The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants
Abstract
:1. Introduction
2. Overview of Dietary Fats
3. Role of Dietary Fatty Acids in the Whole Brain
4. Dietary Fat Regulation of Brain Function and Links to Depression
4.1. Saturated Fatty Acids and the Whole Brain
4.2. Monounsaturated Fatty Acids and the Whole Brain
4.3. Polyunsaturated Fatty Acids and the Whole Brain
4.3.1. Dietary n-3 Polyunsaturated Fatty Acids
4.3.2. Dietary n-6 Polyunsaturated Fatty Acids
5. Role of Dietary Fatty Acids in Specific Brain Regions Involved in Depression
5.1. Dietary Fats, Depression, and the Hippocampus
5.2. Dietary Fats, Depression, and the Hypothalamic-Pituitary-Adrenal (HPA) Axis
5.3. Dietary Fats, Depression, and the Prefrontal Cortex (PFC)
5.4. Dietary Fats, Depression, and the Striatum
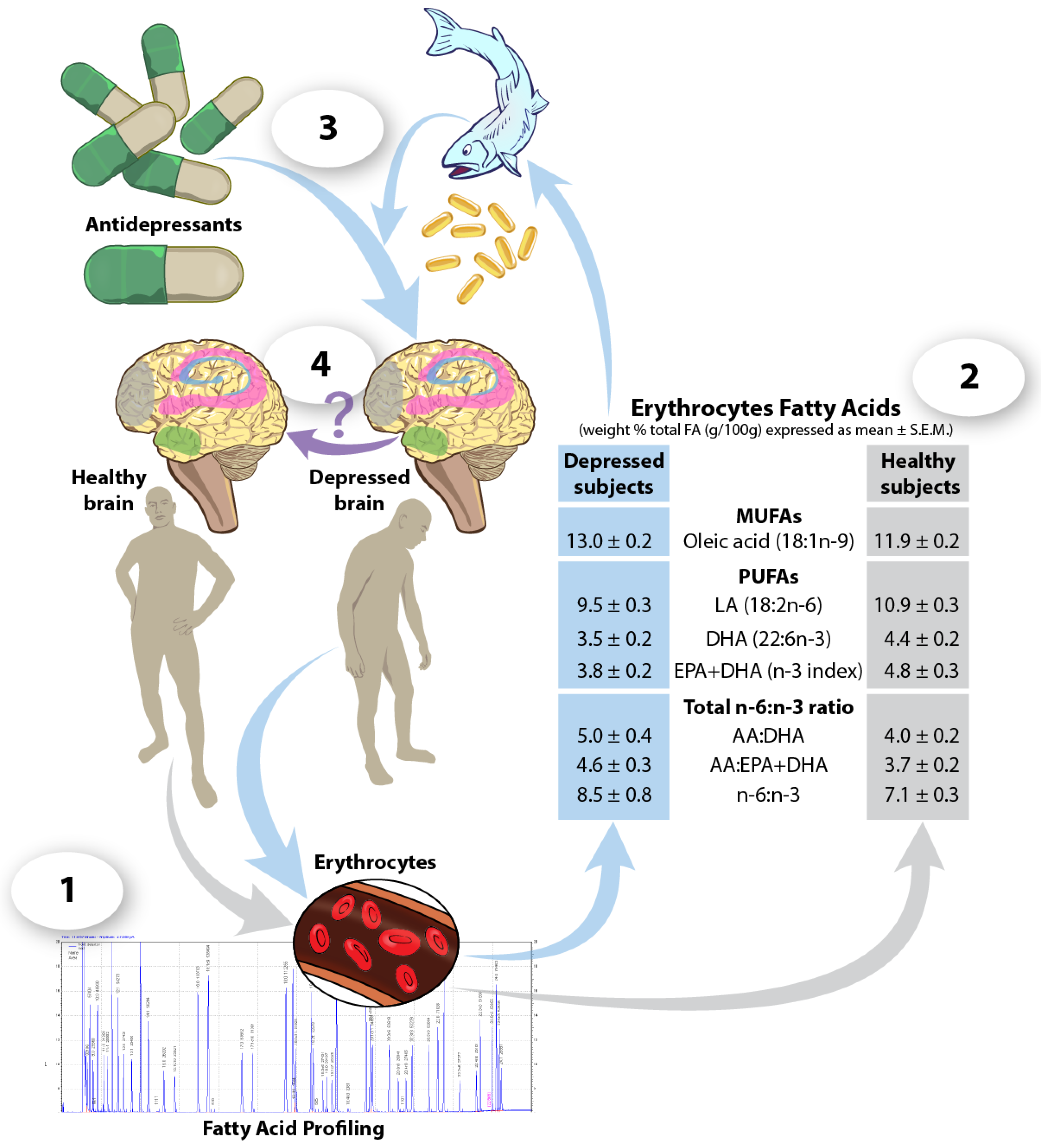
6. Blood Fatty Acids as Predictors of Response to Antidepressant Treatments
7. Future Perspectives
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kessler, R.C. Epidemiology of women and depression. J. Affect. Disord. 2003, 74, 5–13. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Ann. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Levinson, D.F. The genetics of depression: A review. Biol. Psychiatry 2006, 60, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, Y.; Li, F.; Zhang, D. Fruit and vegetable consumption and the risk of depression: A meta-analysis. Nutrition 2016, 32, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rahe, C.; Unrath, M.; Berger, K. Dietary patterns and the risk of depression in adults: A systematic review of observational studies. Eur. J. Nutr. 2014, 53, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.; Asha, M.R.; Ramesh, B.N.; Rao, K.S. Understanding nutrition, depression and mental illnesses. Ind. J. Psychiatry 2008, 50, 77–82. [Google Scholar]
- Tsuboi, H.; Watanabe, M.; Kobayashi, F.; Kimura, K.; Kinae, N. Associations of depressive symptoms with serum proportions of palmitic and arachidonic acids, and alpha-tocopherol effects among male population—A preliminary study. Clin. Nutr. 2013, 32, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.R.; Ogbonna, E.M.; Lim, S.; Li, Y.; Zhang, J. Dietary linoleic and oleic fatty acids in relation to severe depressed mood: 10 years follow-up of a national cohort. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [PubMed]
- Levant, B. N-3 (omega-3) polyunsaturated fatty acids in the pathophysiology and treatment of depression: Pre-clinical evidence. CNS Neurol. Disord. Drug Targets 2013, 12, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Schmitt, F.; Loeffler, J.P.; Gonzalez de Aguilar, J.L. Fatting the brain: A brief of recent research. Front. Cell. Neurosci. 2013, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Population Nutrient Intake Goals for Preventing Diet-Related Chronic Diseases. Available online: http://www.who.int/nutrition/topics/5_population_nutrient/en/index.html (accessed on 6 July 2016).
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Executive summary of the international conference on genetic variation and nutrition. World Rev. Nutr. Diet. 1990, 63, 1–13. [Google Scholar] [PubMed]
- Logan, A.C. Omega-3 fatty acids and major depression: A primer for the mental health professional. Lipids Health Dis. 2004, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Kolacz, P.; Castellano, S.; Galvano, F.; Buscemi, S.; Mistretta, A.; Grosso, G. A review of recent evidence in human studies of n-3 and n-6 pufa intake on cardiovascular disease, cancer, and depressive disorders: Does the ratio really matter? Int. J. Food Sci. Nutr. 2015, 66, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.A.; Hamilton, J.A.; Leaf, A.; Spector, A.A.; Moore, S.A.; Anderson, R.E.; Moser, H.W.; Noetzel, M.J.; Katz, R. Brain uptake and utilization of fatty acids: Applications to peroxisomal biogenesis diseases. J. Mol. Neurosci. 2001, 16, 87–92. [Google Scholar] [CrossRef]
