1. Introduction
Bone development is one of the key processes of intrauterine and postnatal growth [
1]. Preterm infants are at significant risk to develop reduced bone mineral content based on inadequate supply of calcium and phosphorus (Ca-P) [
2,
3]. During normal pregnancies in healthy mothers, there is an active, placental transfer of Ca-P to the fetus leading to a high mineral accretion during the last trimester, while after birth the infant is dependent on nutritional supply of minerals [
4,
5]. In clinical practice, postnatally it is difficult to meet the high fetal needs, because of limited solubility of parenteral fluids, low content of Ca-P of human milk and impaired intestinal absorption through formula feeding [
6,
7,
8,
9]. Studies tried to define nutritional requirements, but, in clinical practice, it is often uncertain whether the nutritional intake of Ca-P provided to preterm infants is sufficient and is actually used for bone mineralization [
10,
11,
12].
Assuming that biochemical parameters of Ca-P homeostasis within a normal range will lead to optimal bone mineralization, evaluation of electrolyte disturbances is standard of care in many neonatal units [
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. However, there is currently neither a consensus on the appropriateness of either parameter or the frequency of measurements [
29,
30]. A recent survey among U.S. neonatologists showed a great lack of consensus and variation in practices regarding definition and screening methods for metabolic bone disease [
31]. Reference values in relation to adequate nutritional intake have not been developed. Urinary excretion of minerals in spot urine samples has been shown to be an easy tool for routine evaluation. Pohlandt proposed to aim for a small “surplus of minerals” in urine samples, while Aladangady et al. developed reference values for urinary Ca-P/creatinine ratios for preterm infants [
17,
23]. Staub et al. compared both methods with regard to an agreement between their results and found neither method to be superior [
32]. None of the studies evaluated the direct effect of nutritional intake on biochemical parameter of Ca-P homeostasis. It is not sure whether biochemical parameters are able to indicate sufficiency of nutritional intake.
The aim of this study was to evaluate changes in biochemical parameters of the Ca-P homeostasis in blood and urine in relation to different nutritional intake during the first 14 days of life in Very Low Birth Weight (VLBW) infants. Our hypothesis was that the nutritional intake of calcium and phosphorus would have an effect on biochemical parameters of the calcium–phosphorus homeostasis.
