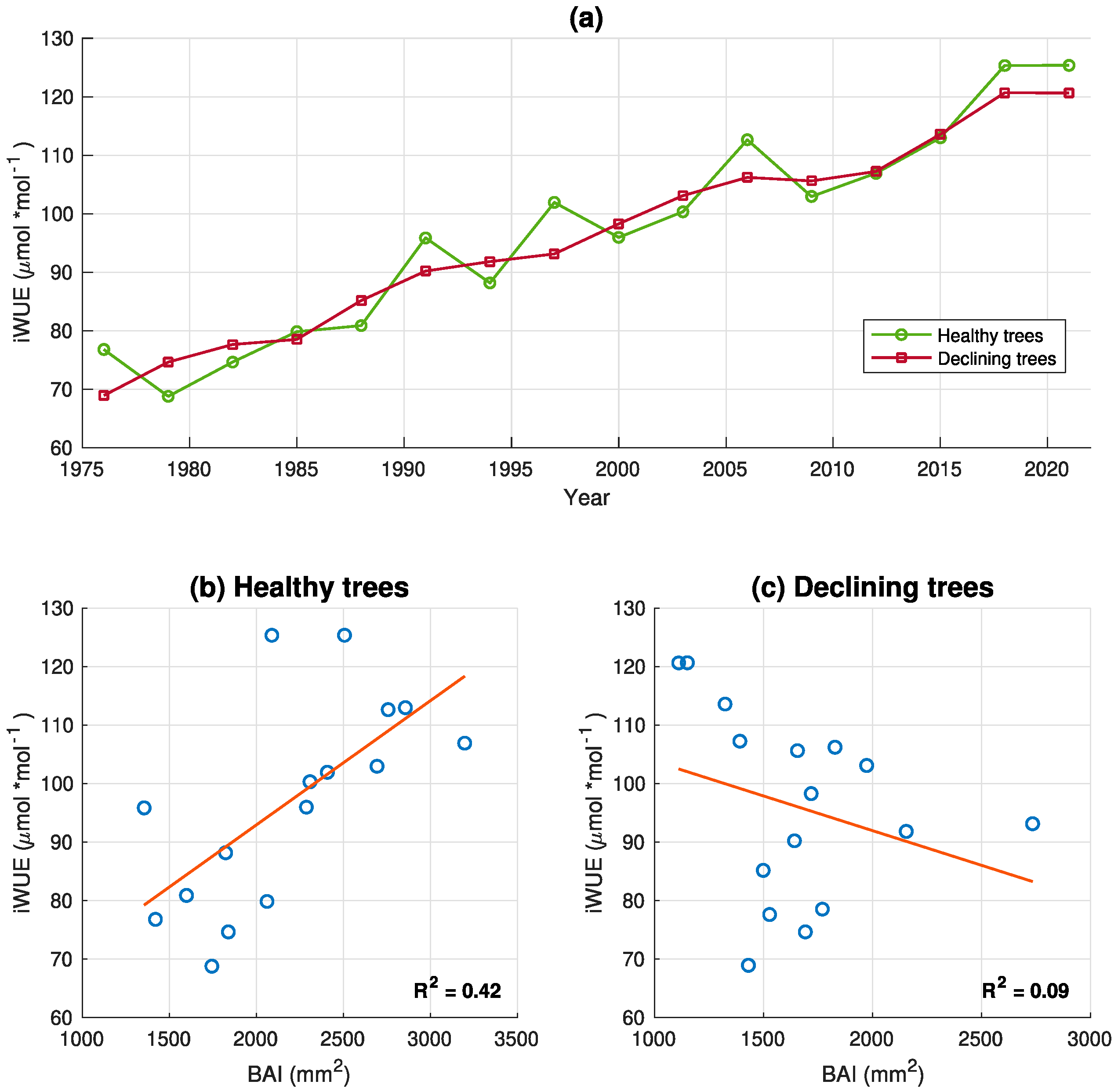
3.2. Isotope Analysis in Tree Rings
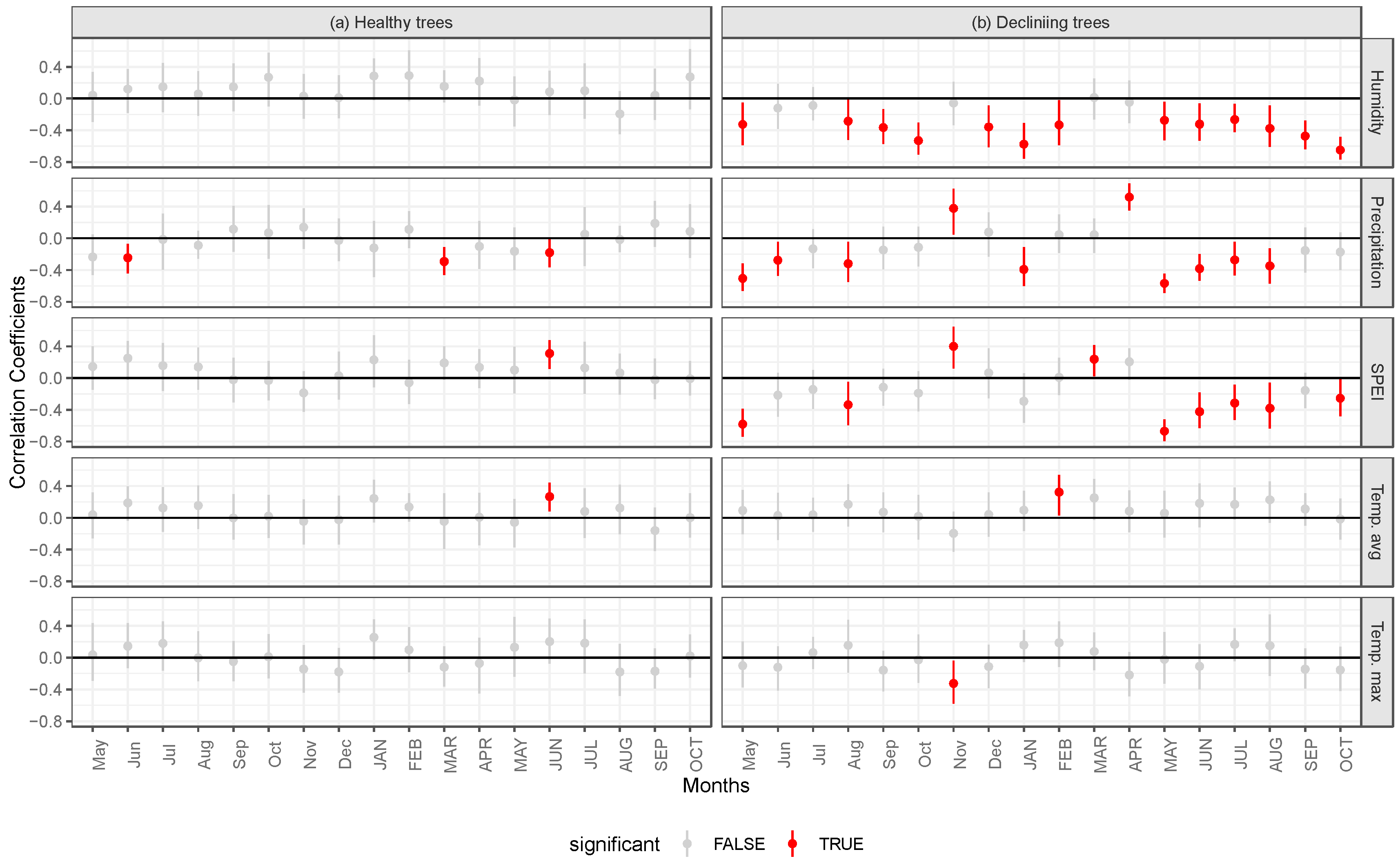
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Morecroft, M.D.; Trisurat, Y. Climate Change 2022: Impacts, Adaptation and Vulnerability; GIEC: Geneva, Switzerland, 2022; Available online: https://hal.science/hal-03774939 (accessed on 11 October 2023).
- Dai, A. Increasing Drought under Global Warming in Observations and Models. Nat. Clim. Chang. 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Senf, C.; Pflugmacher, D.; Zhiqiang, Y.; Sebald, J.; Knorn, J.; Neumann, M.; Hostert, P.; Seidl, R. Canopy mortality has doubled in Europe’s temperate forests over the last three decades. Nat. Commun. 2018, 9, 4978. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Hallsby, G.; Löfvenius, M.O.; Nilsson, M.-C. Synergistic, additive and antagonistic impacts of drought and herbivory on Pinus sylvestris: Leaf, tissue and whole-plant responses and recovery. Tree Physiol. 2013, 33, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.; Starzyk, J.; Kolk, A.; Grodzki, W. Threat of Norway spruce caused by the bark beetle Ips typographus (L.) in the stands of the Forest Promotion Complex “Puszcza Balowieska” in 2000–2002. Lesn. Pr. Badaw. 2004, 3, 5–30. [Google Scholar]
- Bokwa, A.; Klimek, M.; Krzaklewski, P.; Kukułka, W. Drought Trends in the Polish Carpathian Mts in the Years 1991–2020. Atmosphere 2021, 12, 1259. [Google Scholar] [CrossRef]
- Timofeeva, G.; Treydte, K.; Bugmann, H.; Rigling, A.; Schaub, M.; Siegwolf, R.; Saurer, M. Long-term effects of drought on tree-ring growth and carbon isotope variability in Scots pine in a dry environment. Tree Physiol. 2017, 37, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Benisiewicz, B.; Pawelczyk, S.; Niccoli, F.; Kabala, J.; Battipaglia, G. Investigation of Trees’ Sensitivity to Drought: A Case Study in the Opole Region, Poland. Geochronometria 2024, 50, 135–143. [Google Scholar] [CrossRef]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sánchez, G.; Peñuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef]
- Steinkamp, J.; Hickler, T. Is drought-induced forest dieback globally increasing? J. Ecol. 2015, 103, 31–43. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant Cell Environ. 2014, 37, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.D.; Zeppel, M.J.B.; Anderegg, W.R.L.; Hartmann, H.; Landhäusser, S.M.; Tissue, D.T.; Huxman, T.E.; Hudson, P.J.; Franz, T.E.; Allen, C.D.; et al. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 2017, 1, 1285–1291. [Google Scholar] [CrossRef]
