Transparency, Geomorphology and Mixing Regime Explain Variability in Trends in Lake Temperature and Stratification across Northeastern North America (1975–2014)
Abstract
:1. Introduction
2. Materials and Methods
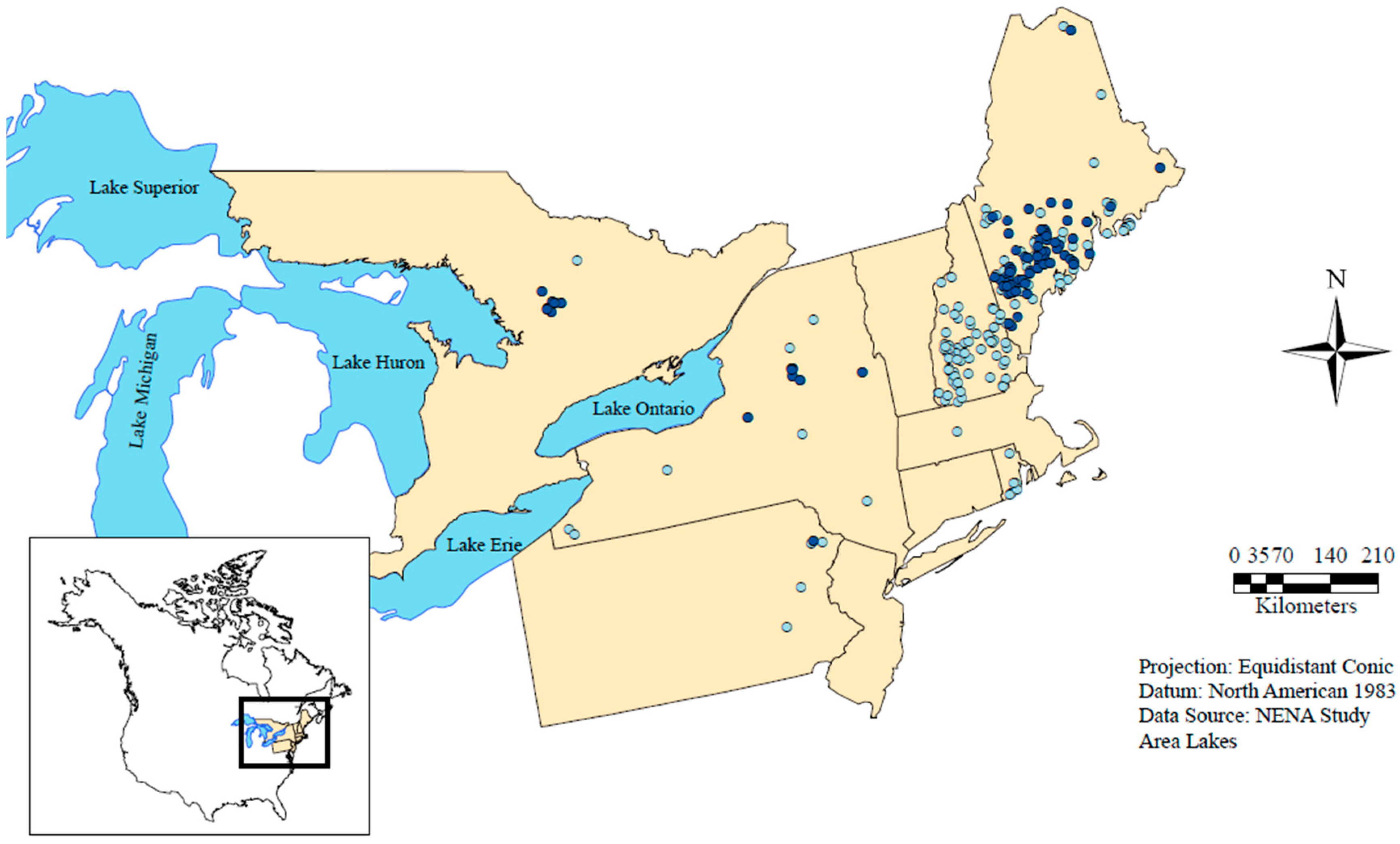
2.1. Data Acquisition and Quality Control
2.2. Annual Profile Selection
2.3. Lake Thermal Metrics
2.4. Data Cohorts
2.5. Temporal Trends in Thermal Metrics
2.6. Air Temperature Data
2.7. Comparison with Air and Lake Near-Surface Temperature Trends
2.8. Median Buoyancy Frequency
2.9. Boosted Regression Tree Analysis
2.10. Meta-Analysis
3. Results
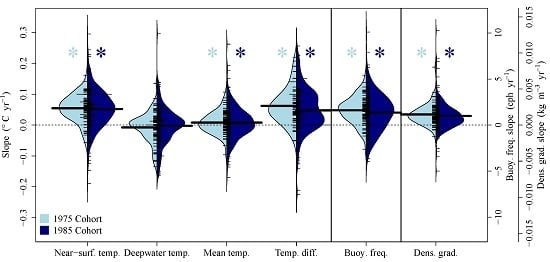
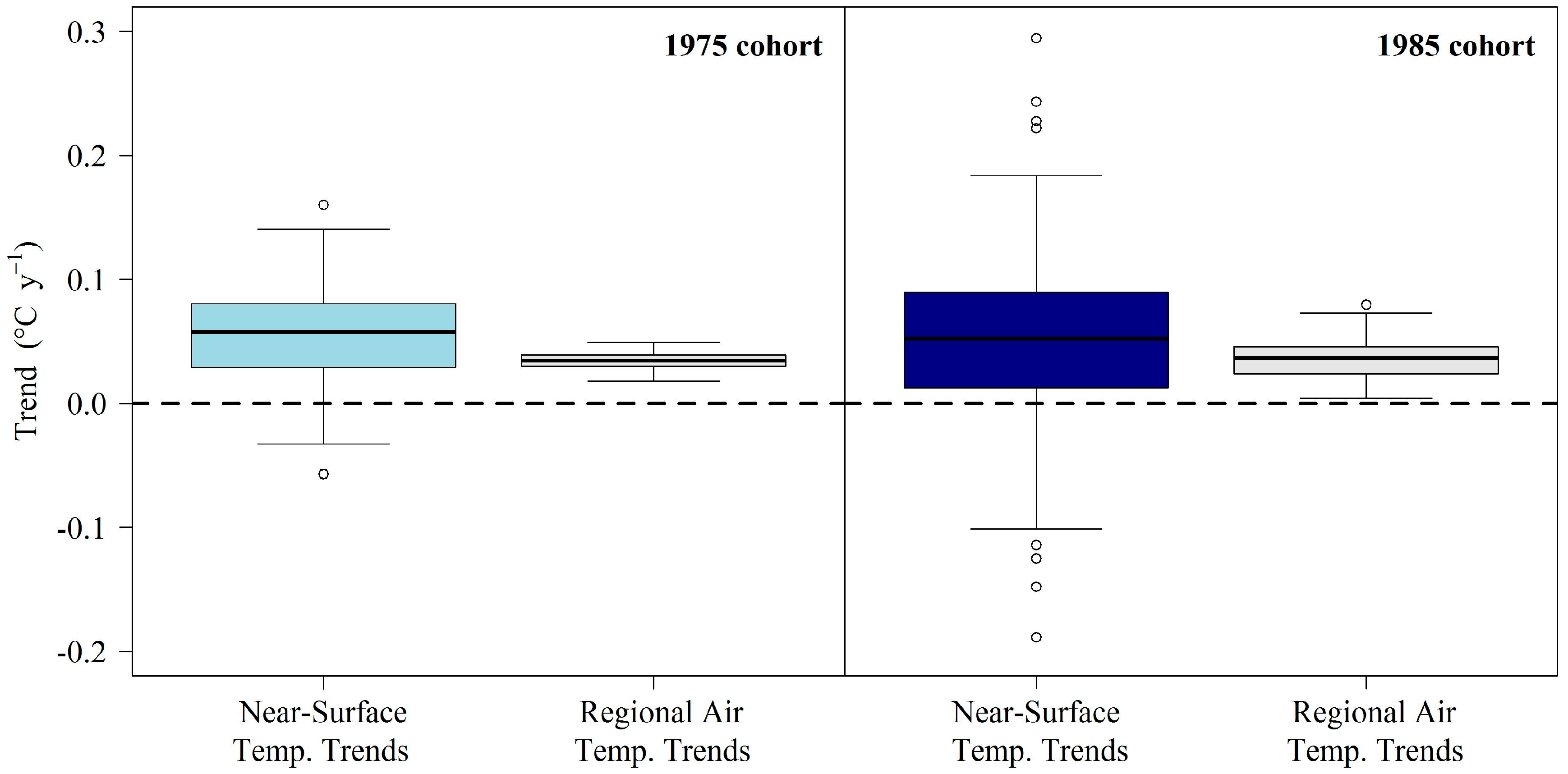
3.1. Comparison of Air and Lake Surface Temperature Trends
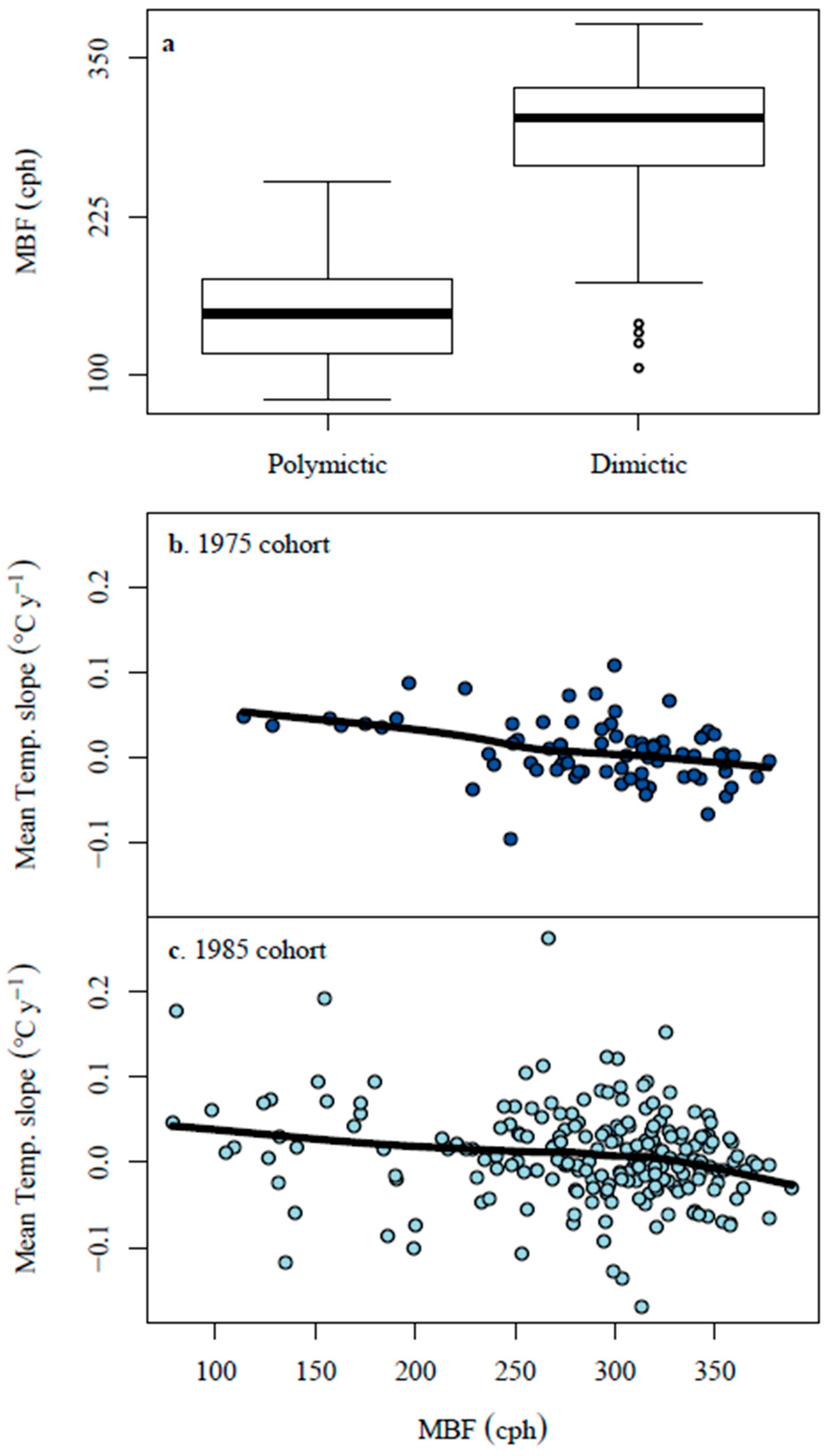
3.2. Median Buoyancy Frequency
3.3. Boosted Regression Tree Models
3.4. Meta-Analysis
4. Discussion
4.1. Near-Surface Warming
4.2. Deepwater Trends
4.3. Thermal Stratification Trends
4.4. Changes in Transparency in the NENA Region
4.5. Ecological Implications
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Messager, M.L.; Lehner, B.; Grill, G.; Nedeva, I.; Schmitt, O. Estimating the volume and age of water stored in global lakes using a geo-statistical approach. Nat. Commun. 2016, 7, 13603. [Google Scholar] [CrossRef] [PubMed]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W. Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Melillo, J.M.; Richmond, T.C.; Yohe, G.W.; Assessment, U.N.C. Climate change impacts in the United States: The third national climate assessment. US Glob. Chang. Res. Progr. 2014. [Google Scholar] [CrossRef]
- Jacobson, G.L.; Fernandez, I.J.; Mayewski, P.A.; Schmitt, C.V. Maine’s Climate Future: An Initial Assessment; University of Maine: Orono, ME, USA, 2009; Available online: http://climatechange.umaine.edu/research/publications/climate-future (accessed on 15 June 2017).
