Protective Effect of Chokeberry (Aronia melanocarpa L.) Extract against Cadmium Impact on the Biomechanical Properties of the Femur: A Study in a Rat Model of Low and Moderate Lifetime Women Exposure to This Heavy Metal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cd Diets
2.3. A. melanocarpa Extract
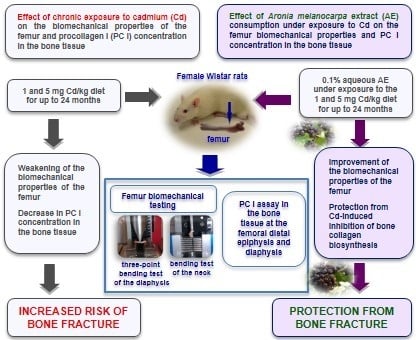
2.4. Study Design
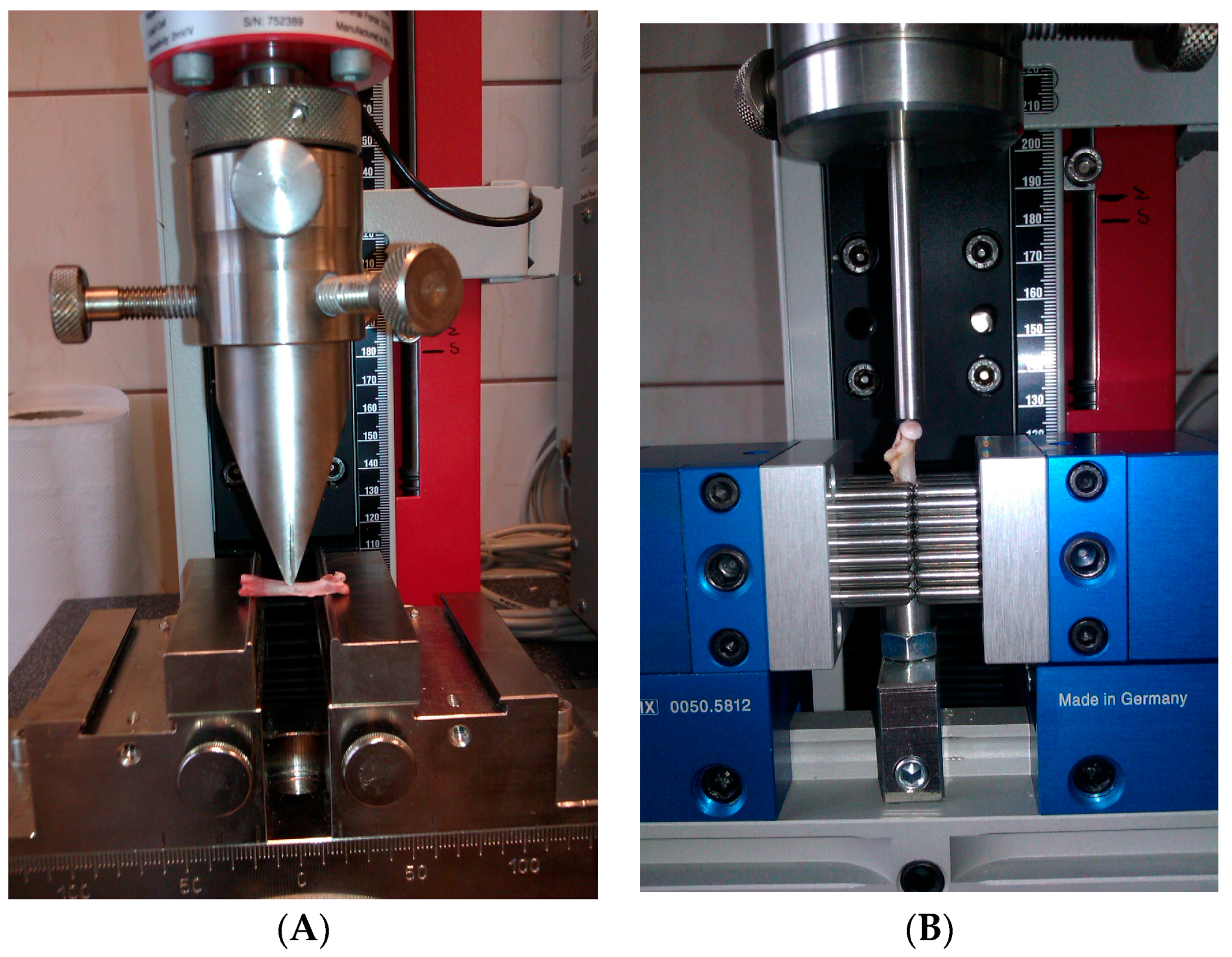
2.5. Bone Biomechanical Testing
2.5.1. Three Point Bending Test of the Femoral Diaphysis
2.5.2. Fracture Test of the Femoral Neck
2.6. Determination of PC I
2.7. Statistical Analysis
3. Results
3.1. Effect of AE on the Femur Weight in Rats Exposed to Cd
3.2. Effect of AE on the Geometric Properties of the Femur in Rats Exposed to Cd
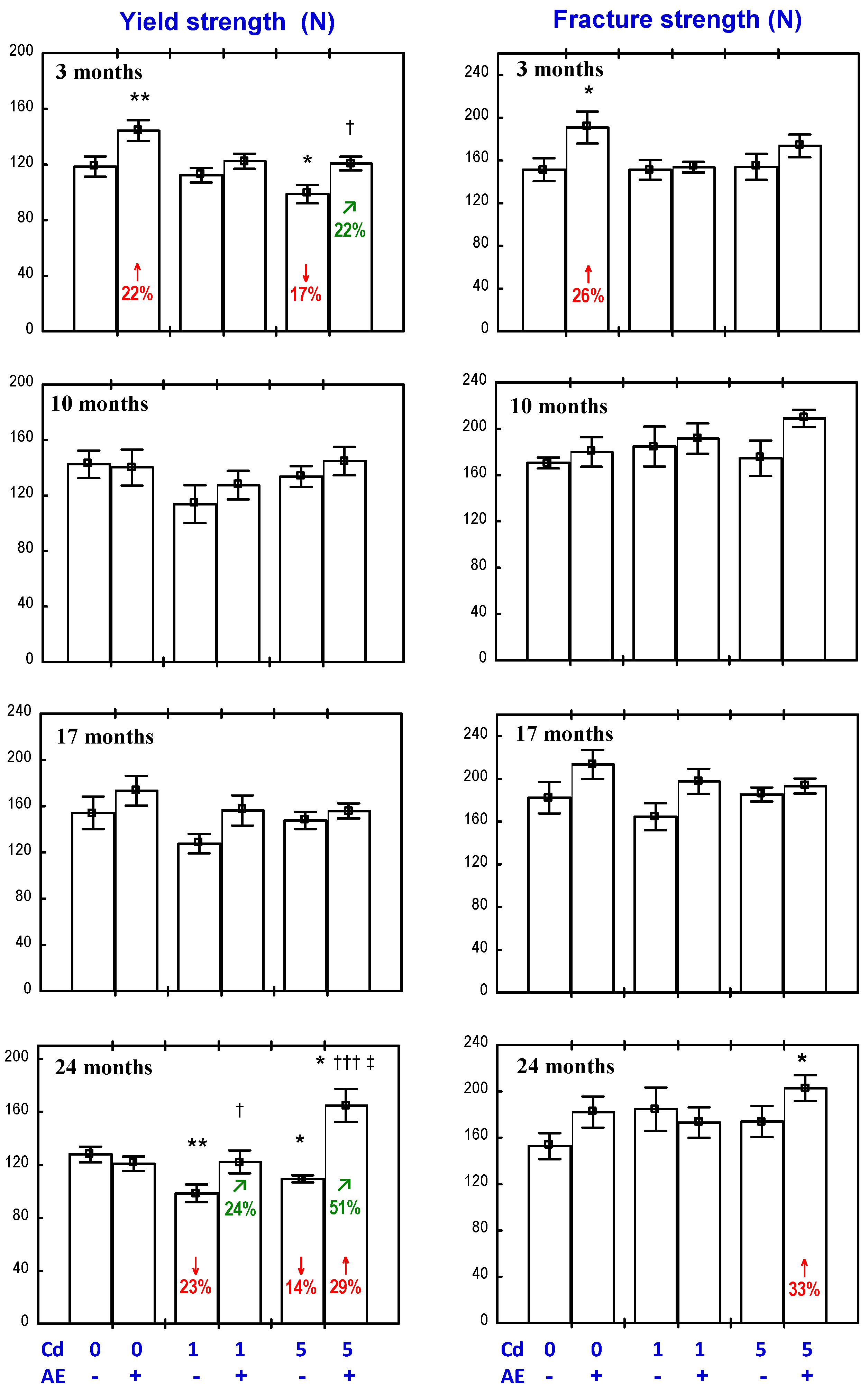
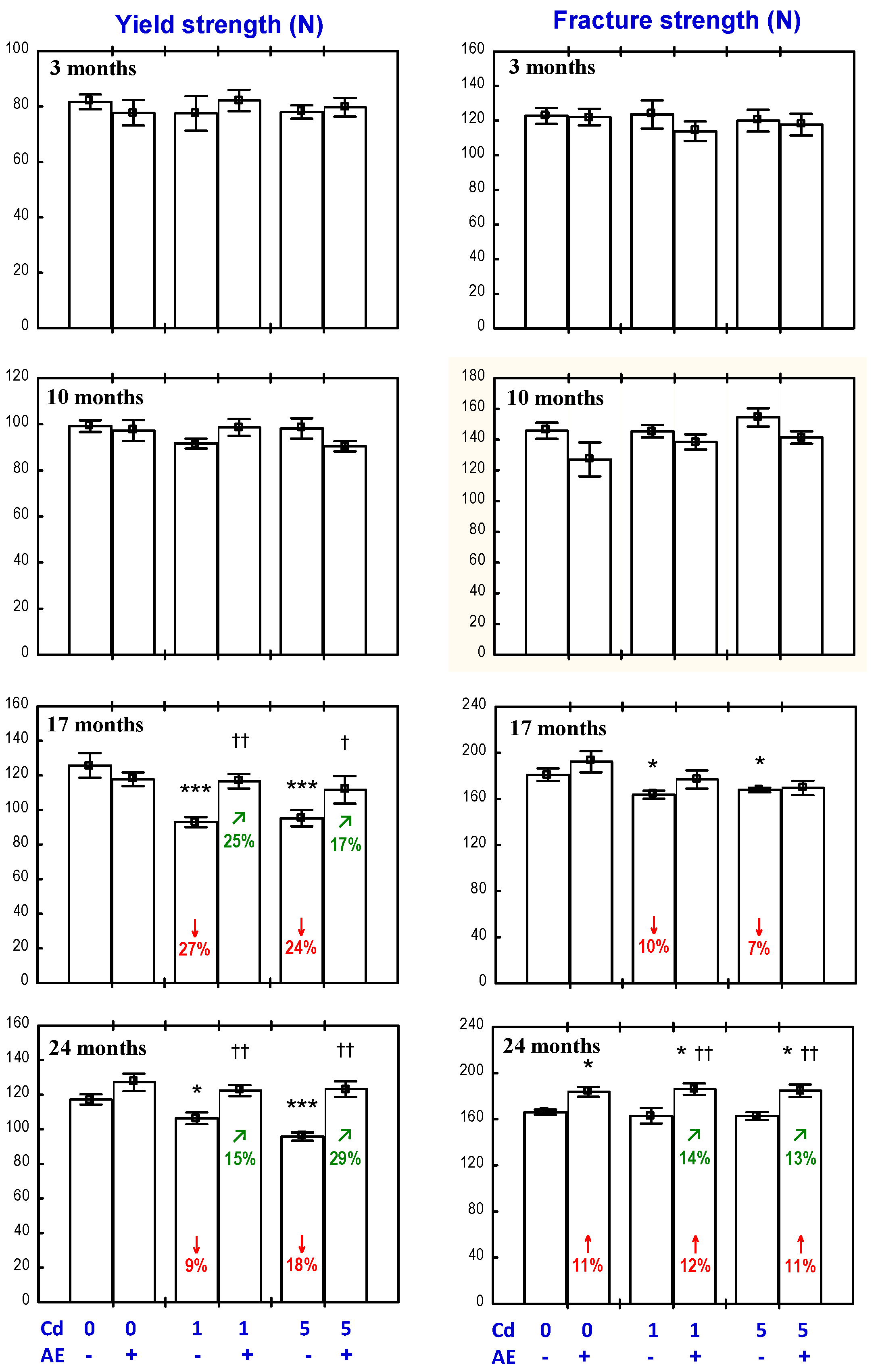
3.3. Effect of AE on the Biomechanical Properties of the Femoral Neck in Rats Exposed to Cd
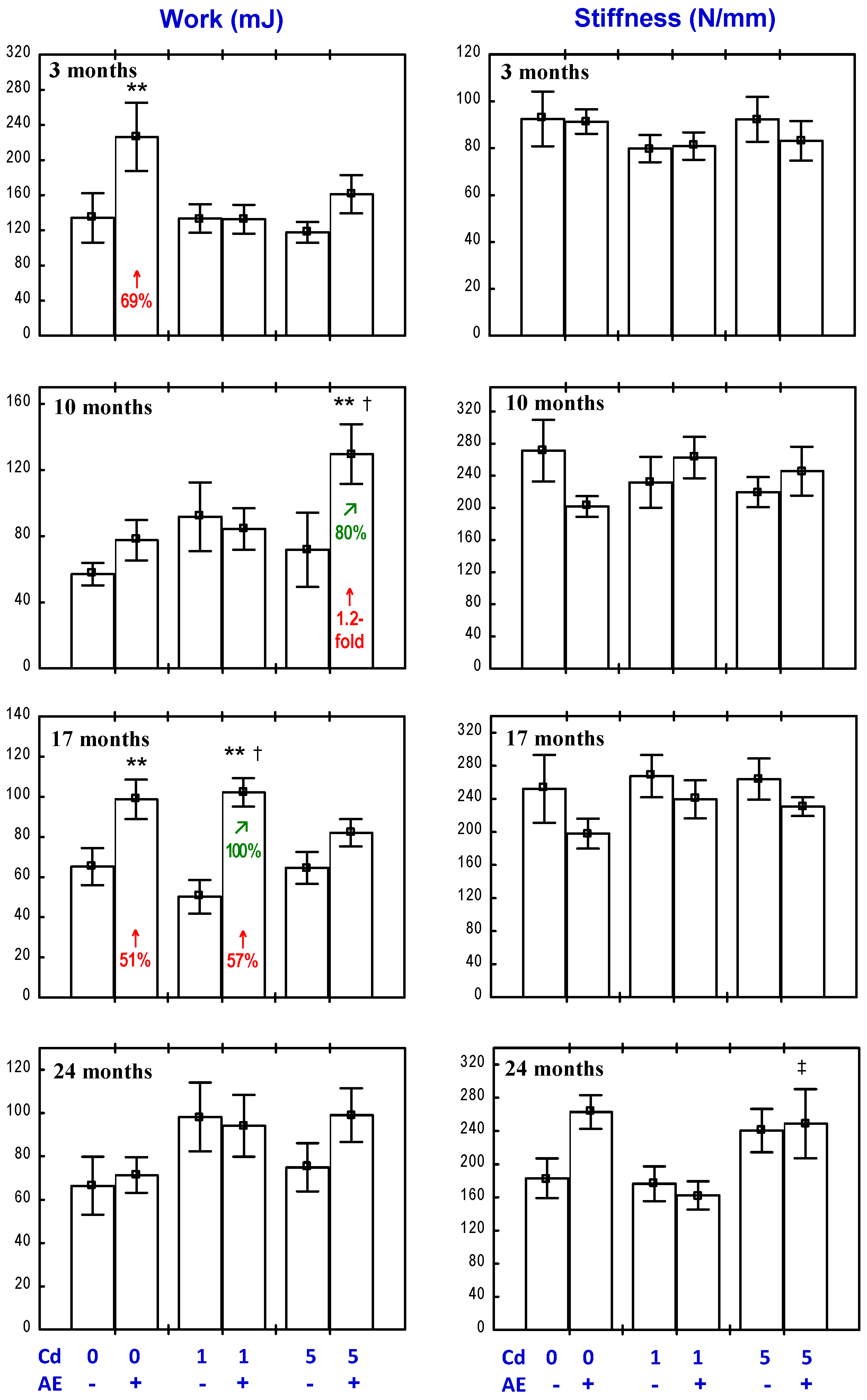
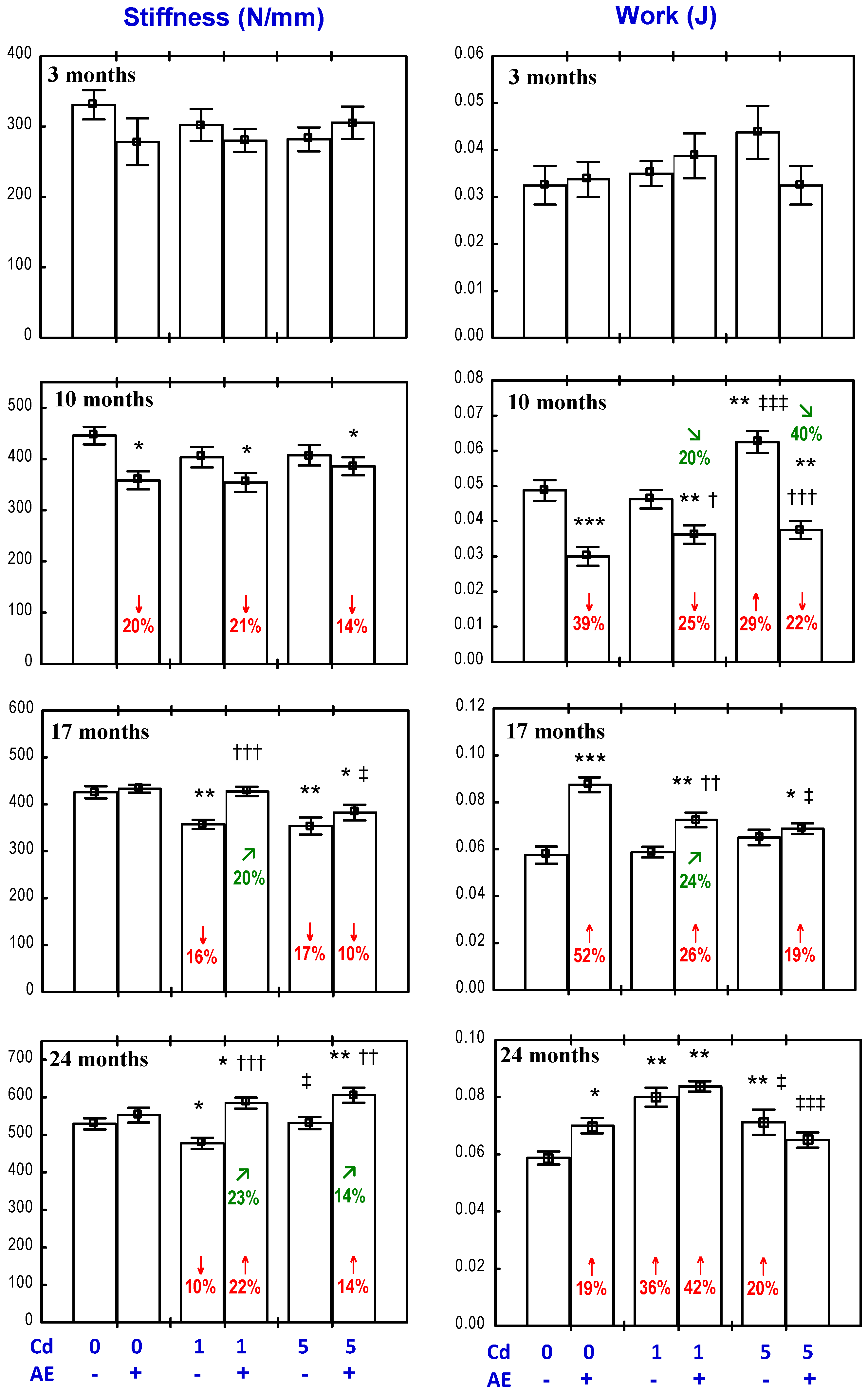
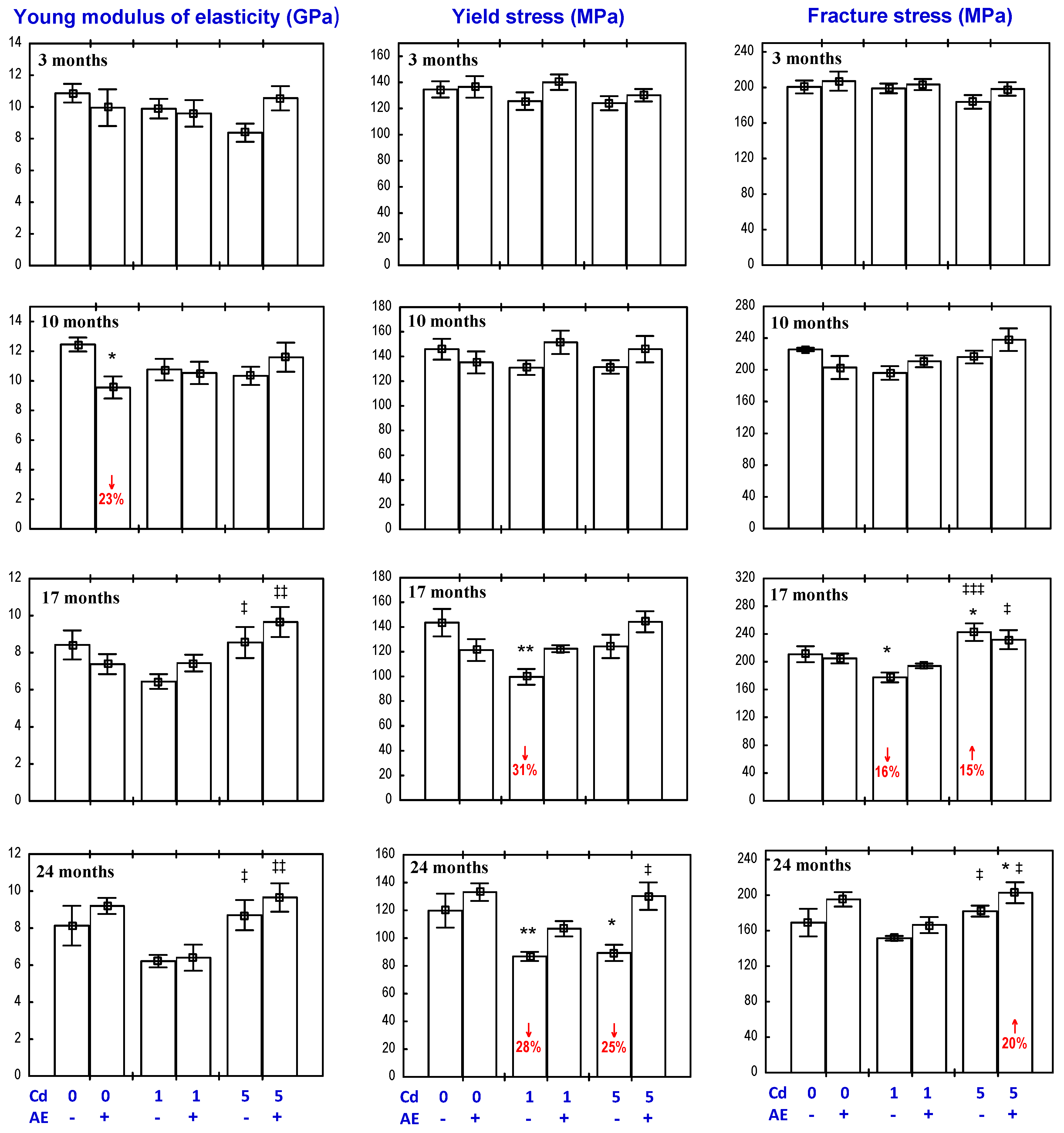
3.4. Effect of AE on the Biomechanical Properties of the Femoral Diaphysis in Rats Exposed to Cd
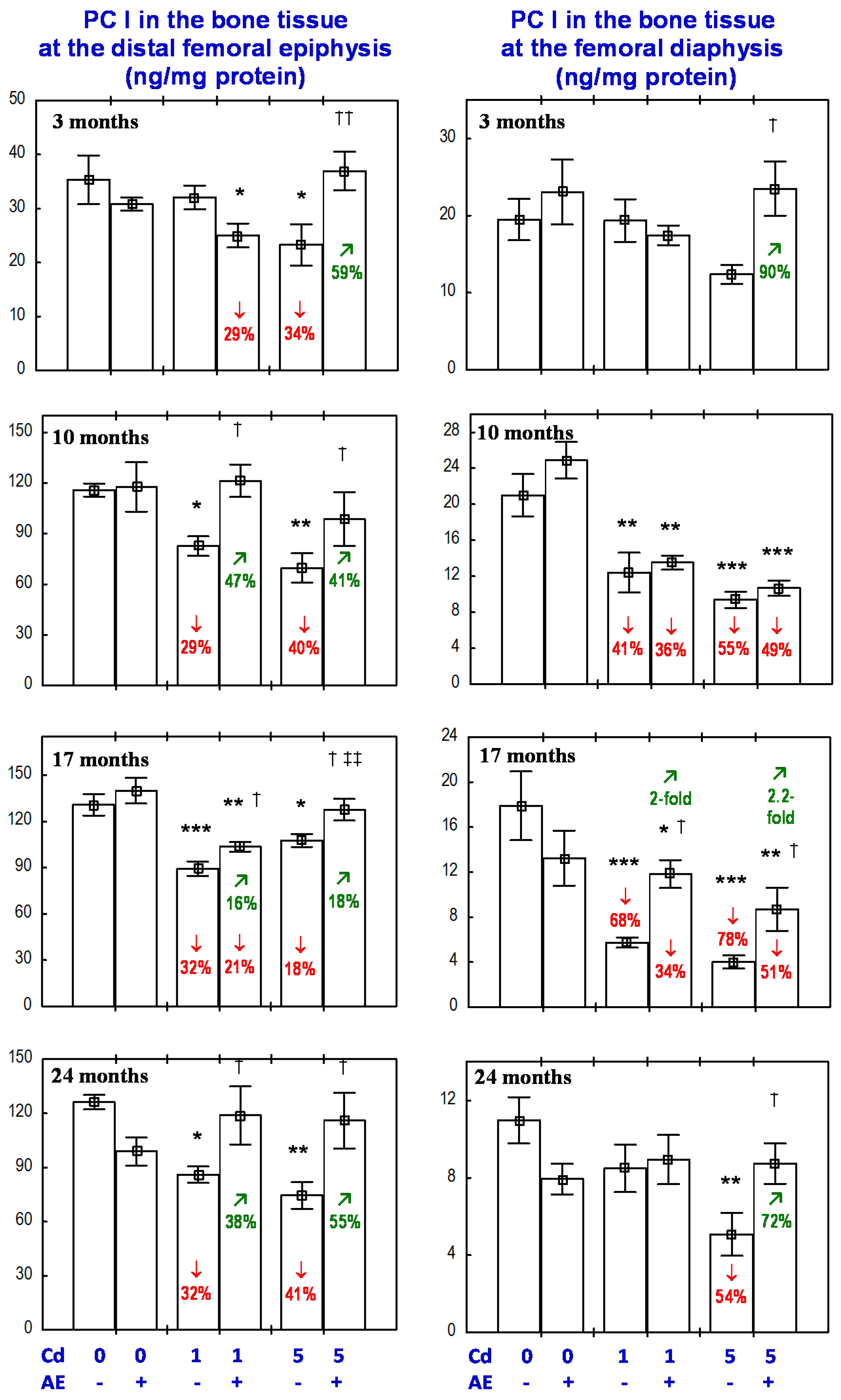
3.5. Effect of AE on PC I Concentration in the Bone Tissue in Rats Exposed to Cd
3.6. Dependence Between the Femur BMD and Its Biomechanical Properties in Rats Exposed to Cd