- Puchi, P.F.; Camarero, J.J.; Battipaglia, G.; Carrer, M. Retrospective analysis of wood anatomical traits and tree-ring isotopes suggests site-specific mechanisms triggering Araucaria araucana drought-induced dieback. Glob. Chang. Biol. 2021, 27, 6394–6408. [Google Scholar] [CrossRef] [PubMed]
- Martín-Gómez, P.; Aguilera, M.; Pemán, J.; Gil-Pelegrín, E.; Ferrio, J.P. Contrasting ecophysiological strategies related to drought: The case of a mixed stand of Scots pine (Pinus sylvestris) and a submediterranean oak (Quercus subpyrenaica). Tree Physiol. 2017, 37, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.C.H.; Hartmann, M.; Conz, R.F.; Six, J.; Solly, E.F. Drought-induced tree mortality in Scots pine mesocosms promotes changes in soil microbial communities and trophic groups. Appl. Soil Ecol. 2024, 194, 105198. [Google Scholar] [CrossRef]
- Shestakova, T.A.; Sin, E.; Gordo, J.; Voltas, J. Tree-ring isotopic imprints on time series of reproductive effort indicate warming-induced co-limitation by sink and source processes in stone pine. Tree Physiol. 2023, 44, tpad147. [Google Scholar] [CrossRef] [PubMed]
- Solly, E.F.; Jaeger, A.C.H.; Barthel, M.; Werner, R.A.; Zürcher, A.; Hagedorn, F.; Six, J.; Hartmann, M. Water limitation intensity shifts carbon allocation dynamics in Scots pine mesocosms. Plant Soil 2023, 490, 499–519. [Google Scholar] [CrossRef] [PubMed]
- Kursar, T.A.; Engelbrecht, B.M.J.; Burke, A.; Tyree, M.T.; EI Omari, B.; Giraldo, J.P. Tolerance to low leaf water status of tropical tree seedlings is related to drought performance and distribution. Funct. Ecol. 2009, 23, 93–102. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- Buras, A.; Schunk, C.; Zeiträg, C.; Herrmann, C.; Kaiser, L.; Lemme, H.; Straub, C.; Taeger, S.; Gößwein, S.; Klemmt, H.-J.; et al. Are Scots pine forest edges particularly prone to drought-induced mortality? Environ. Res. Lett. 2018, 13, 025001. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Galván, J.D.; Sangüesa-Barreda, G.; Gutiérrez, E. Disparate effects of global-change drivers on mountain conifer forests: Warming-induced growth enhancement in young trees vs. CO2 fertilization in old trees from wet sites. Glob. Chang. Biol. 2015, 21, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Etzold, S.; Ziemińska, K.; Rohner, B.; Bottero, A.; Bose, A.K.; Ruehr, N.K.; Zingg, A.; Rigling, A. One Century of Forest Monitoring Data in Switzerland Reveals Species- and Site-Specific Trends of Climate-Induced Tree Mortality. Front. Plant Sci. 2019, 10, 436319. [Google Scholar] [CrossRef] [PubMed]