2. Materials and Methods
2.1. Study Design and Randomization
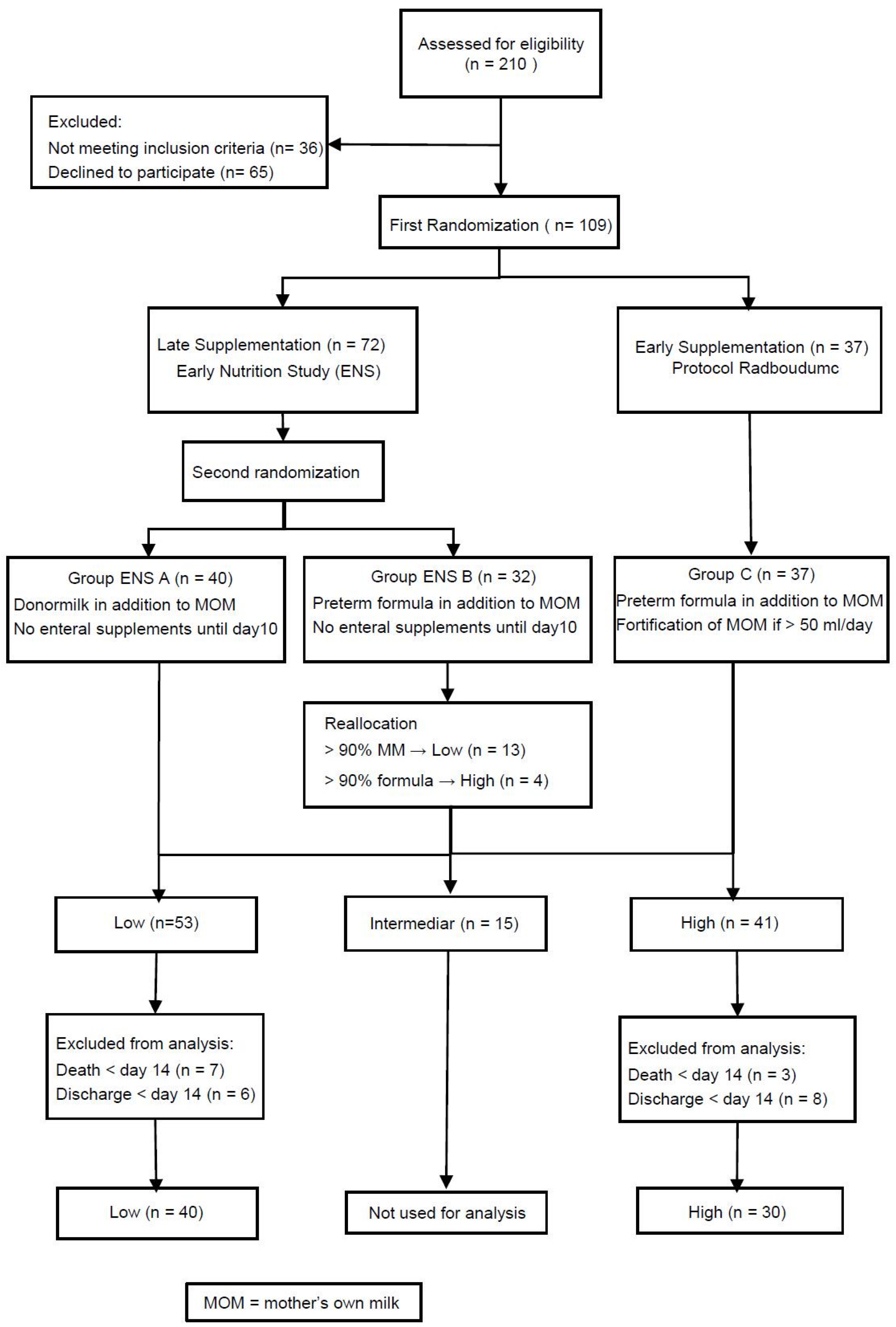
The current study (Early Supplementation Study (ESS)) was part of the Early Nutrition Study (ENS), a multi-center double-blinded randomized controlled trial.While the ENS evaluated the effects of human milk on postnatal outcome, the primary objective of the ESS was bone mineralization in relation to early and late enteral supplementation of minerals [
33]. The studies were approved by the Ethical Committee of the VU university medical center (Amsterdam, The Netherlands), 13 September 2013 (CMO file number: NL37296.029.11, Dutch Trial Registry: NTR 3225). Patients were distributed into three groups through two steps of randomization. The first step randomized eligible infants either into the early supplementation group (High) or the late supplementation group (Low) being part of the ENS. The second step of the randomization was only performed if infants were assigned to late supplementation and randomized them to either “ENS A” or “ENS B” as part of the ENS. Both randomization steps were performed before the first enteral nutrition was administered resulting in basically three groups.
2.2. Study Population
Participants for the ENS/ESS were recruited at the level III neonatal intensive care unit of the Radboud university medical center (Radboudumc), Nijmegen, The Netherlands. The inclusion criteria were a birth weight below 1500 grams and written informed consent of both parents. The exclusion criteria were maternal drugs and/or alcohol use during pregnancy, birth defects, congenital infection within 72 h after birth, perinatal asphyxia with a pH < 7.0 and any intake of cow’s milk based products prior to randomization. For the current study, infants who died or were discharged before the end of the study period of 14 days were excluded from analysis.
2.3. Intervention and Nutritional Protocol
Parenteral nutrition (PN) was started directly within the first hour after birth. The PN solution consisted of 2.5 mmol/dL calcium-gluconate (calcium-gluconate 10%; B. Braun, Melsungen, Germany) and 1.6 mmol/dL sodium-glycerophosphate (Glycophos; Fresenius Kabi BV, Zeist, The Netherlands) and 2.25 grams/dL amino acids (Primene; Clintec, Brussels). Additional parenteral supplementation with 10% calcium-gluconate or sodium glycero-phosphate was administrated depending on biochemical parameters.
Table A1 presents the standard protocol for PN. The decision to start additional supplementation was left to the attending neonatologist based on biochemical parameter as being a standard procedure of our department.