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Dahl, C.; Søgaard, A.J.; Tell, G.S.; Flaten, T.P.; Hongve, D.; Omsland, T.K.; Holvik, K.; Meyer, H.E.; Aamodt, G. Norwegian Epidemiologic Osteoporosis Study (NOREPOS) Core Research Group. Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS study. Biol. Trace Elem. Res. 2014, 157, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, A.J.; Meyer, H.E.; Emaus, N.; Grimnes, G.; Gjesdal, C.G.; Forsmo, S.; Schei, B.; Tell, G.S. Cohort profile: Norwegian Epidemiologic Osteoporosis Studies (NOREPOS). Scand. J. Public Health 2014, 42, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.K.O.; Beattie, K.A.; Bhargava, A.; Cheung, M.; Webber, C.E.; Chettle, D.R.; Papaioannou, A.; Adach, J.D. Bone lead (Pb) content at the tibia is associated with thinner distal tibia cortices and lower volumetric bone density in postmenopausal women. Bone 2015, 79, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Wang, J.S. Green tea and bone metabolism. Nutr. Res. 2009, 29, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Gunn, C.A.; Weber, J.L.; McGill, A.T.; Kruger, M.C. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients 2015, 7, 2499–2517. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between dietary flavonoid intakes and bone health in a Scottish population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Chyu, M.C.; Wang, J.S. Tea and bone health: Steps forward in translational nutrition. Am. J. Clin. Nutr. 2013, 98, 1694S–1699S. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, K.; Wang, Z.; Gan, C.; He, P.; Liang, Y.; Jin, T.; Zhu, G. Effects of lead and cadmium co-exposure on bone mineral density in a Chinese population. Bone 2014, 63, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Callan, A.C.; Devine, A.; Qi, L.; Ng, J.C.; Hinwood, A.L. Investigation of the relationship between low environmental exposure to metals and bone mineral density, bone resorption and renal function. Int. J. Hyg. Environ. Health 2015, 218, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Karlsson, M.K.; Lorentzon, M.; Ohlsson, C.; Mellström, D. Low-level cadmium exposure is associated with decreased bone mineral density and increased risk of incident fractures in elderly men: The MrOS Sweden study. J. Bone Miner. Res. 2015, 31, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Roels, H.A.; Munters, E.; Cuypers, A.; Richart, T.; Ruttens, A.; Smeets, K.; Clijsters, H.; Vangronsveld, J. Cadmium exposure in the population: From health risks to strategies of prevention. Biometals 2010, 23, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe? Environ. Health Perspect. 2017, 125, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Low-level exposure to cadmium during the lifetime increases the risk of osteoporosis and fractures of the lumbar spine in the elderly: Studies on a rat model of human environmental exposure. Toxicol. Sci. 2004, 82, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Moniuszko-Jakoniuk, J. Low-level lifetime exposure to cadmium decreases skeletal mineralization and enhances bone loss in aged rats. Bone 2004, 35, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Majewska, K.; Moniuszko-Jakoniuk, J. Mechanical properties of femoral diaphysis and femoral neck of female rats chronically exposed to various levels of cadmium. Calcif. Tissue Int. 2005, 76, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Majewska, K.; Moniuszko-Jakoniuk, J. Weakness in the mechanical properties of the femur of growing female rats exposed to cadmium. Arch. Toxicol. 2005, 79, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Majewska, K.; Kupraszewicz, E. Effects of low, moderate and relatively high chronic exposure to cadmium on long bones susceptibility to fracture in male rats. Environ. Toxicol. Pharmacol. 2010, 29, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M. Low-level chronic exposure to cadmium enhances the risk of long bone fractures: A study on a female rat model of human lifetime exposure. J. Appl. Toxicol. 2011, 32, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Olgun, O. The effect of dietary cadmium supplementation on performance, egg quality, tibia biomechanical properties and eggshell and bone mineralisation in laying quails. Animal 2015, 9, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Borowska, S.; Tomczyk, M. Antioxidants as a potential preventive and therapeutic strategy for cadmium. Curr. Drug Targets 2016, 17, 1350–1384. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, A.; Sikora, A.; Piątkowska, E.; Borczak, B.; Czech, T. Possible protective role of elderberry fruit lyophilizate against selected effects of cadmium and lead intoxication in Wistar rats. Environ. Sci. Pollut. Res. 2016, 23, 8837–8848. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Shen, C.L.; Wang, P.; Guerrieri, J.; Yeh, J.K.; Wang, J.S. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos. Int. 2008, 19, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Galazyn-Sidorczuk, M.; Jurczuk, M.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols on cadmium accumulation in the body: A study in a rat model of human exposure to this metal. Curr. Drug Targets 2015, 16, 1470–1487. [Google Scholar] [CrossRef]
- Brzóska, M.M.; Rogalska, J.; Galazyn-Sidorczuk, M.; Jurczuk, M.; Roszczenko, A.; Tomczyk, M. Protective effect of Aronia melanocarpa polyphenols against cadmium-induced disorders in bone metabolism: A study in a rat model of lifetime human exposure to this heavy metal. Chem. Biol. Interact. 2015, 229, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Rogalska, J.; Roszczenko, A.; Galazyn-Sidorczuk, A.; Tomczyk, M. The mechanism of the osteoprotective action of a polyphenol-rich Aronia melanocarpa extract during chronic exposure to cadmium is mediated by