- Taeger, S.; Zang, C.; Liesebach, M.; Schneck, V.; Menzel, A. Impact of climate and drought events on the growth of Scots pine (Pinus sylvestris L.) provenances. For. Ecol. Manag. 2013, 307, 30–42. [Google Scholar] [CrossRef]
- Boczoń, A.; Kowalska, A.; Dudzińska, M.; Wróbel, M. Drought in Polish Forests in 2015. Pol. J. Environ. Stud. 2016, 25, 1857–1862. [Google Scholar] [CrossRef]
- Camarero, J.; Colangelo, M.; Gazol, A.; Azorin-Molina, C. Drought and cold spells trigger dieback of temperate oak and beech forests in northern Spain. Dendrochronologia 2021, 66, 125812. [Google Scholar] [CrossRef]
- Bose, A.K.; Gessler, A.; Bolte, A.; Bottero, A.; Buras, A.; Cailleret, M.; Camarero, J.J.; Haeni, M.; Hereş, A.-M.; Hevia, A.; et al. Growth and resilience responses of Scots pine to extreme droughts across Europe depend on predrought growth conditions. Glob. Chang. Biol. 2020, 26, 4521–4537. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, P.; Battipaglia, G.; Innes, J.L. Tree Vitality and Forest Health: Can Tree-Ring Stable Isotopes Be Used as Indicators? Curr. For. Rep. 2021, 7, 69–80. [Google Scholar] [CrossRef]
- Tallieu, C.; Badeau, V.; Allard, D.; Nageleisen, L.-M.; Bréda, N. Year-to-year crown condition poorly contributes to ring width variations of beech trees in French ICP level I network. For. Ecol. Manag. 2020, 465, 118071. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Sørensen, S.J.; Bååth, E.; Bloem, J.; De Ruiter, P.C.; et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity-ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bonner, K.I.; Barker, G.M. Stability of ecosystem properties in response to above-ground functional group richness and composition. Oikos 2000, 89, 11–23. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A. New indices for quantifying the resistance and resilience of soil biota to exogenous disturbances. Soil Biol. Biochem. 2004, 36, 1907–1912. [Google Scholar] [CrossRef]
- Lloret, F.; Keeling, E.G.; Sala, A. Components of tree resilience: Effects of successive low-growth episodes in old ponderosa pine forests. Oikos 2011, 120, 1909–1920. [Google Scholar] [CrossRef]
- Schwarz, J.; Skiadaresis, G.; Kohler, M.; Kunz, J.; Schnabel, F.; Vitali, V.; Bauhus, J. Quantifying Growth Responses of Trees to Drought—A Critique of Commonly Used Resilience Indices and Recommendations for Future Studies. Curr. For. Rep. 2020, 6, 185–200. [Google Scholar] [CrossRef]
- Becker, M.; Bräker, O.; Kenk, G.; Schneider, O.; Schweingruber, F. Crown condition and growth of forest trees over recent decades in the area where Germany, France and Switzerland meet. Allg. Forstz. 1990, 11, 263–274. [Google Scholar]