- Adrian, R.; Reilly, C.M.O.; Zagarese, H.; Baines, S.B.; Hessen, D.O.; Keller, W.; Livingstone, D.M.; Sommaruga, R.; Straile, D.; Van Donk, E. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009, 54, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Dodds, W.; Kratz, T.K.; Palmer, M.A. Lakes and streams as sentinels of environmental change in terrestrial and atmospheric processes. Front. Ecol. Environ. 2008, 6, 247–254. [Google Scholar] [CrossRef]
- Williamson, C.E.; Saros, J.E.; Vincent, W.F.; Smol, J.P. Lakes and reservoirs as sentinels, integrators, and regulators of climate change. Limnol. Oceanogr. 2009, 54, 2273–2282. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C. Global Climate Change Impacts in the United States; Cambridge University Press: New York, NY, USA, 2009; p. 196. [Google Scholar]
- Torbick, N.; Ziniti, B.; Wu, S.; Linder, E. Spatiotemporal lake skin summer temperature trends in the northeast United States. Earth Interact. 2016, 20, 1–21. [Google Scholar] [CrossRef]
- Austin, J.; Colman, S. A century of temperature variability in Lake Superior. Limnol. Oceanogr. 2008, 53, 2724–2730. [Google Scholar] [CrossRef]
- Austin, J.A.; Colman, S.M. Lake Superior summer water temperatures are increasing more rapidly than regional temperatures: A positive ice-albedo feedback. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Rowley, R.J.; Schneider, P.; Lenters, J.D.; Mcintyre, P.B.; Kraemer, B.M. Rapid and highly variable warming of lake surface waters around the globe. Geophys. Res. Lett. 2015, 42, 1–9. [Google Scholar] [CrossRef]
- Schneider, P.; Hook, S.J. Space observations of inland water bodies show rapid surface warming since 1985. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Mackay, M.; Stockwell, J.D.; Thiery, W.; Grossart, H.-P.; Augusto-Silva, P.B.; Baulch, H.M.; de Eyto, E.; Hejzlar, J.; Kangur, K. Citizen science shows systematic changes in the temperature difference between air and inland waters with global warming. Sci. Rep. 2017, 7, 43890. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Gray, D.K.; Read, J.S.; O’Reilly, C.M.; Schneider, P.; Qudrat, A.; Gries, C.; Stefanoff, S.; Hampton, S.E.; Hook, S.; et al. A global database of lake surface temperatures collected by in situ and satellite methods from 1985–2009. Sci. Data 2015, 2, 150008. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, B.M.; Anneville, O.; Chandra, S.; Dix, M.; Kuusisto, E.; Livingstone, D.M.; Rimmer, A.; Schladow, S.G.; Silow, E.; Sitoki, L.M.; et al. Morphometry and average temperature affect lake stratification responses to climate change. Geophys. Res. Lett. 2015, 42, 4981–4988. [Google Scholar] [CrossRef]
- Hondzo, M.; Stefan, H.G. Regional water temperature characteristics of lakes subjected to climate change. Clim. Chang. 1993, 24, 187–211. [Google Scholar] [CrossRef]
- Winslow, L.A.; Read, J.S.; Hansen, G.J.A.; Hanson, P.C. Small lakes show muted climate change signal in deepwater temperatures. Geophys. Res. Lett. 2015, 42, 355–361. [Google Scholar] [CrossRef]
- Butcher, J.B.; Nover, D.; Johnson, T.E.; Clark, C.M. Sensitivity of lake thermal and mixing dynamics to climate change. Clim. Chang. 2015, 129, 295–305. [Google Scholar] [CrossRef]
- Heffernan, J.B.; Soranno, P.A.; Angilletta, M.J.; Buckley, L.B.; Gruner, D.S.; Keitt, T.H.; Kellner, J.R.; Kominoski, J.S.; Rocha, A.V.; Xiao, J. Macrosystems ecology: Understanding ecological patterns and processes at continental scales. Front. Ecol. Environ. 2014, 12, 5–14. [Google Scholar] [CrossRef]
- Jankowski, T.; Livingstone, D.M.; Buhrer, H.; Forster, R.; Niederhauser, P. Consequences of the 2003 European heat wave for lake temperature profiles, thermal stability, and hypolimnetic oxygen depletion: Implications for a warmer world. Limnol. Oceanogr. 2006, 51, 815–819. [Google Scholar] [CrossRef]
- Snucins, E.; Gunn, J. Interannual variation in the thermal structure of clear and colored lakes. Limnol. Oceanogr. 2000, 45, 1639–1646. [Google Scholar] [CrossRef]
- Environmental Systems Research Institute (ESRI). ArcGIS Release 10.2; ESRI: Redlands, CA, USA, 2014. [Google Scholar]
- Martin, J.L.; McCutcheon, S.C. Hydrodynamics and Transport for Water Quality Modeling; Lewis Publishing: New York, NY, USA, 1999. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2016; Available online: https://www.R-project.org/ (accessed on 15 June 2017).
- Winslow, L.A.; Read, J.S.; Woolway, R.; Brentrup, J.A.; Leach, T.H.; Zwart, J. rLakeAnalyzer: Package for the Analysis of Lake Physics. R Package Version 1.7.6. 2015. Available online: http://CRAN.R-project.org/package=rLakeAnalyzer (accessed on 15 June 2017).