- Chang, C.Y.; Ke, D.S.; Chen, J.Y. Essential fatty acids and human brain. Acta Neurol. Taiwan. 2009, 18, 231–241. [Google Scholar] [PubMed]
- Carrie, I.; Clement, M.; de Javel, D.; Frances, H.; Bourre, J.M. Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. J. Lipid Res. 2000, 41, 465–472. [Google Scholar] [PubMed]
- Kishimoto, Y.; Agranoff, B.W.; Radin, N.S.; Burton, R.M. Comparison of the fatty acids of lipids of subcellular brain fractions. J. Neurochem. 1969, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.P.; Reichel, M.; Muhle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain membrane lipids in major depression and anxiety disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: Importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Hatch, G.M. Fatty acid transport into the brain: Of fatty acid fables and lipid tails. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tassoni, D.; Kaur, G.; Weisinger, R.S.; Sinclair, A.J. The role of eicosanoids in the brain. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 220–228. [Google Scholar] [PubMed]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J.; Seutin, V.; Van Gaal, L.F. [Endocannabinoid system in the brain...and elsewhere]. Revue Med. Liege 2008, 63, 364–371. [Google Scholar]
- Naughton, S.S.; Mathai, M.L.; Hryciw, D.H.; McAinch, A.J. Fatty acid modulation of the endocannabinoid system and the effect on food intake and metabolism. Int. J. Endocrinol. 2013, 2013, 361895. [Google Scholar] [CrossRef] [PubMed]
- Hanus, L.; Avraham, Y.; Ben-Shushan, D.; Zolotarev, O.; Berry, E.M.; Mechoulam, R. Short-term fasting and prolonged semistarvation have opposite effects on 2-ag levels in mouse brain. Brain Res. 2003, 983, 144–151. [Google Scholar] [CrossRef]
- Artmann, A.; Petersen, G.; Hellgren, L.I.; Boberg, J.; Skonberg, C.; Nellemann, C.; Hansen, S.H.; Hansen, H.S. Influence of dietary fatty acids on endocannabinoid and n-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta 2008, 1781, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Crozier, G.; Bisogno, T.; Cavaliere, P.; Innis, S.; Di Marzo, V. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding n-acylethanolamines in piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Hryhorczuk, C.; Florea, M.; Rodaros, D.; Poirier, I.; Daneault, C.; Des Rosiers, C.; Arvanitogiannis, A.; Alquier, T.; Fulton, S. Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology 2016, 41, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Fick, L.J.; Fick, G.H.; Belsham, D.D. Palmitate alters the rhythmic expression of molecular clock genes and orexigenic neuropeptide y mrna levels within immortalized, hypothalamic neurons. Biochem. Biophys. Res. Commun. 2011, 413, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Cone, J.J.; Chartoff, E.H.; Potter, D.N.; Ebner, S.R.; Roitman, M.F. Prolonged high fat diet reduces dopamine reuptake without altering dat gene expression. PLoS ONE 2013, 8, e58251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Fulton, S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int. J. Obes. 2013, 37, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, Y.; Gomez-Pinilla, F. High-fat diet transition reduces brain dha levels associated with altered brain plasticity and behaviour. Sci. Rep. 2012, 2, 431. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Kim, Y.K. The roles of bdnf in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010, 7, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.M.; Machaj, A.S.; Chiu, G.S.; Lawson, M.A.; Gainey, S.J.; York, J.M.; Meling, D.D.; Martin, S.A.; Kwakwa, K.A.; Newman, A.F.; et al. Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology 2013, 38, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, T.; Ketterer, C.; Kullmann, S.; Balzer, M.; Rotermund, C.; Binder, S.; Hallschmid, M.; Machann, J.; Schick, F.; Somoza, V.; et al. Monounsaturated fatty acids prevent the aversive effects of obesity on locomotion, brain activity, and sleep behavior. Diabetes 2012, 61, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Kleinridders, A.; Cai, W.; Cappellucci, L.; Ghazarian, A.; Collins, W.R.; Vienberg, S.G.; Pothos, E.N.; Kahn, C.R. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc. Natl. Acad. Sci. USA 2015, 112, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Torre, P.; Antonello, R.M.; Cazzato, G.; Bava, A. Depression and alzheimer’s disease: Symptom or comorbidity? Am. J. Alzheimer’s Dis. Dement. 2002, 17, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhu, M.M.; Yang, J.Y.; Wang, F.; Zhang, R.; Zhang, J.H.; Shen, J.; Tian, H.F.; Wu, C.F. Differential proteomic analysis of the anti-depressive effects of oleamide in a rat chronic mild stress model of depression. Pharmacol. Biochem. Behav. 2015, 131, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Akanmu, M.A.; Adeosun, S.O.; Ilesanmi, O.R. Neuropharmacological effects of oleamide in male and female mice. Behav. Brain Res. 2007, 182, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Navarro, M.A.; Vogler, O.; Perona, J.S.; Osada, J.; Ruiz-Gutierrez, V. Olive oils modulate fatty acid content and signaling protein expression in apolipoprotein e knockout mice brain. Lipids 2010, 45, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Verberne, L.; De Irala, J.; Ruiz-Canela, M.; Toledo, E.; Serra-Majem, L.; Martinez-Gonzalez, M.A. Dietary fat intake and the risk of depression: The sun project. PLoS ONE 2011, 6, e16268. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Villegas, A.; Delgado-Rodriguez, M.; Alonso, A.; Schlatter, J.; Lahortiga, F.; Serra Majem, L.; Martinez-Gonzalez, M.A. Association of the mediterranean dietary pattern with the incidence of depression: The seguimiento universidad de navarra/university of navarra follow-up (sun) cohort. Arch. Gen. Psychiatry 2009, 66, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Gow, R.V.; Hibbeln, J.R. Omega-3 fatty acid and nutrient deficits in adverse neurodevelopment and childhood behaviors. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 555–590. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, G.; Denis, S.; Lavialle, M.; Vancassel, S. Synergistic effects of stress and omega-3 fatty acid deprivation on emotional response and brain lipid composition in adult rats. Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Frances, H.; Drai, P.; Smirnova, M.; Carrie, I.; Debray, M.; Bourre, J.M. Nutritional (n-3) polyunsaturated fatty acids influence the behavioral responses to positive events in mice. Neurosci. Lett. 2000, 285, 223–227. [Google Scholar] [CrossRef]
- De Mello, A.H.; Gassenferth, A.; Schraiber Rde, B.; Souza Lda, R.; Florentino, D.; Danielski, L.G.; Cittadin-Soares Eda, C.; Fortunato, J.J.; Petronilho, F.; Quevedo, J.; et al. Effects of omega-3 on behavioral and biochemical parameters in rats submitted to chronic mild stress. Metab. Brain Dis. 2014, 29, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Ziylan, Z.Y.; Bernard, G.C.; Lefauconnier, J.M.; Durand, G.A.; Bourre, J.M. Effect of dietary n-3 fatty acid deficiency on blood-to-brain transfer of sucrose, alpha-aminoisobutyric acid and phenylalanine in the rat. Neurosci. Lett. 1992, 137, 9–13. [Google Scholar] [CrossRef]