- Adams, M.; Buckley, T.; Turnbull, T. Diminishing CO2-driven gains in water-use efficiency of global forests. Nat. Clim. Chang. 2020, 10, 466–471. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Bryukhanova, M.; Vaganov, E.A.; Mund, M.; Knohl, A.; Linke, P.; Boerner, A.; Schulze, E. Inter-annual and seasonal variability of radial growth, wood density and carbon isotope ratios in tree rings of beech (Fagus sylvatica) growing in Germany and Italy. Trees 2006, 20, 571–586. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef] [PubMed]
- Senf, C.; Buras, A.; Zang, C.S.; Rammig, A.; Seidl, R. Excess forest mortality is consistently linked to drought across Europe. Nat. Commun. 2020, 11, 6200. [Google Scholar] [CrossRef]
- Colangelo, M.; Camarero, J.; Battipaglia, G.; Borghetti, M.; Micco, V.; Gentilesca, T.; Ripullone, F. A multi-proxy assessment of dieback causes in a Mediterranean oak species. Tree Physiol. 2017, 37, 617–631. [Google Scholar] [CrossRef]
- Lloret, F.; García, C. Inbreeding and neighbouring vegetation drive drought-induced die-off within juniper populations. Funct. Ecol. 2016, 30, 1696–1704. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Galiano, L.; Martinez Vilalta, J.; Sabaté, S.; Lloret, F. Determinants of drought effects on crown condition and their relationship with depletion of carbon reserves in a Mediterranean Holm oak forest. Tree Physiol. 2012, 32, 478–489. [Google Scholar] [CrossRef]
- Bigler, C.; Bräker, O.U.; Bugmann, H.; Dobbertin, M.; Rigling, A. Drought as an Inciting Mortality Factor in Scots Pine Stands of the Valais, Switzerland. Ecosystems 2006, 9, 330–343. [Google Scholar] [CrossRef]
- Galiano, L.; Martinez Vilalta, J.; Lloret, F. Drought-Induced Multifactor Decline of Scots Pine in the Pyrenees and Potential Vegetation Change by the Expansion of Co-occurring Oak Species. Ecosystems 2010, 13, 978–991. [Google Scholar] [CrossRef]
- Garcia-Forner, N.; Sala, A.; Biel, C.; Savé, R.; Martínez-Vilalta, J. Individual traits as determinants of time to death under extreme drought in Pinus sylvestris L. Tree Physiol. 2016, 36, 1196–1209. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; González-Gascueña, R.; González-Díaz, P.; Ruiz-Benito, P.; Andivia, E. Reduced growth sensitivity to water availability as potential indicator of drought-induced tree mortality risk in a Mediterranean Pinus sylvestris L. forest. Front. For. Glob. Chang. 2023, 6, 1249246. [Google Scholar] [CrossRef]
- Lasy Państwowe. Bank Danych o Lasach (Forest Data Bank); Lasy Państwowe: Warszawa, Poland, 2023. Available online: https://www.bdl.lasy.gov.pl/portal/ (accessed on 11 October 2023).
