The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies
Abstract
:1. Introduction
2. Methods
2.1. Data Sources
2.2. Study Selection
2.3. Statistical Analysis
3. Results
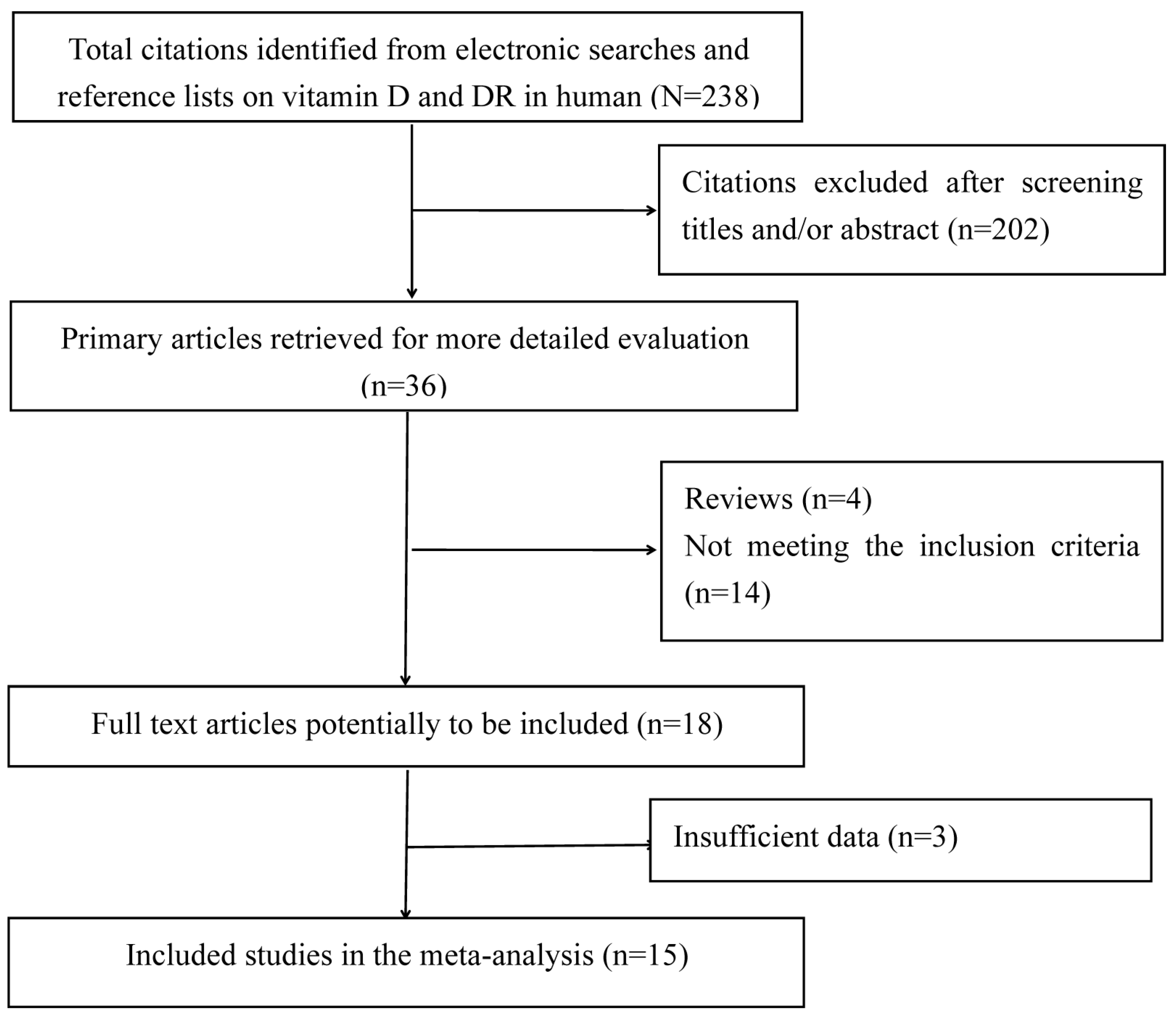
3.1. Search Results
3.2. Characteristics of the Included Studies
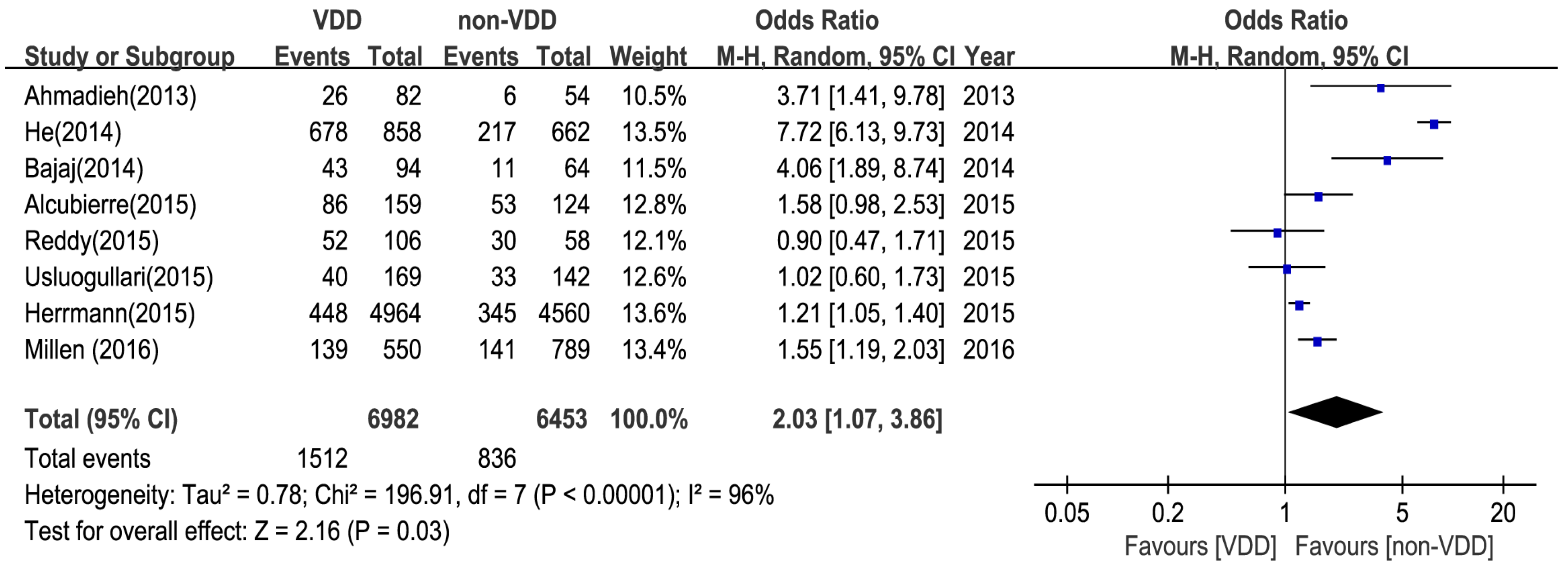
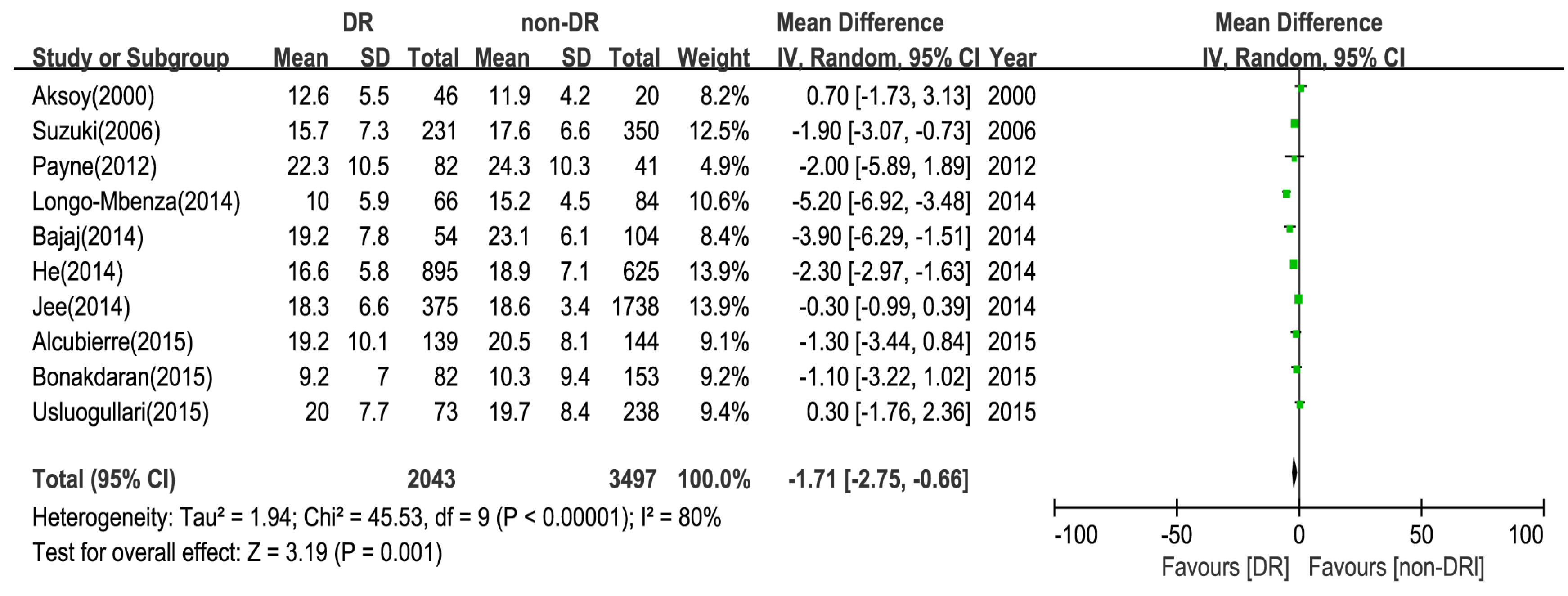
3.3. Main Analysis
3.4. Sensitivity and Subgroup Analysis
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Authors Contributions
Conflicts of Interest
References
- Sherwin, R.; Jastreboff, A.M. Year in diabetes 2012: the diabetes tsunami. J. Clin. Endocrinol. Metab. 2012, 97, 4293–4301. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, M.; Congdon, N. The worldwide epidemic of diabetic retinopathy. Indian J. Ophthalmol. 2012, 60, 428–431. [Google Scholar] [PubMed]
- Stratton, I.M.; Kohner, E.M.; Aldington, S.J.; Turner, R.C.; Holman, R.R.; Manley, S.E.; Matthews, D.R. UKPDS50: Risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia 2001, 44, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Praidou, A.; Harris, M.; Niakas, D.; Labiris, G. Physical activity and its correlation to diabetic retinopathy. J. Diabetes Complicat. 2017, 31, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080s–1086s. [Google Scholar] [CrossRef] [PubMed]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and Type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.Y.; Hwang, Y.C.; Chung, H.Y.; Woo, J.T. Vitamin D and diabetes in Koreans: Analyses based on the Fourth Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2009. Diabet. Med. 2012, 29, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Bonakdaran, S.; Shoeibi, N. Is there any correlation between vitamin D insufficiency and diabetic retinopathy? Int. J. Ophthalmol. 2015, 8, 326–331. [Google Scholar] [PubMed]
- Isaia, G.; Giorgino, R.; Adami, S. High prevalence of hypovitaminosis D in female type diabetic population. Diabetes Care 2001, 24, 1496. [Google Scholar] [CrossRef] [PubMed]
