Feature Papers in ‘Cellular Pathology’
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cellular Pathology".
Viewed by 37910
Share This Topical Collection
Editor
 Prof. Dr. Ritva Tikkanen
Prof. Dr. Ritva Tikkanen
 Prof. Dr. Ritva Tikkanen
Prof. Dr. Ritva Tikkanen
E-Mail
Website
Collection Editor
Institute of Biochemistry, Medical Faculty, Justus-Liebig University of Giessen, Friedrichstrasse 24, D-35392 Giessen, Germany
Interests: lysosomal storage disorders; vesicular trafficking; endosomal sorting; lysosome biogenesis; mitochondrial diseases; autoimmune disorders
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection “Feature Papers in Cellular Pathology” aims at collecting high-quality research articles, review articles and communications in all fields of Cellular Pathology with a focus on cell biological research. The aim of this Topical Collection is to illustrate, through selected works, frontier research in various aspects of cellular pathology in diseases. We especially encourage the Editorial Board Members of Cells to contribute papers reflecting the latest progress in their research field. In addition, further experts in relevant research fields are welcome to submit their work to this Topical Collection.
Topics include but are not limited to:
- mechanisms of genetic diseases;
- mechanisms of infectious diseases;
- mechanisms of acquired diseases;
- autoimmune disorders;
- ageing;
- cellular damage;
- cellular pathways relevant to pathogenic processes.
Prof. Dr. Ritva Tikkanen
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Published Papers (8 papers)
Open AccessReview
Using Zebrafish to Study Multiciliated Cell Development and Disease States
by
Thanh Khoa Nguyen, Sophia Baker, John-Michael Rodriguez, Liana Arceri and Rebecca A. Wingert
Viewed by 536
Abstract
Multiciliated cells (MCCs) serve many important functions, including fluid propulsion and chemo- and mechanosensing. Diseases ranging from rare conditions to the recent COVID-19 global health pandemic have been linked to MCC defects. In recent years, the zebrafish has emerged as a model to
[...] Read more.
Multiciliated cells (MCCs) serve many important functions, including fluid propulsion and chemo- and mechanosensing. Diseases ranging from rare conditions to the recent COVID-19 global health pandemic have been linked to MCC defects. In recent years, the zebrafish has emerged as a model to investigate the biology of MCCs. Here, we review the major events in MCC formation including centriole biogenesis and basal body docking. Then, we discuss studies on the role of MCCs in diseases of the brain, respiratory, kidney and reproductive systems, as well as recent findings about the link between MCCs and SARS-CoV-2. Next, we explore why the zebrafish is a useful model to study MCCs and provide a comprehensive overview of previous studies of genetic components essential for MCC development and motility across three major tissues in the zebrafish: the pronephros, brain ependymal cells and nasal placode. Taken together, here we provide a cohesive summary of MCC research using the zebrafish and its future potential for expanding our understanding of MCC-related disease states.
Full article
►▼
Show Figures
Open AccessArticle
Mapping Cell-in-Cell Structures in Oral Squamous Cell Carcinoma
by
Leonardo de Oliveira Siquara da Rocha, Bruno Solano de Freitas Souza, Ricardo Della Coletta, Daniel W. Lambert and Clarissa A. Gurgel Rocha
Cited by 3 | Viewed by 2023
Abstract
Cell-in-cell (CIC) structures contribute to tumor aggressiveness and poor prognosis in oral squamous cell carcinoma (OSCC). In vitro 3D models may contribute to the understanding of the underlying molecular mechanisms of these events. We employed a spheroid model to study the CIC structures
[...] Read more.