- Van Loon, A.F.; Stahl, K.; Di Baldassarre, G.; Clark, J.; Rangecroft, S.; Wanders, N.; Gleeson, T.; Van Dijk, A.I.J.M.; Tallaksen, L.M.; Hannaford, J.; et al. Drought in a human-modified world: Reframing drought definitions, understanding, and analysis approaches. Hydrol. Earth Syst. Sci. 2016, 20, 3631–3650. [Google Scholar] [CrossRef]
- Somorowska, U. Changes in Drought Conditions in Poland over the Past 60 Years Evaluated by the Standardized Precipitation-Evapotranspiration Index. Acta Geophys. 2016, 64, 2530–2549. [Google Scholar] [CrossRef]
- Liu, C.; Yang, C.; Yang, Q.; Wang, J. Spatiotemporal drought analysis by the standardized precipitation index (SPI) and standardized precipitation evapotranspiration index (SPEI) in Sichuan Province, China. Sci. Rep. 2021, 11, 1280. [Google Scholar] [CrossRef]
- Shekhar, A.; Shapiro, C.A. What do meteorological indices tell us about a long-term tillage study? Soil Tillage Res. 2019, 193, 161–170. [Google Scholar] [CrossRef]
- Zespół Ochrony Lasu w Opolu. Sośniny w Dalszym Ciągu Zagrożone (Sośniny Are Still Under Threat). 2019. Available online: https://www.zolopole.lasy.gov.pl (accessed on 14 April 2023).
- RDLP Katowice. Ochrona Lasów na Terenie RDLP w Katowicach (Protection of Forests in the RDLP in Katowice). 2022. Available online: https://www.katowice.lasy.gov.pl/ochrona-lasu1#.VqXyuFIXukQ (accessed on 14 April 2023).
- Swidrak, I.; Gruber, A.; Kofler, W.; Oberhuber, W. Effects of environmental conditions on onset of xylem growth in Pinus sylvestris under drought. Tree Physiol. 2011, 31, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Polish Institute of Meteorology and Water Management (IMGW-PIB). 2022. Available online: https://danepubliczne.imgw.pl (accessed on 3 December 2022).
- Beguería, S.; Serrano, S.M.V.; Reig-Gracia, F.; Garcés, B.L. SPEIbase v.2.9 (Dataset). DIGITAL.CSIC. Version 2.9. 2023. Available online: http://hdl.handle.net/10261/332007 (accessed on 17 December 2023).
- Leonelli, G.; Battipaglia, G.; Cherubini, P.; Saurer, M.; Siegwolf, R.T.W.; Maugeri, M.; Stenni, B.; Fusco, S.; Maggi, V.; Pelfini, M. Larix decidua δ18O tree-ring cellulose mainly reflects the isotopic signature of winter snow in a high-altitude glacial valley of the European Alps. Sci. Total Environ. 2017, 579, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, G.; Pelfini, M.; Panseri, S.; Battipaglia, G.; Vezzola, L.; Giorgi, A. Tree-ring stable isotopes, growth disturbances and needles volatile organic compounds as environmental stress indicators at the debris covered miage glacier (Monte Bianco Massif, European Alps). Geogr. Fis. Din. Quat. 2015, 37, 101–111. [Google Scholar] [CrossRef]
- Eckstein, D.; Bauch, J. Beitrag zur Rationalisierung eines dendrochronologischen Verfahrens und zur Analyse seiner Aussagesicherheit. Forstwiss. Cent. 1969, 88, 230–250. [Google Scholar] [CrossRef]
- Niccoli, F.; Pelleri, F.; Manetti, M.C.; Sansone, D.; Battipaglia, G. Effects of thinning intensity on productivity and water use efficiency of Quercus robur L. For. Ecol. Manag. 2020, 473, 118282. [Google Scholar] [CrossRef]