- Jassby, A.D.; Cloern, J.E. Wq: Some Tools for Exploring Water Quality Monitoring Data. R Package Version 0.4.8. 2016. Available online: https://cran.r-project.org/src/contrib/Archive/wq/ (accessed on 15 June 2017).
- Brohan, P.; Kennedy, J.J.; Harris, I.; Tett, S.F.; Jones, P.D. Uncertainty estimates in regional and global observed temperature changes: A new data set from 1850. J. Geophys. Res. Atmos. 2006, 111, D12106. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Phillips, S.; Leathwick, J.; Elith, J. Dismo: Species Distribution Modeling. R package Version 1.1-1. 2016. Available online: https://CRAN.R-project.org/package=dismo (accessed on 15 June 2017).
- Ridgeway, G. Gbm: Generalized Boosted Regression Models. R package Version 2.1.1. 2015. Available online: https://CRAN.R-project.org/package=gbm (accessed on 15 June 2017).
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Lewin, W.C.; Mehner, T.; Ritterbusch, D.; Brämick, U. The influence of anthropogenic shoreline changes on the littoral abundance of fish species in German lowland lakes varying in depth as determined by boosted regression trees. Hydrobiologia 2014, 724, 293–306. [Google Scholar] [CrossRef]
- Schneider, P.; Hook, S.J.; Radocinski, R.G.; Corlett, G.K.; Hulley, G.C.; Schladow, S.G.; Steissberg, T.E. Satellite observations indicate rapid warming trend for lakes in California and Nevada. Geophys. Res. Lett. 2009, 36, L22402. [Google Scholar] [CrossRef]
- Mason, L.A.; Riseng, C.M.; Gronewold, A.D.; Rutherford, E.S.; Wang, J.; Clites, A.; Smith, S.D.P.; McIntyre, P.B. Fine-scale spatial variation in ice cover and surface temperature trends across the surface of the Laurentian Great Lakes. Clim. Chang. 2016, 138, 71–83. [Google Scholar] [CrossRef]
- Palmer, M.E.; Yan, N.D.; Somers, K.M. Climate change drives coherent trends in physics and oxygen content in North American lakes. Clim. Chang. 2014, 124, 285–299. [Google Scholar] [CrossRef]
- Pareeth, S.; Bresciani, M.; Buzzi, F.; Leoni, B.; Lepori, F.; Ludovisi, A.; Morabito, G.; Adrian, R.; Neteler, M.; Salmaso, N. Warming trends of perialpine lakes from homogenised time series of historical satellite and in-situ data. Sci. Total Environ. 2017, 578, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kirillin, G.; Wen, L.; Shatwell, T. Seasonal thermal regime and climatic trends in lakes of Tibetan Highlands. Hydrol. Earth Syst. Sci. Discuss. 2017, 21, 1895–1909. [Google Scholar] [CrossRef]
- Schmid, M.; Koster, O. Excess warming of a central European lake driven by solar brightening. Water Resour. Res. 2016, 52, 8103–8116. [Google Scholar] [CrossRef]
- Livingstone, D.M. Thermal structure of a large temperate central European lake. Clim. Chang. 2003, 57, 205–225. [Google Scholar] [CrossRef]
- Burns, N.M.; Rockwell, D.C.; Bertram, P.E.; Dolan, D.M.; Ciborowski, J.J.H. Trends in temperature, secchi depth, and dissolved oxygen depletion rates in the central basin of Lake Erie, 1983–2002. J. Great Lakes Res. 2005, 31, 35–49. [Google Scholar] [CrossRef]
- Coats, R.; Perez-Losada, J.; Schladow, G.; Richards, R.; Goldman, C. The warming of Lake Tahoe. Clim. Chang. 2006, 76, 121–148. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Hook, S.; Huttula, T.; Kotilainen, P.; O’Reilly, C.M.; Peltonen, A.; Plisnier, P.-D.; Sarvala, J.; Tamatamah, R.; Vadeboncoeur, Y.; et al. Century-long warming trends in the upper water column of Lake Tanganyika. PLoS ONE 2015, 10, e0132490. [Google Scholar] [CrossRef] [PubMed]
- Hampton, S.E.; Izmest’eva, L.R.; Moore, M.V.; Katz, S.L.; Dennis, B.; Silow, E.A. Sixty years of environmental change in the world’s largest freshwater lake—Lake Baikal, Siberia. Glob. Chang. Biol. 2008, 14, 1947–1958. [Google Scholar] [CrossRef]
- Pareeth, S.; Salmaso, N.; Adrian, R.; Neteler, M. Homogenised daily lake surface water temperature data generated from multiple satellite sensors: A long-term case study of a large sub-Alpine lake. Sci. Rep. 2016, 6, 31251. [Google Scholar] [CrossRef] [PubMed]