Enteral feeding was started on the first day of life with daily increments, while PN was gradually reduced to maintain a daily fluid intake within the protocol range. Late supplementation was assumed to provide a low intake of nutrients because “Group Low”, comprising of group ENS A and ENS B, received no additional enteral supplementation or fortification of human milk during the first 10 days of life. Group ENS A received donor milk if mother’s own milk (MOM) was not available. Group ENS B received preterm formula (Hero Baby Prematuur Start; Hero Kindervoeding, Breda, The Netherlands) if MOM was not available, containing 2.4 mmol/dL calcium, 1.7 mmol/dL phosphorus and 2.6 grams/dL proteins. The additional nutrition in ENS A and ENS B was blinded to all caretakers and parents. After 10 days, all infants received nutrition according to the standard protocol of the Radboudumc.
Early supplementation was assumed to provide a high intake (Group High). This group received enteral nutrition from Day 1 onwards according to the local protocol. They received additional enteral supplementation and human milk fortifier (Nutrilon Neonatal BMF; Nutricia, Zoetermeer, The Netherlands; BMF) by the time the enteral intake was 50 mL per day. The human milk fortifier added 1.65 mmol/dL calcium, 1.22 mmol/dL phosphorus and 0.8 grams/dL protein to human milk. They received preterm formula if MOM was not available. The decision to start additional enteral supplementation was left to the attending neonatologist based on biochemical parameter and postnatal growth as being a standard procedure of our department. The additional enteral supplementation could comprise of either a supplement of protein (Nutrilon Nenatal Protein Fortifier; Nutricia, Zoetermeer, The Netherlands) or a potassium phosphate (KPO4) and calcium chloride (CaCl2) suspension for enteral supplementation.
Group ENS A received 100% human milk during the first 10 days and reflected a group with low intake of minerals and protein, because human milk has a very low nutrient content. Group High reflected a high intake of nutrients, because human milk was enriched with minerals and protein as soon as possible. Group ENS B could be considered as intermediate depending on the amount of MOM or preterm formula an infant received, since preterm formula contained approximately the same amount of minerals as fortified human milk.
2.4. Biochemical Parameters of Bone Mineralization
For this study, blood and urine samples were analyzed according to the local protocol of the department. Samples were taken on Days 1, 3, 5, 7, 10 and 14 after birth. Urine was collected through spot samples [
25]. The following parameters were analyzed: serum calcium (sCa), serum phosphorus (sP), serum alkaline phosphatase (ALP), urine calcium (uCa), urine phosphorus (uP), urine calcium/creatinin ratio (uCa/Creat), urine phosphorus/creatinin ratio (uP/Creat), and tubular reabsorption of phosphorus (TrP).
2.5. Data Registration and Handling
Patient characteristics, clinical course, growth and intake of all nutrients were recorded daily from the patient records and abstracted for this study. Amounts of enteral, parenteral and additional supplementation (parenteral and enteral) of all nutrients were calculated separately for each patient. The total intakes were calculated per kg per day per infant. The intake through human milk was calculated based on the reference of Gidrewicz et al. [
34]. The calcium/phosphorus ratio was calculated per day by dividing the daily intake of calcium in mmol/kg through the daily intake of phosphorus in mmol/kg.
After closure of patient enrollment and de-blinding of the ENS, we performed a reallocation procedure for the intermediate group ENS B. Infants who received more than 90% MOM were considered to reflect a low intake of minerals and were allocated to group Low together with the infants of group ENS A. Infants who received more than 90% of preterm formula were considered to reflect a high intake of minerals and were allocated to High. Infants in between these extremes were not included in the analyses.
2.6. Statistical Analysis
The primary objective of the ESS was bone mineralization in relation to mineral supplementation, and the original power calculation was based on bone mineral content at term corrected age. For the evaluation of changes in biochemical parameters in relation to nutritional intake the power calculation was based on sP. In a previous evaluation of our nutritional protocol performed at our department, we found a mean 1.7 mmol/L of sP during the first week [
35]. A concentration of 2.0 mmol/L was defined as target for optimal bone mineralization by Hellstern et al. [
20]. Assuming an expected mean of 1.7 mmol/L, we determined that 24 infants were required in each group to find a difference of 0.3 mmol/L in sP between High and Low with α = 0.05 (two-sided) and a power of β = 0.80.