- Bunn, A.G. A dendrochronology program library in R (dplR). Dendrochronologia 2008, 26, 115–124. [Google Scholar] [CrossRef]
- Holmes, R.L. Computer-Assisted Quality Control in Tree-Ring Dating and Measurement. Tree-Ring Bull. 1983, 43, 51–67. [Google Scholar]
- John, E. The Balance of Nature? Ecological Issues in the Conservation of Species and Communities by Stuart L. Pimm (University of Chicago Press, Chicago and London, 1992, ISBN 0 226 66830 4, 434 pp., SB £21.50/$26.95). Oryx 1993, 27, 125–126. [Google Scholar] [CrossRef]
- McCarroll, D.; Loader, N.J. Stable isotopes in tree rings. Quat. Sci. Rev. 2004, 23, 771–801. [Google Scholar] [CrossRef]
- Zang, C.; Biondi, F. Treeclim: An R package for the numerical calibration of proxy-climate relationships. Ecography 2015, 38, 431–436. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Anand, M.; Leithead, M.D. Recent widespread tree growth decline despite increasing atmospheric CO2. PLoS ONE 2010, 5, e11543. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Siegwolf, R.; Saurer, M.; Eilmann, B.; Rigling, A. Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol. 2014, 203, 94–109. [Google Scholar] [CrossRef]
- Pińskwar, I.; Choryński, A.; Kundzewicz, Z.W. Severe Drought in the Spring of 2020 in Poland—More of the Same? Agronomy 2020, 10, 1646. [Google Scholar] [CrossRef]
- Petrucco, L.; Nardini, A.; von Arx, G.; Saurer, M.; Cherubini, P. Isotope signals and anatomical features in tree rings suggest a role for hydraulic strategies in diffuse drought-induced die-back of Pinus nigra. Tree Physiol. 2017, 37, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Das, A.J.; Battles, J.J.; Stephenson, N.L.; van Mantgem, P.J. The relationship between tree growth patterns and likelihood of mortality: A study of two tree species in the Sierra Nevada. Can. J. For. Res. 2007, 37, 580–597. [Google Scholar] [CrossRef]
- Cavin, L.; Mountford, E.P.; Peterken, G.F.; Jump, A.S. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Johnstone, J.F.; Allen, C.D.; Franklin, J.F.; Frelich, L.E.; Harvey, B.J.; Higuera, P.E.; Mack, M.C.; Meentemeyer, R.K.; Metz, M.R.; Perry, G.L.W.; et al. Changing disturbance regimes, ecological memory, and forest resilience. Front. Ecol. Environ. 2016, 14, 369–378. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Pinkard, E.A.; Brodribb, T.J.; Arndt, S.K.; Blackman, C.J.; Duursma, R.A.; Fensham, R.J.; Hilbert, D.W.; Nitschke, C.R.; et al. An ecoclimatic framework for evaluating the resilience of vegetation to water deficit. Glob. Chang. Biol. 2016, 22, 1677–1689. [Google Scholar] [CrossRef]
- Trugman, A.T.; Detto, M.; Bartlett, M.K.; Medvigy, D.; Anderegg, W.R.L.; Schwalm, C.; Schaffer, B.; Pacala, S.W. Tree carbon allocation explains forest drought-kill and recovery patterns. Ecol. Lett. 2018, 21, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Joseph, J.; Peter, M.; Luster, J.; Pritsch, K.; Geppert, U.; Kerner, R.; Molinier, V.; Egli, S.; Schaub, M.; et al. Recovery of trees from drought depends on belowground sink control. Nat. Plants 2016, 2, 16111. [Google Scholar] [CrossRef] [PubMed]