- Ellis, E.F.; Police, R.J.; Dodson, L.Y.; McKinney, J.S.; Holt, S.A. Effect of dietary n-3 fatty acids on cerebral microcirculation. Am. J. Physiol. 1992, 262, H1379–H1386. [Google Scholar] [PubMed]
- de Wilde, M.C.; Farkas, E.; Gerrits, M.; Kiliaan, A.J.; Luiten, P.G. The effect of n-3 polyunsaturated fatty acid-rich diets on cognitive and cerebrovascular parameters in chronic cerebral hypoperfusion. Brain Res. 2002, 947, 166–173. [Google Scholar] [CrossRef]
- Ito, H.; Kawashima, R.; Awata, S.; Ono, S.; Sato, K.; Goto, R.; Koyama, M.; Sato, M.; Fukuda, H. Hypoperfusion in the limbic system and prefrontal cortex in depression: Spect with anatomic standardization technique. J. Nucl. Med. 1996, 37, 410–414. [Google Scholar] [PubMed]
- Yazici, K.M.; Kapucu, O.; Erbas, B.; Varoglu, E.; Gulec, C.; Bekdik, C.F. Assessment of changes in regional cerebral blood flow in patients with major depression using the 99mTc-HMPAO single photon emission tomography method. Eur. J. Nucl. Med. 1992, 19, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Kakutani, S.; Rogi, T.; Kawashima, H.; Shibata, H.; Ando, F.; et al. Cross-sectional association between serum concentrations of n-3 long-chain pufa and depressive symptoms: Results in japanese community dwellers. Br. J. Nutr. 2016, 115, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Huang, S.Y.; Su, K.P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.C.; Scott, K.M.; Silvers, K.M. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. J. Affect. Disord. 2006, 90, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Mizoue, T.; Sasaki, S.; Ohta, M.; Sato, M.; Matsushita, Y.; Mishima, N. Dietary intake of folate, other b vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in japanese adults. Nutrition 2008, 24, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Frasure-Smith, N.; Lesperance, F.; Julien, P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biol. Psychiatry 2004, 55, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Sontrop, J.; Campbell, M.K. Omega-3 polyunsaturated fatty acids and depression: A review of the evidence and a methodological critique. Prev. Med. 2006, 42, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Evolutionary aspects of diet: The omega-6/omega-3 ratio and the brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef] [PubMed]
- De la Presa Owens, S.; Innis, S.M. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J. Nutr. 1999, 129, 2088–2093. [Google Scholar] [PubMed]
- Vetrivel, U.; Ravichandran, S.B.; Kuppan, K.; Mohanlal, J.; Das, U.N.; Narayanasamy, A. Agonistic effect of polyunsaturated fatty acids (PUFAs) and its metabolites on brain-derived neurotrophic factor (BDNF) through molecular docking simulation. Lipids Health Dis. 2012, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.P.; Srinivas, G.S.; E, Y.M.; Malla, L.; Rao, A.A. Agonistic approach of omega-3, omega-6 and its metabolites with BDNF: An in-silico study. Bioinformation 2013, 9, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Gispan-Herman, I.; Yadid, G. Increased arachidonic acid concentration in the brain of flinders sensitive line rats, an animal model of depression. J. Lipid Res. 2005, 46, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Tanaka, A.; Nishizaki, T. Linoleic acid derivative DCP-LA ameliorates stress-induced depression-related behavior by promoting cell surface 5-HT1A receptor translocation, stimulating serotonin release, and inactivating gsk-3beta. Mol. Neurobiol. 