- Taverna, M.J.; Selam, J.L.; Slama, G. Association between a protein polymorphism in the start codon of the vitamin D receptor gene and severe diabetic retinopathy in C-peptide-negative type 1 diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 4803–4808. [Google Scholar] [CrossRef] [PubMed]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol is a potent inhibitor of retinal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Patrick, P.A.; Visintainer, P.F.; Shi, Q.; Weiss, I.A.; Brand, D.A. Vitamin D and Retinopathy in Adults with Diabetes Mellitus. Arch. Ophthalmol. 2012, 130, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Inukai, T.; Fujiwara, Y.; Tayama, K.; Aso, Y.; Takemura, Y. Alterations in serum levels of 1 alpha, 25(OH)2 D3 and osteocalcin in patients with early diabetic nephropathy. Diabetes Res. Clin. Pract. 1997, 38, 53–59. [Google Scholar] [CrossRef]
- Alam, U.; Amjad, Y.; Chan, A.W.; Asghar, O.; Petropoulos, I.N.; Malik, R.A. Vitamin D Deficiency Is Not Associated with Diabetic Retinopathy or Maculopathy. J. Diabetes Res. 2016, 2016, 6156217. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (moose) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Duckitt, K.; Harrington, D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ (Clin. Res. Ed.) 2005, 330, 565. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, H.; Akçay, F.; Kurtul, N.; Baykal, O.; Avci, B. Serum 1,25 Dihydroxy Vitamin D (1,25(OH)2D3), 25 Hydroxy Vitamin D (25(OH)D) and Parathormone Levels in Diabetic Retinopathy. Clin. Biochem. 2000, 33, 47–51. [Google Scholar] [CrossRef]
- Suzuki, A.; Kotake, M.; Ono, Y.; Kato, T.; Oda, N.; Hayakawa, N.; Hashimoto, S.; Itoh, M. Hypovitaminosis D in type 2 diabetes mellitus: Association with microvascular complications and type of treatment. Endocr. J. 2006, 53, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.F.; Ray, R.; Watson, D.G.; Delille, C.; Rimler, E.; Cleveland, J.; Lynn, M.J.; Tangpricha, V.; Srivastava, S.K. Vitamin D Insufficiency in Diabetic Retinopathy. Endocr. Pract. 2012, 18, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Ahmadieh, H.; Azar, S.T.; Lakkis, N.; Arabi, A. Hypovitaminosis D in Patients with Type 2 Diabetes Mellitus: A Relation to Disease Control and Complications. ISRN Endocrinol. 2013, 2013, 641098. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Singh, R.P.; Dwivedi, N.C.; Singh, K.; Gupta, A.; Mathur, M. Vitamin D levels and microvascular complications in type 2 diabetes. Indian J. Endocrinol. Metab. 2014, 18, 537–541. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Shen, J.; Liu, F.; Zeng, H.; Li, L.; Yu, H.; Lu, H.; Lu, F.; Wu, Q.; Jia, W. Vitamin D deficiency increases the risk of retinopathy in Chinese patients with Type 2 diabetes. Diabet. Med. 2014, 31, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Jee, D.; Han, K.; Kim, E.C. Inverse Association between High Blood 25 Hydroxyvitamin D Levels and Diabetic Retinopathy in a Representative Korean Population. PLoS ONE 2014, 9, e115199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo-Mbenza, B.; Mvitu Muaka, M.; Masamba, W.; Muizila Kini, L.; Longo Phemba, I.; Kibokela Ndembe, D.; Tulomba Mona, D. Retinopathy in non diabetics, diabetic retinopathy and oxidative stress: A new phenotype in Central Africa? Int. J. Ophthalmol. 2014, 7, 293–301. [Google Scholar] [PubMed]
- Alcubierre, N.; Valls, J.; Rubinat, E.; Cao, G.; Esquerda, A.; Traveset, A.; Granado-Casas, M.; Jurjo, C.; Mauricio, D. Vitamin D Deficiency Is Associated with the Presence and Severity of Diabetic Retinopathy in Type 2 Diabetes Mellitus. J. Diabetes Res. 2015, 2015, 374178. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Sullivan, D.R.; Veillard, A.S.; McCorquodale, T.; Straub, I.R.; Scott, R.; Laakso, M.; Topliss, D.; Jenkins, A.J.; Blankenberg, S.; Burton, A.; et al. Serum 25-Hydroxyvitamin D: A Predictor of Macrovascular and Microvascular Complications in Patients With Type 2 Diabetes. Diabetes Care 2015, 38, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.B.; Sivaprasad, M.; Shalini, T.; Satyanarayana, A.; Seshacharyulu, M.; Balakrishna, N.; Viswanath, K.; Sahay, M. Plasma vitamin D status in patients with type 2 diabetes with and without retinopathy. Nutrition 2015, 31, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Usluogullari, C.A.; Balkan, F.; Caner, S.; Ucler, R.; Kaya, C.; Ersoy, R.; Cakir, B. The relationship between microvascular complications and vitamin D deficiency in type 2 diabetes mellitus. BMC Endocr. Disord. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, G.; Galletti, A.; Targher, G.; Brangani, C.; Pichiri, I.; Trombetta, M.; Negri, C.; de Santi, F.; Stoico, V.; Cacciatori, V.; et al. Lower levels of 25-hydroxyvitamin D3 are associated with a higher prevalence of microvascular complications in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2015, 3, e000058. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Sahli, M.W.; Nie, J.; LaMonte, M.J.; Lutsey, P.L.; Klein, B.E.; Mares, J.A.; Meyers, K.J.; Andrews, C.A.; Klein, R. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc. Diabetol. 2016, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Koerfer, R. Protective and toxic effects of vitamin D on vascular calcification: Clinical implications. Mol. Asp. Med. 2008, 29, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Zenari, L.; Scala, L.; Cigolini, M.; Arcaro, G. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima–media thickness among Type 2 diabetic patients. Clin. Endocrinol. (Oxf.) 2006, 65, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The impact of 1,25-dihydroxy vitamin D3 on the expressions of vascular endothelial growth factor and transforming growth factor-b1 in the retinas of rats with diabetes. Diabetes Res. Clin. Pract. 2012, 98, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, J.C. Vitamin D and cardiovascular disease. J. Am. Coll. Nutr. 2011, 30, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Gysemans, C.; Giulietti, A.; Bouillon, R. Vitamin D and diabetes. Diabetologia 2005, 48, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Michos, E.D. Vitamin D deficiency and the risk of incident Type 2 diabetes. Future Cardiol. 2009, 5, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Danescu, L.G.; Levy, S.; Levy, J. Vitamin D and diabetes mellitus. Endocrine 2009, 35, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Delanaye, P.; Souberbielle, J.C.; Radermecker, R.P. Vitamin D and type 2 diabetes mellitus: where do we stand? Diabetes Metab. 