- Izmest’eva, L.R.; Moore, M.V.; Hampton, S.E.; Ferwerda, C.J.; Gray, D.K.; Woo, K.H.; Pislegina, H.V.; Krashchuk, L.S.; Shimaraeva, S.V.; Silow, E.A. Lake-wide physical and biological trends associated with warming in Lake Baikal. J. Great Lakes Res. 2016, 42, 6–17. [Google Scholar] [CrossRef]
- Schmid, M.; Hunziker, S.; Wüest, A. Lake surface temperatures in a changing climate: A global sensitivity analysis. Clim. Chang. 2014, 124, 301–315. [Google Scholar] [CrossRef]
- Fink, G.; Schmid, M.; Wahl, B.; Wolf, T.; Wuest, A. Heat fluxmodifications related to climate-induced warming of large European lakes. Water Resour. Res. 2014, 50, 2072–2085. [Google Scholar] [CrossRef]
- Eastman, R.; Warren, S.G.; Eastman, R. A 39-year survey of cloud changes from land stations worldwide 1971–2009. J. Clim. 2013, 26, 1286–1303. [Google Scholar] [CrossRef]
- Hodgkins, G.A.; James, I.C.; Huntington, T.G. Historical changes in lake ice-out dates as indicators of climate change in New England, 1850–2000. Int. J. Climatol. 2002, 22, 1819–1827. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Robertson, D.M.; Benson, B.J.; Wynne, R.H.; Livingstone, D.M.; Arai, T.; Assel, R.A.; Barry, R.G.; Card, V.M.; Kuusisto, E.; et al. Historical trends in lake and river ice-cover in the Northern Hemisphere. Science 2000, 289, 1743–1746. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Notaro, M.; Vavrus, S.J.; Foster, M.J. Recent accelerated warming of the Laurentian Great Lakes: Physical drivers. Limnol. Oceanogr. 2016. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Yan, N.D.; Keller, B.; Girard, R.; Heneberry, J.; Gunn, J.M.; Hamilton, D.P.; Taylor, P.A. Cooling lakes while the world warms: Effects of forest regrowth and increased dissolved organic matter on the thermal regime of a temperate, urban lake. Limnol. Oceanogr. 2008, 53, 404–410. [Google Scholar]
- Valerio, G.; Pilotti, M.; Barontini, S.; Leoni, B. Sensitivity of the multiannual thermal dynamics of a deep pre-alpine lake to climatic change. Hydrol. Process. 2015, 29, 767–779. [Google Scholar] [CrossRef]
- Heiskanen, J.J.; Mammarella, I.; Ojala, A.; Stepanenko, V.; Erkkila, K.-M.; Miettinen, H.; Sandstrom, H.; Eugster, W.; Lepparanta, M.; Jarvinen, H.; et al. Effects of water clarity on lake stratification and lake-atmosphere heat exchange. J. Geophys. Res. Atmos. 2015, 120, 4282–4303. [Google Scholar] [CrossRef]
- Rose, K.C.; Winslow, L.A.; Read, J.S.; Hansen, G.J. Climate-induced warming of lakes can be either amplified or suppressed by trends in water clarity. Limnol. Oceanogr. Lett. 2016, 1, 44–53. [Google Scholar] [CrossRef]
- Read, J.S.; Rose, K.C. Physical responses of small temperate lakes to variation in dissolved organic carbon concentrations. Limnol. Oceanogr. 2013, 58, 921–931. [Google Scholar] [CrossRef]
- Thompson, J.R.; Carpenter, D.N.; Cogbill, C.V.; Foster, D.R. Four centuries of change in northeastern United States forests. PLoS ONE 2013, 8, e72540. [Google Scholar] [CrossRef] [PubMed]
- Fee, E.J.; Hecky, R.E.; Kasian, S.E.M.; Cruikshank, D.R. Physical and chemical responses of lakes and streams. Limnol. Oceanogr. 1996, 41, 912–920. [Google Scholar] [CrossRef]
- Read, J.S.; Hamilton, D.P.; Desai, A.R.; Rose, K.C.; MacIntyre, S.; Lenters, J.D.; Smyth, R.L.; Hanson, P.C.; Cole, J.J.; Staehr, P.A.; et al. Lake-size dependency of wind shear and convection as controls on gas exchange. Geophys. Res. Lett. 2012, 39, 1–5. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Long-term lake water temperature and ice cover simulations/measurements. Cold Reg. Sci. Technol. 1996, 24, 289–304. [Google Scholar] [CrossRef]
- Vollmer, M.K.; Bootsma, H.A.; Hecky, R.E.; Patterson, G.; Halfman, J.D.; Edmond, J.M.; Eccles, D.H.; Weiss, R.F. Deep-water warming trend in Lake Malawi, East Africa. Limnol. Oceanogr. 2005, 50, 727–732. [Google Scholar] [CrossRef]