2015, 51, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Vaz, J.S.; Kac, G.; Nardi, A.E.; Hibbeln, J.R. Omega-6 fatty acids and greater likelihood of suicide risk and major depression in early pregnancy. J. Affect. Disord. 2014, 152–154, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Pandya, M.; Altinay, M.; Malone, D.A., Jr.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, I.; Tuckwell, V.; Ames, D.; O’Brien, J. Structural neuroimaging studies in late-life depression: A review. World J. Biol. Psychiatry 2001, 2, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kronmuller, K.T.; Pantel, J.; Kohler, S.; Victor, D.; Giesel, F.; Magnotta, V.A.; Mundt, C.; Essig, M.; Schroder, J. Hippocampal volume and 2-year outcome in depression. Br. J. Psychiatry 2008, 192, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012, 233, 398–404. [Google Scholar] [CrossRef] [PubMed]
- McMillan, L.; Owen, L.; Kras, M.; Scholey, A. Behavioural effects of a 10-day mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite 2011, 56, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Manku, M.S.; Horrobin, D.F. Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 2008, 138, 954–963. [Google Scholar] [PubMed]
- Tang, M.; Zhang, M.; Cai, H.; Li, H.; Jiang, P.; Dang, R.; Liu, Y.; He, X.; Xue, Y.; Cao, L.; et al. Maternal diet of polyunsaturated fatty acid altered the cell proliferation in the dentate gyrus of hippocampus and influenced glutamatergic and serotoninergic systems of neonatal female rats. Lipids Health Dis. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Choi, K.H.; Kim, H.Y. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: No changes in docosahexaenoic acid species. J. Psychiatr. Res. 2010, 44, 688–693. [Google Scholar] [CrossRef] [PubMed]
- du Bois, T.M.; Deng, C.; Bell, W.; Huang, X.F. Fatty acids differentially affect serotonin receptor and transporter binding in the rat brain. Neuroscience 2006, 139, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; Cherbuin, N.; Anstey, K.J.; Sachdev, P.; Butterworth, P. Western diet is associated with a smaller hippocampus: A longitudinal investigation. BMC Med. 2015, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Ruhe, H.G.; Assies, J.; Lok, A.; Koeter, M.W.; Visser, I.; Bockting, C.L.; Schene, A.H. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology 2013, 38, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Grayson, B.; Takahashi, D.; Robertson, N.; Maier, A.; Bethea, C.L.; Smith, M.S.; Coleman, K.; Grove, K.L. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 2010, 30, 3826–3830. [Google Scholar] [CrossRef] [PubMed]
- Singh, M. Mood, food, and obesity. Front. Psychol. 2014, 5, 925. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Su, H.M. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. J. Nutr. Biochem. 2013, 24, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.; Keshavarz, S.A.; Tehrani-Doost, M.; Djalali, M.; Hosseini, M.; Amini, H.; Chamari, M.; Djazayery, A. Effects of eicosapentaenoic acid and fluoxetine on plasma cortisol, serum interleukin-1beta and interleukin-6 concentrations in patients with major depressive disorder. Psychiatry Res. 2010, 178, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Lanfumey, L.; Mongeau, R.; Cohen-Salmon, C.; Hamon, M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci. Biobehav. Rev. 2008, 32, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Raygada, M.; Cho, E.; Hilakivi-Clarke, L. High maternal intake of polyunsaturated fatty acids during pregnancy in mice alters offsprings’ aggressive behavior, immobility in the swim test, locomotor activity and brain protein kinase c activity. J. Nutr. 1998, 128, 2505–2511. [Google Scholar] [PubMed]