2011, 37, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Kang, E.S.; Ji, M.J.; Choi, H.J.; Oh, T.; Koong, S.S.; Jeon, H.J. Association between Bsm1 Polymorphism in Vitamin D Receptor Gene and Diabetic Retinopathy of Type 2 Diabetes in Korean Population. Endocrinol. Metab. (Seoul) 2015, 30, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, C.; Beauchet, O.; Bartha, R.; Graffe, A.; Milea, D.; Montero-Odasso, M. Association between serum 25-hydroxyvitamin D concentration and optic chiasm volume. J. Am. Geriatr. Soc. 2013, 61, 1026–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bućan, K.; Ivanisević, M.; Zemunik, T.; Boraska, V.; Skrabić, V.; Vatavuk, Z.; Galetović, D.; Znaor, L. Retinopathy and nephropathy in type 1 diabetic patients association with polymorphysms of vitamin D-receptor, TNF, Neuro-D and IL-1 receptor 1 genes. Coll. Antropol. 2009, 33, 99–105. [Google Scholar] [PubMed]
- Zhong, X.; Du, Y.; Lei, Y.; Liu, N.; Guo, Y.; Pan, T. Effects of vitamin D receptor gene polymorphism and clinical characteristics on risk of diabetic retinopathy in Han Chinese type 2 diabetes patients. Gene 2015, 566, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chen, R. Vitamin D as an analgesic for patients with type 2 diabetes and neuropathic pain. Arch. Intern. Med. 2008, 168, 771–772. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Country | Study Design | Sample Size (n) | VD Assay Method | DR Diagnosis | VDD Prevalence (%) | Mean 25(OH)D ng/mL (SD) | Significant | Adjustment | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DR NDR | DR NDR | ||||||||||
| Aksoy (2000) [19] | Turkey | Cross-sectional | 66 | RIA | Ophthalmologists | NA | NA | 12.6 ± 5.5 | 11.9 ± 4.2 | Yes | No |
| Suzuki (2006) [20] | Japan | Case–control | 581 | RIA | Ophthalmologists | NA | NA | 15.7 ± 7.3 | 17.6 ± 6.6 | Yes | Age, BMI, duration, HbA1c, treatment |
| Payne (2012) [21] | US | Cross-sectional | 123 | CL | Ophthalmologists | NA | NA | 22.3 ± 10.5 | 24.3 ± 10.3 | Yes | Multivitamin use |
| Ahmadieh (2013) [22] | Lebanon | Cross-sectional | 136 | RIA | Ophthalmologists | 78.8 | 53.8 | NA | NA | Yes | BMI, duration, smoking |
| Bajaj (2014) [23] | Indian | Case–control | 158 | NA | Ophthalmologists | 79.6 | 49.0 | NA | NA | Yes | No |
| He (2014) [24] | China | Cross-sectional | 1520 | CL | The International Clinical DR Severity Scale | 75.7 | 63.6 | 16.6 ± 5.8 | 18.9 ± 7.1 | Yes | Age, sex, duration |