- Poyatos, R.; Aguadé, D.; Galiano, L.; Mencuccini, M.; Martínez-Vilalta, J. Drought-induced defoliation and long periods of near-zero gas exchange play a key role in accentuating metabolic decline of Scots pine. New Phytol. 2013, 200, 388–401. [Google Scholar] [CrossRef]
- Shestakova, T.; Voltas, J.; Saurer, M.; Berninger, F.; Esper, J.; Andreu-Hayles, L.; Daux, V.; Helle, G.; Leuenberger, M.; Loader, N.; et al. Spatio-temporal patterns of tree growth as related to carbon isotope fractionation in European forests under changing climate. Glob. Ecol. Biogeogr. 2019, 28, 1295–1309. [Google Scholar] [CrossRef]
- Hemming, D.L.; Switsur, V.R.; Waterhouse, J.S.; Heaton, T.H.E.; Carter, A.H.C. Climate variation and the stable carbon isotope composition of tree ring cellulose: An intercomparison of Quercus robur, Fagus sylvatica and Pinus silvestris. Tellus Ser. B Chem. Phys. Meteorol. 1998, 50, 25–33. [Google Scholar] [CrossRef]
- Gessler, A.; Ferrio, J.P.; Hommel, R.; Treydte, K.; Werner, R.A.; Monson, R.K. Stable isotopes in tree rings: Towards a mechanistic understanding of isotope fractionation and mixing processes from the leaves to the wood. Tree Physiol. 2014, 34, 796–818. [Google Scholar] [CrossRef]
- Farquhar, G.; O’Leary, M.H.O.; Berry, J. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Nagavciuc, V.; Kern, Z.; Ionita, M.; Hartl, C.; Konter, O.; Esper, J.; Popa, I. Climate signals in carbon and oxygen isotope ratios of Pinus cembra tree-ring cellulose from the Călimani Mountains, Romania. Int. J. Climatol. 2020, 40, 2539–2556. [Google Scholar] [CrossRef]
- Olano, J.; Linares, J.; García-Cervigón, A.; Arzac, A.; Delgado Huertas, A.; Rozas, V. Drought-induced increase in water-use efficiency reduces secondary tree growth and tracheid wall thickness in a Mediterranean conifer. Oecologia 2014, 176, 273–283. [Google Scholar] [CrossRef]
- Waterhouse, J.S.; Switsur, V.R.; Barker, A.C.; Carter, A.H.; Hemming, D.L.; Loader, N.J.; Robertson, I. Northern European trees show a progressive diminishing response to increasing atmospheric carbon dioxide concentrations. Quat. Sci. Rev. 2004, 23, 803–810. [Google Scholar] [CrossRef]
- Saurer, M.; Spahni, R.; Frank, D.C.; Joos, F.; Leuenberger, M.; Loader, N.J.; McCarroll, D.; Gagen, M.; Poulter, B.; Siegwolf, R.T.; et al. Spatial variability and temporal trends in water-se efficiency of European forest. Glob. Chang. Biol. 2014, 20, 3700–3712. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Horwath, W. Explaining Global Increases in Water Use Efficiency: Why Have We Overestimated Responses to Rising Atmospheric CO2 in Natural Forest Ecosystems? PLoS ONE 2013, 8, e53089. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Li, Z.; Han, S.; Wang, X. Climate change increased the intrinsic water use efficiency of Larix gmelinii in permafrost degradation areas, but did not promote its growth. Agric. For. Meteorol. 2022, 320, 108957. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Linares, J.C.; Julio Camarero, J. Drought and mistletoe reduce growth and water-use efficiency of Scots pine. For. Ecol. Manag. 2013, 296, 64–73. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J. From pattern to process: Linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Chang. Biol. 2012, 18, 1000–1015. [Google Scholar] [CrossRef]