The statistical analyses were performed using IBM SPSS statistics 22.0 for Windows (IBM SPSS Inc., Chicago, IL, USA). Differences in patient characteristics, nutritional characteristics and biochemical parameters between the High and Low group were determined using the Mann-Whitney U test or the chi-square test, depending on the variable under examination. Due to non-normality of the continuous variables, the data were presented as median (with interquartile range (IQR)), unless otherwise indicated. A p-value < 0.05 was considered statistically significant.
To account for repeated outcome measurements, we used a mixed model analysis to determine the effects of daily nutritional intake of calcium and phosphorus on each biochemical parameter. We included the total intake of Ca/P and protein, the percentage of enteral amount of Ca/P intake, and a number of clinical parameter that could affect the Ca/P homeostasis such as birth weight, gestational age, gender, caesarian section, multiple births, sepsis, and days of caffeine, furosemide, steroids, and sedation during the first two weeks as co-variables in the initial models. Necrotizing enterocolitis was not included as co-variable because of small numbers. Using manual backward selection, variables were kept in the model when they contributed statistically significantly with a p-value < 0.1.
4. Discussion
In this observational study of initially three randomized groups providing different nutritional intake to VLBW infants during the first 10 days of life, we found no differences between groups Low and High concerning the biochemical parameters of Ca-P homeostasis. However, the mixed model analysis showed that the intake of calcium was associated with increased urinary calcium excretion and tubular reabsorption of phosphorus and a decrease in the ALP, while the nutritional intake of phosphorus was associated with a decreased sCa and an increase in sP, uP and uP/creat ratio. The nutritional intake of calcium and phosphorus affected the TrP and ALP in opposite directions. Protein intake was greatly associated with a decrease in sP, uP and an increase in ALP, sCa, and uCa, while, in addition, gestational age and male gender affected especially the phosphorus metabolism.
VLBW infants belong to one of the most vulnerable patient groups for whom adequate postnatal nutritional intake has life-long consequences [
41]. Therefore, intervention studies with different nutritional intakes could be seen as unethical in the light of the right of optimal treatment for every patient. On the other hand, in clinical practice a great variation in clinical guidelines has been reported, often based on rather low evidence [
42]. While fortification of human milk is generally seen as necessary nowadays, there is also concern about possible risks of introducing cow-milk based products too early [
43,
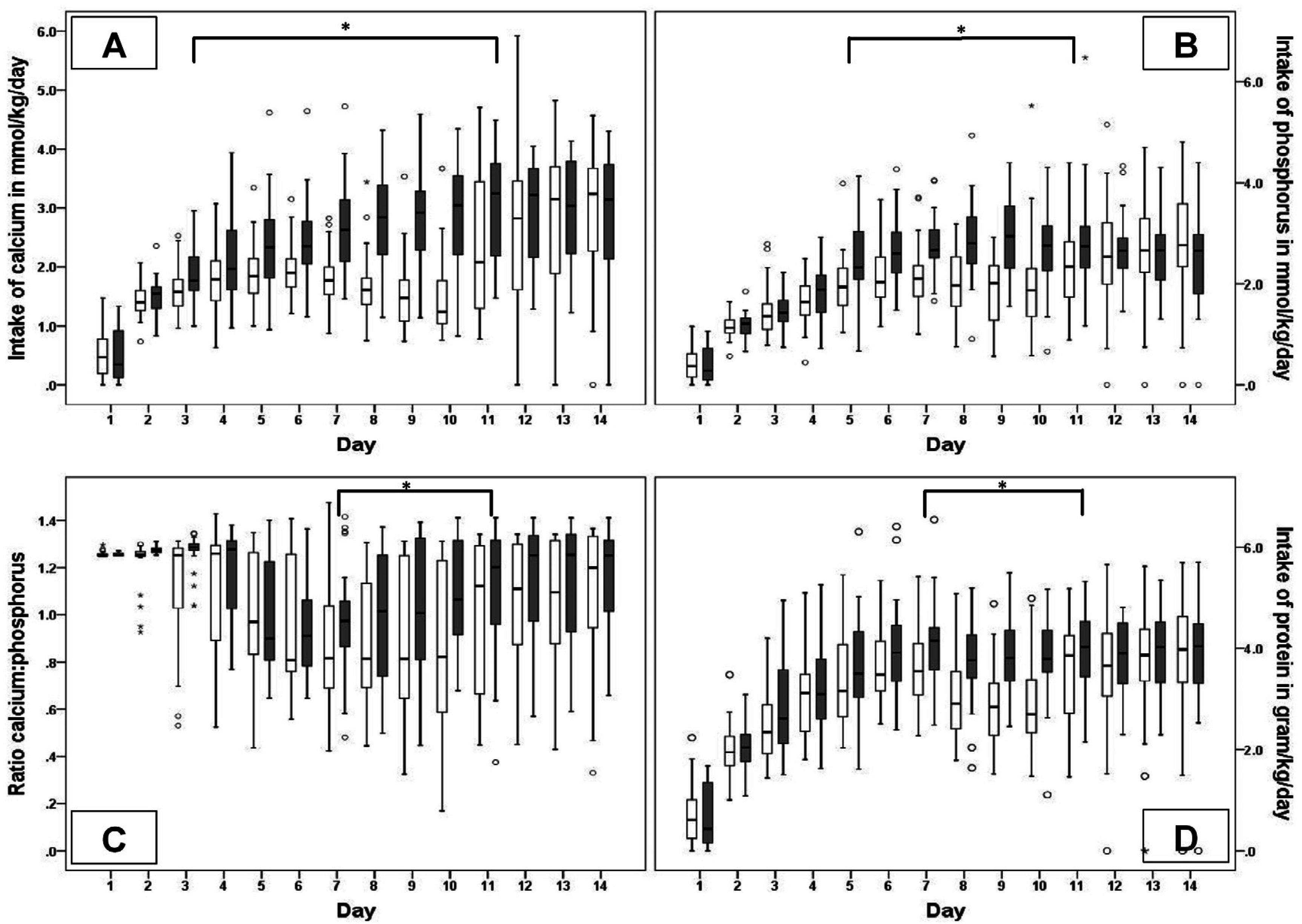
44]. According to our local protocol, fortification is introduced early and additional mineral supplementation is provided based on laboratory results. The intention is to optimize postnatal growth and bone mineralization but the efficacy of our protocol has not been proven. The combination of the Early Nutrition Study and the Early Supplementation Study provided the opportunity to evaluate two different nutritional concepts within the range of nutritional guidelines and therefore within the ethical limits. On the other hand, all infants participating