- Markfort, C.D.; Perez, A.L.S.; Thill, J.W.; Jaster, D.A.; Porté-Agel, F.; Stefan, H.G. Wind sheltering of a lake by a tree canopy or bluff topography. Water Resour. Res. 2010, 46, 1–13. [Google Scholar] [CrossRef]
- Pryor, S.; Barthelmie, R.; Young, D.; Takle, E.; Arritt, R.; Flory, D.; Gutowski, W.; Nunes, A.; Roads, J. Wind speed trends over the contiguous United States. J. Geophys. Res. Atmos. 2009, 114. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). The physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Mooij, W.M.; Hülsmann, S.; De Senerpont Domis, L.N.; Nolet, B.A.; Bodelier, P.L.E.; Boers, P.C.M.; Dionisio Pires, L.M.; Gons, H.J.; Ibelings, B.W.; Noordhuis, R.; et al. The impact of climate change on lakes in the Netherlands: A review. Aquat. Ecol. 2005, 39, 381–400. [Google Scholar] [CrossRef]
- Evans, C.D.; Chapman, P.J.; Clark, J.M.; Monteith, D.T.; Cresser, M.S. Alternative explanations for rising dissolved organic carbon export from organic soils. Glob. Chang. Biol. 2006, 12, 2044–2053. [Google Scholar] [CrossRef]
- Monteith, D.T.; Stoddard, J.L.; Evans, C.D.; de Wit, H.A.; Forsius, M.; Høgåsen, T.; Wilander, A.; Skjelkvåle, B.L.; Jeffries, D.S.; Vuorenmaa, J.; et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 2007, 450, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Turner, M.A. Biological, chemical and physical responses of lakes to experimental acidification. Water Air Soil Pollut. 1981, 18, 259–271. [Google Scholar] [CrossRef]
- Raymond, P.A.; Saiers, J.E. Event controlled DOC export from forested watersheds. Biogeochemistry 2010, 100, 197–209. [Google Scholar] [CrossRef]
- Roulet, N.; Moore, T. Browning the waters. Nature 2006, 444, 283–284. [Google Scholar] [CrossRef] [PubMed]
- SanClements, M.D.; Oelsner, G.P.; McKnight, D.M.; Stoddard, J.L.; Nelson, S.J. New insights into the source of decadal increases of dissolved organic matter in acid-sensitive lakes of the Northeastern United States. Environ. Sci. Technol. 2012, 46, 3212–3219. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.E.; Overholt, E.P.; Pilla, R.M.; Leach, T.H.; Brentrup, J.A.; Knoll, L.B.; Mette, E.M.; Moeller, R.E. Ecological consequences of long-term browning in lakes. Sci. Rep. 2015, 5, 18666. [Google Scholar] [CrossRef] [PubMed]
- Strock, K.E.; Nelson, S.J.; Kahl, J.S.; Saros, J.E.; McDowell, W.H. Decadal trends reveal recent acceleration in the rate of recovery from acidification in the northeastern US. Environ. Sci. Technol. 2014, 48, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Couture, S.; Houle, D.; Gagnon, C. Increases of dissolved organic carbon in temperate and boreal lakes in Quebec, Canada. Environ. Sci. Pollut. Res. 2012, 19, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Strock, K.E.; Saros, J.E.; Nelson, S.J.; Birkel, S.D.; Kahl, J.S.; McDowell, W.H. Extreme weather years drive episodic changes in lake chemistry: Implications for recovery from sulfate deposition and long-term trends in dissolved organic carbon. Biogeochemistry 2016, 127, 353–365. [Google Scholar] [CrossRef]
- Sleeter, B.M.; Sohl, T.L.; Loveland, T.R.; Auch, R.F.; Acevedo, W.; Drummond, M.A.; Sayler, K.L.; Stehman, S.V. Land-cover change in the conterminous United States from 1973 to 2000. Glob. Environ. Chang. 2013, 23, 733–748. [Google Scholar] [CrossRef]
- Dodds, W.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Policy analysis eutrophication of U.S. Freshwaters: Damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.; Vander Zanden, M. What a difference a species makes: A meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecol. Monogr. 2010, 80, 179–196. [Google Scholar] [CrossRef]
- Zhu, B.; Fitzgerald, D.G.; Mayer, C.M.; Rudstam, L.G.; Mills, E.L. Alteration of ecosystem function by zebra mussels in Oneida Lake: Impacts on submerged macrophytes. Ecosystems 2006, 9, 1017–1028. [Google Scholar] [CrossRef]