- Peñuelas, J.; Hunt, J.M.; Ogaya, R.; Jump, A.S. Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: Increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob. Chang. Biol. 2008, 14, 1076–1088. [Google Scholar] [CrossRef]
- Hentschel, R.; Rosner, S.; Kayler, Z.E.; Andreassen, K.; Børja, I.; Solberg, S.; Tveito, O.E.; Priesack, E.; Gessler, A. Norway spruce physiological and anatomical predisposition to dieback. For. Ecol. Manag. 2014, 322, 27–36. [Google Scholar] [CrossRef]
- Urrutia-Jalabert, R.; Malhi, Y.; Barichivich, J.; Lara, A.; Delgado Huertas, A.; Rodríguez, C.; Cuq, E. Increased water use efficiency but contrasting tree-growth patterns in Fitzroya cupressoides forests of southern Chile during recent decades. J. Geophys. Res. Biogeosci. 2015, 120, 2505–2524. [Google Scholar] [CrossRef]

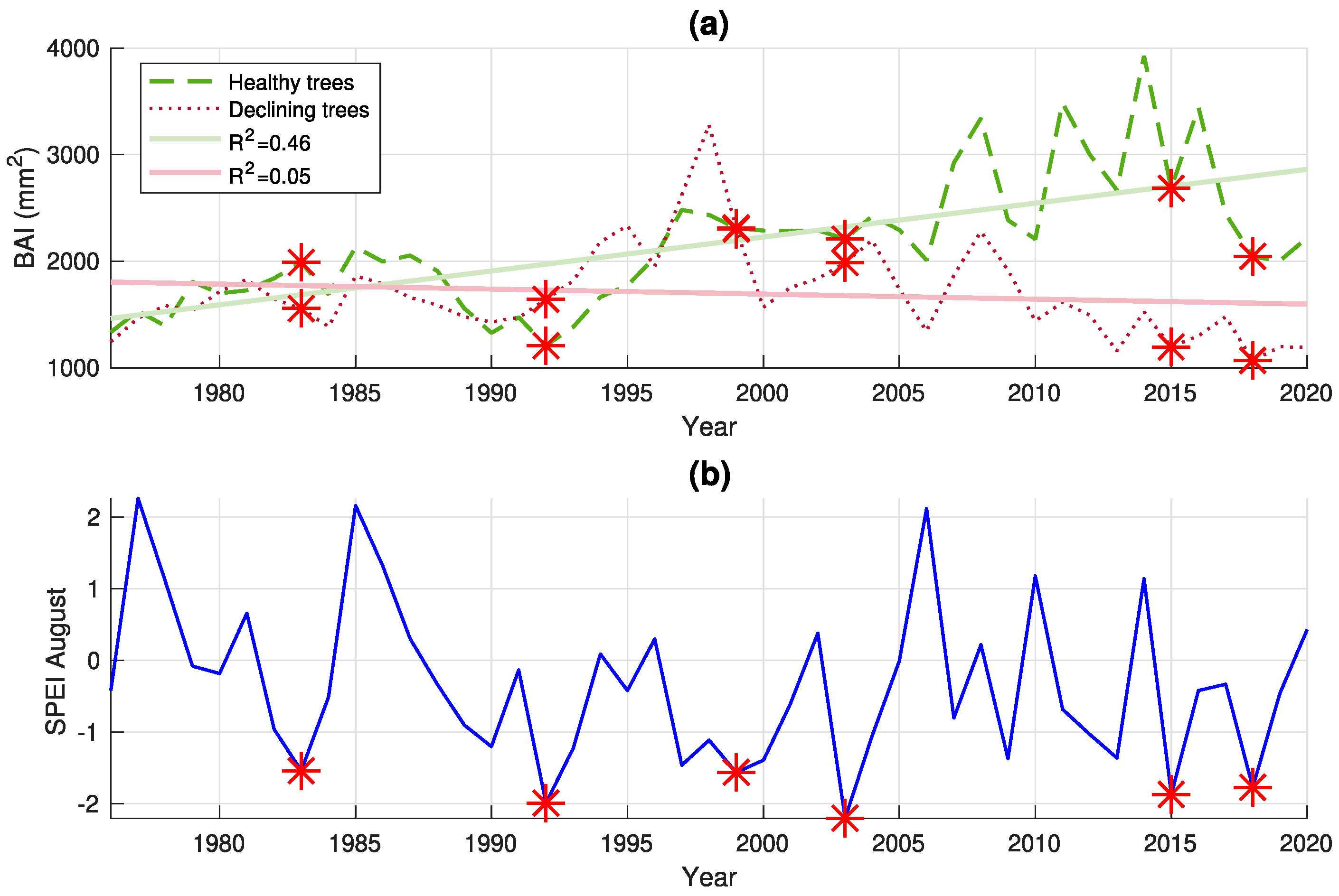
| Group | Resistance Index (Rt) | Resilience Index (Rs) | Recovery Index (Rc) | Year with Lowest August SPEI |
|---|---|---|---|---|
| Healthy | 1.18 (±0.11) | 1.16 (±0.11) | 0.98 (±0.08) | 1983 |
| Declining | 0.94 (±0.06) | 1.00 (±0.13) | 1.06 (±0.10) | |
| Healthy | 0.73 (±0.12) | 1.12 (±0.23) | 1.55 (±0.13) | 1992 |
| Declining | 1.08 (±0.07) | 1.43 (±0.11) | 1.33 (±0.11) | |
| Healthy | 1.11 (±0.18) | 1.11 (±0.15) | 0.99 (±0.03) | 1999 |
| Declining | 0.93 (±0.19) | 0.76 (±0.32) | 0.81 (±0.16) | |
| Healthy | 0.95 (±0.02) | 1.12 (±0.16) | 1.18 (±0.16) | 2003 |
| Declining | 0.93 (±0.30) | 0.88 (±0.43) | 0.95 (±0.21) | |
| Healthy | 0.88 (±0.17) | 0.80 (±0.37) | 0.91 (±0.24) | 2015 |
| Declining | 0.83 (±0.10) | 0.86 (±0.20) | 1.04 (±0.12) | |
| Healthy | 0.67 (±0.12) | 0.76 (±0.27) | 1.13 (±0.09) | 2018 |
| Declining | 0.80 (±0.10) | 0.87 (±0.16) | 1.08 (±0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benisiewicz, B.; Pawełczyk, S.; Niccoli, F.; Kabala, J.P.; Battipaglia, G. Drought Impact on Eco-Physiological Responses and Growth Performance of Healthy and Declining Pinus sylvestris L. Trees Growing in a Dry Area of Southern Poland. Forests 2024, 15, 741. https://doi.org/10.3390/f15050741
Benisiewicz B, Pawełczyk S, Niccoli F, Kabala JP, Battipaglia G. Drought Impact on Eco-Physiological Responses and Growth Performance of Healthy and Declining Pinus sylvestris L. Trees Growing in a Dry Area of Southern Poland. Forests. 2024; 15(5):741. https://doi.org/10.3390/f15050741
Chicago/Turabian StyleBenisiewicz, Barbara, Sławomira Pawełczyk, Francesco Niccoli, Jerzy Piotr Kabala, and Giovanna Battipaglia. 2024. "Drought Impact on Eco-Physiological Responses and Growth Performance of Healthy and Declining Pinus sylvestris L. Trees Growing in a Dry Area of Southern Poland" Forests 15, no. 5: 741. https://doi.org/10.3390/f15050741