the oxidative defense system. Planta Med. 2016, 82, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Follet, H.; Boivin, G.; Rumelhart, C.; Meunier, P.J. The degree of mineralization is a determinant of bone strength: A study on human calcanei. Bone 2004, 34, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamarae, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Inj. Int. J. Care Inj. 2016, 47, S11–S20. [Google Scholar] [CrossRef]
- Garnero, P. The role of collagen organization on the properties of bone. Calcif. Tissue Int. 2015, 97, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- Kokotkiewicz, A.; Jaremicz, Z.; Łuczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Brzóska, M.M.; Galażyn-Sidorczuk, M.; Rogalska, J.; Roszczenko, A.; Jurczuk, M.; Majewska, K.; Moniuszko-Jakoniuk, J. Beneficial effect of zinc supplementation on biomechanical properties of femoral distal end and femoral diaphysis of male rats chronically exposed to cadmium. Chem. Biol. Interact. 2008, 171, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Galicka, A.; Brzóska, M.M.; Średzińska, K.; Gindzieński, A. Effect of cadmium on collagen content and solubility in rat bone. Acta Biochim. Polon. 2004, 51, 825–829. [Google Scholar] [PubMed]
- Burr, D.B. The contribution of the organic matrix to bone’s material properties. Bone 2002, 31, 8–11. [Google Scholar] [CrossRef]