in the ESS, independent of group allocation, received the standard treatment according to the local practice, which frequently led to additional parenteral supplementation of nutrients in case of electrolyte disturbances or impaired growth. This practice may have ameliorated the differences between the groups and therefore affected the results. By reallocating infants from group ENS B to either group Low or High and excluding infants with intermediate intake from further analysis, we tried to maximize the differences in nutritional intake between the two remaining groups. The reallocation of infants did not change the baseline patient characteristics. The detailed analysis of nutritional intake showed that additional supplementation was not different between the groups and differences in intake were mainly based on enteral nutrition.
Even though the two groups had a maximum difference in nutritional intake, the comparison of daily concentrations of the biochemical parameters showed no differences between groups High and Low, probably by leveling out inter-individual differences on group level. In contrast, the linear mixed model analysis took into account both intra- and inter-individual fluctuations, and thereby enabled us to specify effects of various co-variables.
Despite an increasing intake of phosphorus, sP remained below our target concentration during the first week. Recently, a randomized trial, evaluating nutritional support according to current recommendations in VLBW infants, observed hypophosphatemia in relation to high protein intake [
45]. Jamin et al. observed electrolyte disturbances, especially hypophosphatemia and hypokalemia, in low-birth weight piglets with a high protein diet [
46]. Hypophosphatemia is the hallmark of the refeeding syndrome and a well-known complication in relation to parenteral nutrition of malnourished patients [
47,
48,
49]. Bonsante et al. proposed the concept of Placental Incompletely Restored Feeding (PI-Refeeding) syndrome for electrolyte disturbances found in VLBW infants [
50]. This syndrome is said to be caused by an imbalanced nutritional intake of amino acids and phosphorus. Amino acids and energy are needed to maintain an anabolic state of the cell, while phosphorus is necessary for a number of cellular functions, energy homeostasis as well as for bone mineralization. Phosphorus in blood will preferably be transferred to the cell regardless of bone mineral status. A higher intake of amino acids will enhance the need for phosphorus in growing cells, and in case of low concentrations of phosphorus in blood it will be released from bone. Simultaneously with the release of phosphorus, calcium will also be released from the bone because of an unfavorable Ca/P ratio and will consecutively be excreted in urine if the sP concentrations are too low. Our results are in agreement with this concept. According to the mixed model analyses, we found that an increasing amount of protein was associated with an increase in the sCa, uCa, ALP and uCa/Creat ratio, whereas it was associated with a decrease in sP, uP and the uP/Creat ratio. Remarkably, in our study, an increase in a sP concentration of 0.13 mmol/L occurred per 1 mmol/kg intake of phosphorus and a decrease of −0.13 mmol/L per 1 gram/kg protein intake, meaning that 1 gram/kg of protein intake should be accompanied by 1 mmol/kg of phosphorus in nutrition of VLBW infants to maintain adequate sP concentrations.