- Hahn, C.G.; Friedman, E. Abnormalities in protein kinase c signaling and the pathophysiology of bipolar disorder. Bipolar Disord. 1999, 1, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D.; et al. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Lalovic, A.; Levy, E.; Canetti, L.; Sequeira, A.; Montoudis, A.; Turecki, G. Fatty acid composition in postmortem brains of people who completed suicide. J. Psychiatry Neurosci. 2007, 32, 363–370. [Google Scholar] [PubMed]
- Delion, S.; Chalon, S.; Herault, J.; Guilloteau, D.; Besnard, J.C.; Durand, G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotoninergic neurotransmission in rats. J. Nutr. 1994, 124, 2466–2476. [Google Scholar] [PubMed]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; De Smedt-Peyrusse, V.; Labrousse, V.F.; Bretillon, L.; Matute, C.; et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, T.; Madore, C.; Joffre, C.; Laye, S. Nutritional n-3 polyunsaturated fatty acids deficiency alters cannabinoid receptor signaling pathway in the brain and associated anxiety-like behavior in mice. J. Physiol. Biochem. 2012, 68, 671–681. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K. Dha deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J. Nutr. 2010, 140, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Bambico, F.R.; Cassano, T.; Dominguez-Lopez, S.; Katz, N.; Walker, C.D.; Piomelli, D.; Gobbi, G. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology 2010, 35, 2083–2100. [Google Scholar] [CrossRef] [PubMed]
- Hamazaki, K.; Hamazaki, T.; Inadera, H. Abnormalities in the fatty acid composition of the postmortem entorhinal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2013, 210, 346–350. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Hahn, C.G.; Jandacek, R.; Rider, T.; Tso, P.; Stanford, K.E.; Richtand, N.M. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol. Psychiatry 2007, 62, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; Ely, B.A.; Li, Q.; Bangaru, S.D.; Panzer, A.M.; Alonso, C.M.; Castellanos, F.X.; Milham, M.P. Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.H.; McMain, S.; Kennedy, S.H.; Korman, L.; Brown, G.M.; DaSilva, J.N.; Wilson, A.A.; Blak, T.; Eynan-Harvey, R.; Goulding, V.S.; et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am. J. Psychiatry 2003, 160, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.K.; Leonard, S.; Reddy, R.D. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr. Res. 2000, 42, 7–17. [Google Scholar] [CrossRef]
- Davis, P.F.; Ozias, M.K.; Carlson, S.E.; Reed, G.A.; Winter, M.K.; McCarson, K.E.; Levant, B. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): Interactions with reproductive status. Nutr. Neurosci. 2010, 13, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, D.; Yasui, Y.; Yamada, K.; Ohara, N.; Okuyama, H. Regional differences of the mouse brain in response to an alpha-linolenic acid-restricted diet: Neurotrophin content and protein kinase activity. Life Sci. 2010, 87, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Labermaier, C.; Masana, M.; Muller, M.B. Biomarkers predicting antidepressant treatment response: How can we advance the field? Dis. Markers 2013, 35, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Zheng, P.; Zhao, X.; Zhou, C.; Hu, C.; Hou, X.; Wang, H.; Xie, P.; Xu, G. Plasma lipidomics reveals potential lipid markers of major depressive disorder. Anal. Bioanal. Chem. 2016, 408, 6497–6507. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.H.; Hanstock, T.L.; Hirneth, S.J.; Kable, C.J.; Garg, M.L.; Hazell, P.L. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 2008, 43, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Jandacek, R.; Rider, T.; Tso, P.; Dwivedi, Y.; Pandey, G.N. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J. Affect. Disord. 2010, 126, 303–311. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Jandacek, R.; Tso, P.; Blom, T.J.; Welge, J.A.; Strawn, J.R.; Adler, C.M.; Strakowski, S.M.; DelBello, M.P. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: A candidate prodromal risk biomarker. Early Interv. Psychiatry 2016, 10, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Miller, G.E.; Carrier, E.J.; Gorzalka, B.B.; Hillard, C.J. Circulating endocannabinoids and n-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology 2009, 34, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Miller, G.E.; Ho, W.S.; Gorzalka, B.B.; Hillard, C.J. Serum endocannabinoid content is altered in females with depressive disorders: A preliminary report. Pharmacopsychiatry 2008, 41, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Buttar, H.S.; Li, T.; Ravi, N. Prevention of cardiovascular diseases: Role of exercise, dietary interventions, obesity and smoking cessation. Exp. Clin. Cardiol. 2005, 10, 229–249. [Google Scholar] [PubMed]
- Jazayeri, S.; Tehrani-Doost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Zeal. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Gertsik, L.; Poland, R.E.; Bresee, C.; Rapaport, M.H. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 2012, 32, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of omega-3 fatty acids in the treatment of depressive disorders: A comprehensive meta-analysis of randomized clinical trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Lesperance, F.; Frasure-Smith, N.; St-Andre, E.; Turecki, G.; Lesperance, P.; Wisniewski, S.R. The efficacy of omega-3 supplementation for major depression: A randomized controlled trial. J. Clin. Psychiatry 2011, 72, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.; Horrobin, D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry 2002, 59, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhe, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, S.I.; Ramadan, E.; Basselin, M. Docosahexaenoic acid (DHA) incorporation into the brain from plasma, as an in vivo biomarker of brain dha metabolism and neurotransmission. Prostaglandins Lipid Mediat. 2011, 96, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Umhau, J.C.; Zhou, W.; Carson, R.E.; Rapoport, S.I.; Polozova, A.; Demar, J.; Hussein, N.; Bhattacharjee, A.K.; Ma, K.; Esposito, G.; et al. Imaging incorporation of circulating docosahexaenoic acid into the human brain using positron emission tomography. J. Lipid Res. 2009, 50, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Giovacchini, G.; Liow, J.S.; Bhattacharjee, A.K.; Greenstein, D.; Schapiro, M.; Hallett, M.; Herscovitch, P.; Eckelman, W.C.; Carson, R.E.; et al. Imaging neuroinflammation in alzheimer’s disease with radiolabeled arachidonic acid and pet. J. Nucl. Med. 2008, 49, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.F.; Mutch, D.M.; Leri, F. The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants. Nutrients 2017, 9, 298. https://doi.org/10.3390/nu9030298
Fernandes MF, Mutch DM, Leri F. The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants. Nutrients. 2017; 9(3):298. https://doi.org/10.3390/nu9030298
Chicago/Turabian StyleFernandes, Maria Fernanda, David M. Mutch, and Francesco Leri. 2017. "The Relationship between Fatty Acids and Different Depression-Related Brain Regions, and Their Potential Role as Biomarkers of Response to Antidepressants" Nutrients 9, no. 3: 298. https://doi.org/10.3390/nu9030298