- Benson, B.J.; Magnuson, J.J.; Jensen, O.P.; Card, V.M.; Hodgkins, G.; Korhonen, J.; Livingstone, D.M.; Stewart, K.M.; Weyhenmeyer, G.A.; Granin, N.G. Extreme events, trends, and variability in Northern Hemisphere lake-ice phenology (1855–2005). Clim. Chang. 2012, 112, 299–323. [Google Scholar] [CrossRef]
- Jensen, O.P.; Benson, B.J.; Magnuson, J.J.; Card, V.M.; Futter, M.N.; Soranno, P.A.; Stewart, K.M. Spatial analysis of ice phenology trends across the Laurentian Great Lakes region during a recent warming period. Limnol. Oceanogr. 2007, 52, 2013–2026. [Google Scholar] [CrossRef]
- Van Bocxlaer, B.; Schultheiß, R.; Plisnier, P.D.; Albrecht, C. Does the decline of gastropods in deep water herald ecosystem change in Lakes Malawi and Tanganyika? Freshw. Biol. 2012, 57, 1733–1744. [Google Scholar] [CrossRef]
- O’Reilly, C.M.; Alin, S.R.; Plisnier, P.-D.D.; Cohen, A.S.; McKee, B.A. Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature 2003, 424, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Tierney, J.E.; Mayes, M.T.; Meyer, N.; Johnson, C.; Swarzenski, P.W.; Cohen, A.S.; Russell, J.M. Late-twentieth-century warming in Lake Tanganyika unprecedented since AD 500. Nat. Geosci. 2010, 3, 422–425. [Google Scholar] [CrossRef]
- Cohen, A.S.; Gergurich, E.L.; Kraemer, B.M.; McGlue, M.M.; McIntyre, P.B.; Russell, J.M.; Simmons, J.D.; Swarzenski, P.W. Climate warming reduces fish production and benthic habitat in Lake Tanganyika, one of the most biodiverse freshwater ecosystems. Proc. Natl. Acad. Sci. USA 2016, 113, 9563–9568. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, K.L.; Ewing, H.A.; Greer, M.L.; Carey, C.C.; Weathers, K.C. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 2015, 6, 1–19. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Paul, V.J. Climate change: Links to global expansion of harmful cyanobacteria. Water Res. 2012, 46, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.A.; Bianco, G.; Ekvall, M.; Heuschele, J.; Hylander, S.; Yang, X. Instantaneous threat escape and differentiated refuge demand among zooplankton taxa. Ecology 2016, 97, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Larsson, P.; Lampert, W. Experimental evidence of a low-oxygen refuge for large zooplankton. Limnol. Oceanogr. 2011, 56, 1682–1688. [Google Scholar] [CrossRef]
- Manca, M.; DeMott, W.R. Response of the invertebrate predator Bythotrephes to a climate-linked increase in the duration of a refuge from fish predation. Limnol. Oceanogr. 2009, 54, 2506–2512. [Google Scholar] [CrossRef]
- Kraemer, B.M.; Chandra, S.; Dell, A.I.; Dix, M.; Kuusisto, E.; Livingstone, D.M.; Schladow, S.G. Global patterns in lake ecosystem responses to warming based on the temperature dependence of metabolism. Glob. Chang. Biol. 2016, 23, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Robillard, M.; Fox, M. Historical changes in abundance and community structure of warmwater piscivore communities associated with changes in water clarity, nutrients, and temperature. Can. J. Fish. Aquat. Sci. 2006, 63, 798–809. [Google Scholar] [CrossRef]
- Rudstam, L.G.; Jackson, J.R.; Hetherington, A.L. Concluding remarks: Forecasting the future of Oneida Lake and its fishery in an era of climate change and biological invasions. In Oneida Lake: Long-Term Dynamics of a Managed Ecosystem and Its Fishery; Rudstam, L.G., Mills, E.L., Jackson, J.R., Stewart, D.J., Eds.; American Fisheries Society: Bethesda, NY, USA, 2016. [Google Scholar]
- Downing, J.; Prairie, Y.; Cole, J.; Duarte, C.; Tranvik, L.; Striegl, R.; McDowell, W.; Kortelainen, P.; Caraco, N.; Melack, J. The global abundance and size distribution of lakes, ponds, and impoundments. Limnol. Oceanogr. 2006, 51, 2388–2397. [Google Scholar] [CrossRef]

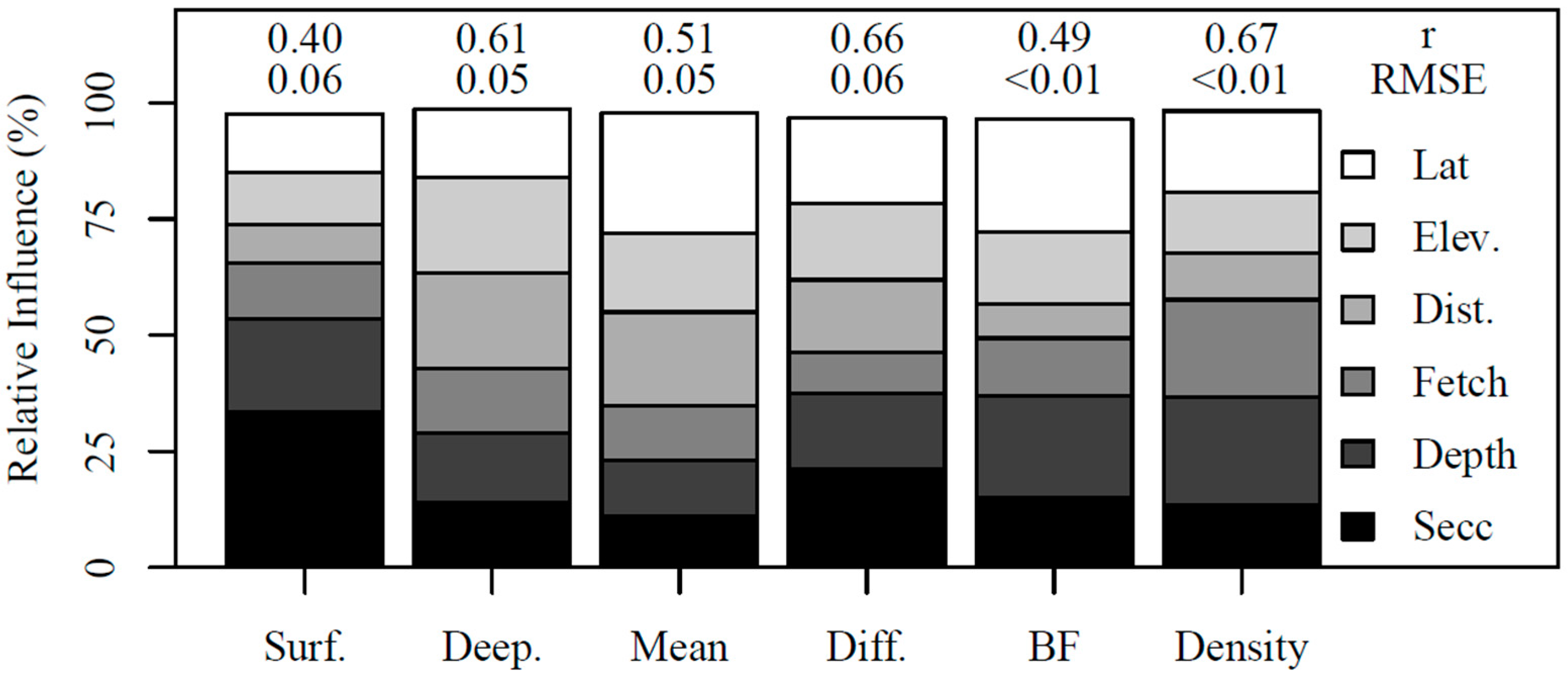
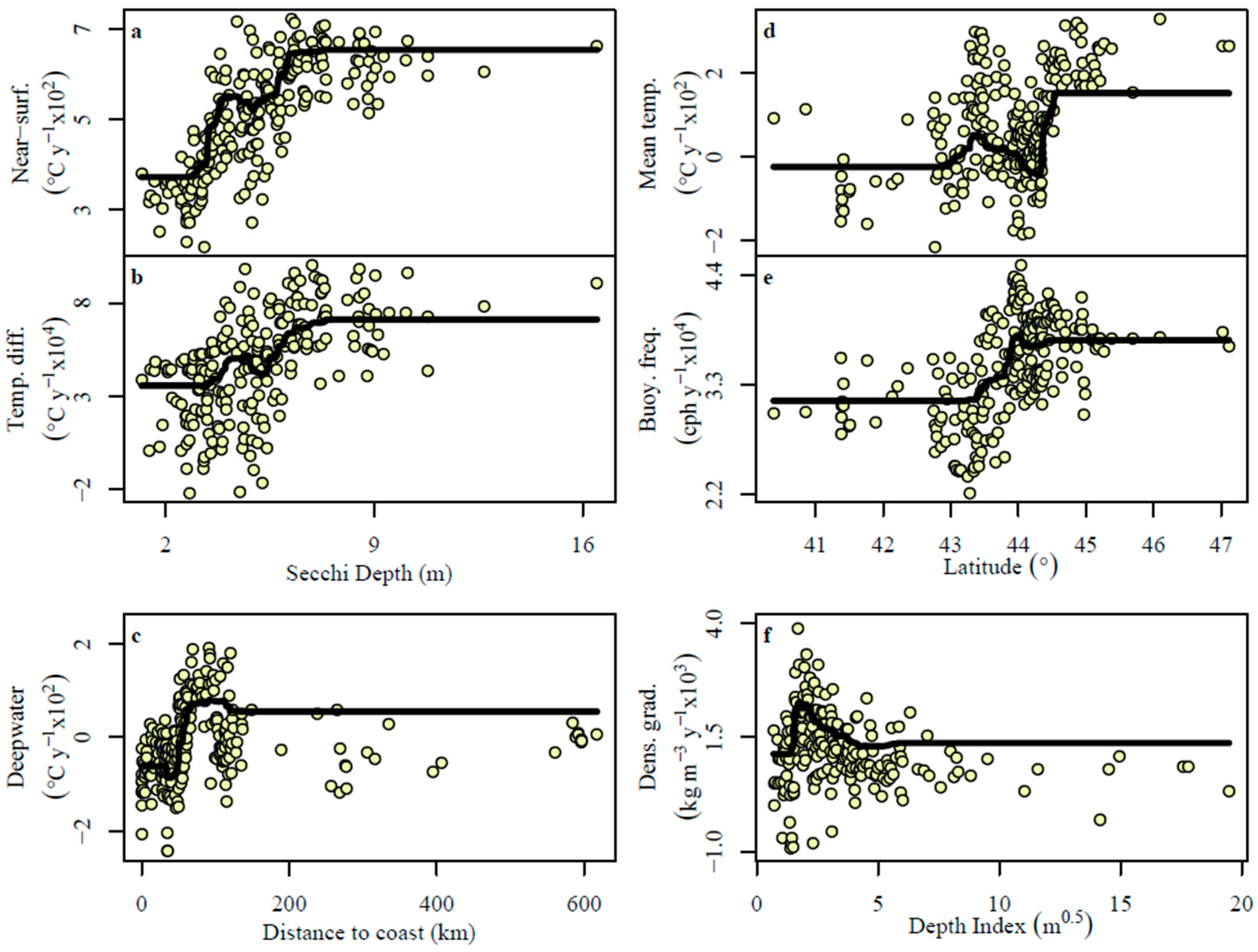
| Variable | Units | Abbrev. | Min. | Max. | Median | BRT |
|---|---|---|---|---|---|---|
| Chlorophyll a | μg/L | Chl a | 0.3 | 42.3 | 3.9 | N |
| Distance to coast | km | Dist. | 0.0 | 617.1 | 58.1 | Y |
| Elevation | m asl | Elev | 3.1 | 667.0 | 137.5 | Y |