The role of ALP in bone mineralization is controversial, but an increase is usually associated with poor bone mineralization [
30,
51]. According to our results, an increasing intake of protein was associated with an increase in ALP. Again, following the above mentioned mechanisms higher protein intake enhanced the cellular need of phosphorus and thereby decreased the sP concentration and the availability of phosphorus for bone mineralization, leading to activation of ALP. We also found that an increased ALP was associated with increasing phosphorus intake, while one would expect lowering of ALP. An explanation for this phenomenon could be a relatively insufficient intake of calcium in combination with phosphorus intake, since an increasing calcium intake was associated with decrease in ALP concentrations. In this study, for both groups, the calcium/phosphorus ratio was below recommendations, meaning that relatively more phosphorus than calcium was administered.
Gestational age at birth seemed to be an important determinant for the phosphorus metabolism in our study, meaning that infants with a lower gestational age had a higher renal excretion of phosphorus, irrespective of nutritional intake. Immaturity of the kidneys at lower gestational age has been shown to cause impaired tubular reabsorption of phosphorus [
15]. Renal losses of minerals may then compromise the effect of nutritional intake on bone mineralization. However, current recommendations for nutritional intake of calcium and phosphorus usually do not take into account differences in renal function based on gestational age.
Further, we found that male gender was related to low serum phosphorus concentrations, low tubular reabsorption and increased renal excretion of phosphorus and uP/creat ratio. We speculate a retardation in maturation of the renal function in male infants compared to females as is known for the development of the pulmonary function [
52].
All parameters evaluated in this study are regularly used to monitor either electrolyte homeostasis or bone mineralization. Practices among units vary greatly, measurements may be performed at later age and greater intervals and not standardized or in combination, leading to inconsistent results and handling. An explanation for the inconsistency in results of other studies could be the underestimation of the effects of inter-relationships between various co-variates. In our opinion, these associations can only be discovered with standardized repeated measurements taking into account other clinical factors. To our knowledge, this is the first study evaluating changes in biochemical parameters of the calcium-phosphorus homeostasis based on standardized repeated measurements and daily changes in nutritional intake in a mixed model linear regression analysis including also clinical factors.
Our data show that standardized repeated measurements of blood and urine samples can provide useful information with regard to the Ca-P homeostasis. This does not result in a clear advice for nutritional intake. Nevertheless, this study is a first step and its importance lies in the description and quantification of changes in a more “physiological way” that will further enable us to develop new guidelines to improve bone mineral status in preterm infants. Notwithstanding, we confirmed the relationship between the intake of protein and phosphorus, and demonstrated the effect of renal immaturity and gender. Thus, a second step could be, to relate the current results to bone mineralization and provide recommendations for nutritional intake and a third step to develop a concept of target values for biochemical parameter so that these can be used to monitor nutritional intake to achieve optimal bone mineralization in daily practice.
This study had several limitations. The mixed model analysis assumes that the effects of the different variables are linear which has not been proven yet. In addition, the biochemical parameters may have been influenced by factors that were not taken into account in our analysis. Daily sampling of biochemical parameter would have been optimal, but this was judged unethical regarding the amount of blood volume needed. Nevertheless, measurements were performed in a standardized manner and therefore provided a good reflection of changes in blood and urine concentrations for the complete study period. Further, in comparison to other studies, both groups had relatively high daily intakes. This may partly explain the small variations in biochemical parameters. This study only investigated the biochemical parameters during the first 14 days of life. Maturational changes in renal function may alter the results; however, repeated measurements will indicate these changes and thereby can be used as guide for optimal supplementation of minerals.