| Jee (2014) [25] | Korea | Cross-sectional | 2113 | RIA | The Early Treatment Diabetic Retinopathy Study severity scale | NA | NA | 18.3 ± 6.6 | 18.7 ± 3.4 | Yes | Sex |
| Longo-Mbenza (2014) [26] | Congo | Case–control | 150 | HPLC | The modified Airlie House classification system | NA | NA | 10 ± 5.9 | 15.2 ± 4.5 | Yes | No |
| Alcubierre (2015) [27] | Spain | Case–control | 283 | CL | Ophthalmologists | 61.9 | 50.7 | 19.2 ± 10.1 | 20.5 ± 8.1 | Yes | Race, season, physical activity |
| Bonakdaran (2015) [10] | Iran | Cross-sectional | 235 | RIA | Ophthalmologists | NA | NA | 9.2 ± 7.0 | 10.3 ± 9.4 | No | Age, sex, duration, BMI, HbA1c,BMI, sex, HbA1c |
| Herrmann (2015) [28] | Australia, New Zealand, and Finland | Prospective | 9524 | CL | Ophthalmologists | 56.5 | 51.7 | NA | NA | Yes | Age, sex, et al. * |
| Reddy (2015) [29] | Indian | Case–control | 164 | HPLC | The modified Airlie House classification system | 27.0 | 23.0 | NA | NA | No | Duration |
| Usluogullari (2015) [30] | Turkey | Retrospective | 557 | HPLC | Ophthalmologists | 45.2 | 54.2 | 20.0 ± 7.7 | 19.7 ± 8.4 | No | Age, BMI, sex, HbA1c |
| Zoppini (2015) [31] | Italy | Cross-sectional | 715 | CL | Ophthalmologists | NA | NA | NA | NA | Yes | Age |
| Millen (2016) [32] | US | Prospective | 1339 | LC-MS | The modified Airlie House classification system | 49.6 | 38.8 | NA | NA | Yes | Race, duration, HbA1c, hypertension |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.-A.; Gao, F.; Qin, L.-L. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2017, 9, 307. https://doi.org/10.3390/nu9030307
Luo B-A, Gao F, Qin L-L. The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients. 2017; 9(3):307. https://doi.org/10.3390/nu9030307
Chicago/Turabian StyleLuo, Bang-An, Fan Gao, and Lu-Lu Qin. 2017. "The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies" Nutrients 9, no. 3: 307. https://doi.org/10.3390/nu9030307
APA StyleLuo, B.-A., Gao, F., & Qin, L.-L. (2017). The Association between Vitamin D Deficiency and Diabetic Retinopathy in Type 2 Diabetes: A Meta-Analysis of Observational Studies. Nutrients, 9(3), 307. https://doi.org/10.3390/nu9030307