| Fetch | m | Fetch | 350 | 51269 | 2466 | Y |
| Latitude | ° | Lat | 40.4 | 47.1 | 44.0 | Y |
| Maximum depth | m | MaxD | 2.7 | 60.0 | 13.5 | N |
| Mean depth | m | Depth | 1.4 | 21.3 | 5.6 | Y * |
| Secchi depth | m | Secc | 1.2 | 16.5 | 4.9 | Y |
| Surface area | km2 | Sarea | 0.016 | 207.0 | 1.4 | N |
| Total phosphorus | μg/L | TP | 1.9 | 95.4 | 9.3 | N |
| Citation | Type | Location | LSWT Trends (°C Decade−1) | AT Trends (°C Decade−1) | Years |
|---|---|---|---|---|---|
| This study | IS (85 a) | R: northeastern North America | 0.54 ± 0.05 a | 0.35 ± 0.01 a | 1975–2014 a |
| IS (226 b) | 0.52 ± 0.04 b | 0.35 ± 0.01 b | 1985–2014 b | ||
| [13] | SAT (167) | Global | 0.37 | NA | 1991–2009 |
| [12] | IS (118) | Global | 0.34 ± 0.09 | 0.24 | 1985–2009 |
| SAT (128) | |||||
| [16] | IS (26) | Global | 0.20 ± 0.04 | NA | 1970–2010 |
| [34] | SAT (6) | R: Nev./Cal. U.S. | 1.10 ± 0.20 | 0.60 | 1992–2008 |
| [35] | SAT (5) | R: Great Lakes | 0.96 ± 0.13 | NA | 1994–2013 |
| US/CA | |||||
| [36] | IS (7 c) | R: Ont., CA c | 0.80 ± 0.10 c | 1.00 c | 1981–2005 |
| IS (5 d) | Wis., U.S. d | 0.90 ± 0.10 d | 0.40 d | ||
| [9] | SAT (3955) | R: Northeast U.S. | 0.80 | 0.46 | 1984–2012 |
| [18] | IS (118 e) | R: Wis., U.S. | 0.56 e | 0.67 | 1990–2013 |
| IS (24 f) | 0.30 f | ||||
| [37] | SAT (5) | R: Alps, IT | 0.32 ± 0.04 | NA | 1986–2015 |
| [38] | IS & SAT (2) | R: Tibet, CN | 0.32 | 0.28 | 1982–2012 |
| [11] | IS (1 *) | S:L. Superior, U.S. | 1.10 ± 0.66 | 0.59 ± 0.17 | 1979–2006 |
| [10] | IS (1 *) | S:L. Superior, U.S. | 0.27 ± 0.04 g | ||
| 1.10 ± 0.40 h,i | 0.09 ± 0.02 g | 1906–2005 g | |||
| 1.00 ± 0.70 j | 0.60 ± 0.20 h,i,j | 1980–2005 h,i,j | |||
| [39] | IS (1 *) | S:L. Zurich, CH | 0.41 | 0.50 k | 1981–2013 |
| [40] | IS (1 *) | S:L. Zurich, CH | 0.41 | 0.11l | 1947–1998 |
| [41] | IS (1 *) | S:L. Erie, U.S./CA | 0.37 | NA | 1983–2002 |
| [42] | IS (1 *) | S:L. Tahoe, U.S. | 0.23 | NA | 1970–2002 |
| [43] | IS k,l | S:L. Tanganyika | 0.13 ± 0.02 m | NA | 1912–2013 k |
| 0.16 ± 0.08 n | |||||
| SAT m (1 *) | TZ/CD/BI/ZM | 1985–2011 l,m | |||
| 0.23 ± 0.11 ° | |||||
| [44] | IS (1) | S:L. Baikal, RU | 0.20 | 0.12 | 1946–2006 |
| [45] | SAT (1 *) | S:L. Garda, IT | 0.20 | NA | 1986–2015 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richardson, D.C.; Melles, S.J.; Pilla, R.M.; Hetherington, A.L.; Knoll, L.B.; Williamson, C.E.; Kraemer, B.M.; Jackson, J.R.; Long, E.C.; Moore, K.; et al. Transparency, Geomorphology and Mixing Regime Explain Variability in Trends in Lake Temperature and Stratification across Northeastern North America (1975–2014). Water 2017, 9, 442. https://doi.org/10.3390/w9060442
Richardson DC, Melles SJ, Pilla RM, Hetherington AL, Knoll LB, Williamson CE, Kraemer BM, Jackson JR, Long EC, Moore K, et al. Transparency, Geomorphology and Mixing Regime Explain Variability in Trends in Lake Temperature and Stratification across Northeastern North America (1975–2014). Water. 2017; 9(6):442. https://doi.org/10.3390/w9060442
Chicago/Turabian StyleRichardson, David C., Stephanie J. Melles, Rachel M. Pilla, Amy L. Hetherington, Lesley B. Knoll, Craig E. Williamson, Benjamin M. Kraemer, James R. Jackson, Elizabeth C. Long, Karen Moore, and et al. 2017. "Transparency, Geomorphology and Mixing Regime Explain Variability in Trends in Lake Temperature and Stratification across Northeastern North America (1975–2014)" Water 9, no. 6: 442. https://doi.org/10.3390/w9060442
APA StyleRichardson, D. C., Melles, S. J., Pilla, R. M., Hetherington, A. L., Knoll, L. B., Williamson, C. E., Kraemer, B. M., Jackson, J. R., Long, E. C., Moore, K., Rudstam, L. G., Rusak, J. A., Saros, J. E., Sharma, S., Strock, K. E., Weathers, K. C., & Wigdahl-Perry, C. R. (2017). Transparency, Geomorphology and Mixing Regime Explain Variability in Trends in Lake Temperature and Stratification across Northeastern North America (1975–2014). Water, 9(6), 442. https://doi.org/10.3390/w9060442