- Hubert, P.A.; Lee, S.G.; Lee, S.K.; Chun, O.K. Dietary polyphenols, berries, and age-related bone loss: A review based on human, animal, and cell studies. Antioxidants 2014, 3, 144–158. [Google Scholar] [CrossRef] [PubMed]
| Group | Experiment Duration | |||
|---|---|---|---|---|
| 3 Months | 10 Months | 17 Months | 24 Months | |
| WT (mm) | ||||
| Control | 0.718 ± 0.047 | 0.691 ± 0.010 | 0.786 ± 0.036 | 0.799 ± 0.018 |
| AE | 0.636 ± 0.021 | 0.693 ± 0.017 | 0.860 ± 0.034 | 0.798 ± 0.019 |
| Cd1 | 0.613 ± 0.019 * | 0.678 ± 0.018 | 0.848 ± 0.021 | 0.899 ± 0.038 |
| Cd1 + AE | 0.636 ± 0.032 * | 0.744 ± 0.023 † | 0.756 ± 0.158 | 0.826 ± 0.051 |
| Cd5 | 0.591 ± 0.020 ** | 0.721 ± 0.020 | 0.753 ± 0.023 | 0.819 ± 0.043 |
| Cd5 + AE | 0.633 ± 0.015 | 0.651 ± 0.020 †,‡‡ | 0.821 ± 0.054 | 0.869 ± 0.028 |
| CSA (mm2) | ||||
| Control | 7.610 ± 0.386 | 7.650 ± 0.083 | 9.114 ± 0.428 | 9.571 ± 0.247 |
| AE | 6.937 ± 0.365 | 7.642 ± 0.299 | 10.011 ± 0.478 | 9.082 ± 0.214 |
| Cd1 | 6.419 ± 0.274 * | 7.871 ± 0.235 | 9.788 ± 0.246 | 10.810 ± 0.275 * |
| Cd1 + AE | 6.902 ± 0.335 | 8.362 ± 0.440 | 8.999 ± 0.250 | 9.926 ± 0.407 |
| Cd5 | 6.602 ± 0.221 * | 8.122 ± 0.286 | 8.485 ± 0.449 | 9.616 ± 0.457 ‡ |
| Cd5 + AE | 6.775 ± 0.278 | 7.277 ± 0.396 ‡ | 9.178 ± 0.584 | 10.039 ± 0.433 |
| CSMI | ||||
| Control | 3.681 ± 0.208 | 4.342 ± 0.274 | 6.338 ± 0.382 | 8.615 ± 1.682 |
| AE | 4.080 ± 0.414 | 4.980 ± 0.472 | 7.660 ± 0.612 | 7.442 ± 0.175 |
| Cd1 | 3.606 ± 0.168 | 4.882 ± 0.200 | 6.963 ± 0.609 | 9.850 ± 0.524 |
| Cd1 + AE | 3.778 ± 0.205 | 5.201 ± 0.381 | 7.061 ± 0.442 | 9.271 ± 0.200 |
| Cd5 | 4.395 ± 0.390 | 4.793 ± 0.188 | 5.239 ± 0.516 ‡ | 8.943 ± 0.726 |
| Cd5 + AE | 3.721 ± 0.185 | 4.772 ± 0.356 | 5.801 ± 0.613 | 7.762 ± 0.670 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzóska, M.M.; Roszczenko, A.; Rogalska, J.; Gałażyn-Sidorczuk, M.; Mężyńska, M. Protective Effect of Chokeberry (Aronia melanocarpa L.) Extract against Cadmium Impact on the Biomechanical Properties of the Femur: A Study in a Rat Model of Low and Moderate Lifetime Women Exposure to This Heavy Metal. Nutrients 2017, 9, 543. https://doi.org/10.3390/nu9060543
Brzóska MM, Roszczenko A, Rogalska J, Gałażyn-Sidorczuk M, Mężyńska M. Protective Effect of Chokeberry (Aronia melanocarpa L.) Extract against Cadmium Impact on the Biomechanical Properties of the Femur: A Study in a Rat Model of Low and Moderate Lifetime Women Exposure to This Heavy Metal. Nutrients. 2017; 9(6):543. https://doi.org/10.3390/nu9060543
Chicago/Turabian StyleBrzóska, Małgorzata M., Alicja Roszczenko, Joanna Rogalska, Małgorzata Gałażyn-Sidorczuk, and Magdalena Mężyńska. 2017. "Protective Effect of Chokeberry (Aronia melanocarpa L.) Extract against Cadmium Impact on the Biomechanical Properties of the Femur: A Study in a Rat Model of Low and Moderate Lifetime Women Exposure to This Heavy Metal" Nutrients 9, no. 6: 543. https://doi.org/10.3390/nu9060543