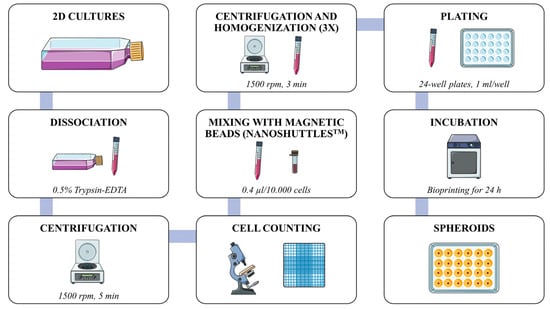
Cell-in-cell (CIC) structures contribute to tumor aggressiveness and poor prognosis in oral squamous cell carcinoma (OSCC). In vitro 3D models may contribute to the understanding of the underlying molecular mechanisms of these events. We employed a spheroid model to study the CIC structures in OSCC. Spheroids were obtained from OSCC (HSC3) and cancer-associated fibroblast (CAF) lines using the Nanoshuttle-PL
TM bioprinting system (Greiner Bio-One). Spheroid form, size, and reproducibility were evaluated over time (Evos
TM XL; ImageJ version 1.8). Slides were assembled, stained (hematoxylin and eosin), and scanned (Axio Imager Z2/VSLIDE) using the OlyVIA System (Olympus Life Science) and ImageJ software (NIH) for cellular morphology and tumor zone formation (hypoxia and/or proliferative zones) analysis. CIC occurrence, complexity, and morphology were assessed considering the spheroid regions. Well-formed spheroids were observed within 6 h of incubation, showing the morphological aspects of the tumor microenvironment, such as hypoxic (core) and proliferative zone (periphery) formation. CIC structures were found in both homotypic and heterotypic groups, predominantly in the proliferative zone of the mixed HSC3/CAF spheroids. “Complex cannibalism” events were also noted. These results showcase the potential of this model in further studies on CIC morphology, formation, and relationship with tumor prognosis.
Full article
►▼
Show Figures
Open AccessReview
Beyond SMARCB1 Loss: Recent Insights into the Pathobiology of Epithelioid Sarcoma
by
Elisa Del Savio and Roberta Maestro
Cited by 13 | Viewed by 2999
Abstract
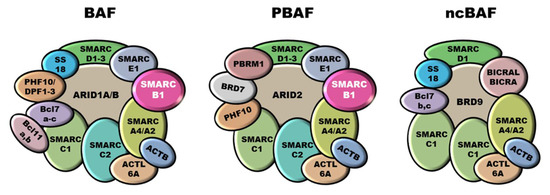
Epithelioid sarcoma (ES) is a very rare and aggressive mesenchymal tumor of unclear origin and uncertain lineage characterized by a prevalent epithelioid morphology. The only recurrent genetic alteration reported in ES as yet is the functional inactivation of SMARCB1 (SWI/SNF-related matrix-associated actin-dependent regulator
[...] Read more.
Epithelioid sarcoma (ES) is a very rare and aggressive mesenchymal tumor of unclear origin and uncertain lineage characterized by a prevalent epithelioid morphology. The only recurrent genetic alteration reported in ES as yet is the functional inactivation of SMARCB1 (SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1), a key component of the SWI/SNF (SWItch/Sucrose Non-Fermentable) chromatin remodeling complexes. How SMARCB1 deficiency dictates the clinicopathological characteristics of ES and what other molecular defects concur to its malignant progression is still poorly understood. This review summarizes the recent findings about ES pathobiology, including defects in chromatin remodeling and other signaling pathways and their role as therapeutic vulnerabilities.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Formins in Human Disease
by
Leticia Labat-de-Hoz and Miguel A. Alonso
Cited by 26 | Viewed by 6445
Abstract
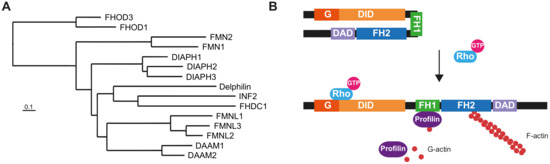
Almost 25 years have passed since a mutation of a formin gene,
DIAPH1, was identified as being responsible for a human inherited disorder: a form of sensorineural hearing loss. Since then, our knowledge of the links between formins and disease has deepened
[...] Read more.
Almost 25 years have passed since a mutation of a formin gene,
DIAPH1, was identified as being responsible for a human inherited disorder: a form of sensorineural hearing loss. Since then, our knowledge of the links between formins and disease has deepened considerably. Mutations of
DIAPH1 and six other formin genes (
DAAM2,
DIAPH2,
DIAPH3,
FMN2,
INF2 and
FHOD3) have been identified as the genetic cause of a variety of inherited human disorders, including intellectual disability, renal disease, peripheral neuropathy, thrombocytopenia, primary ovarian insufficiency, hearing loss and cardiomyopathy. In addition, alterations in formin genes have been associated with a variety of pathological conditions, including developmental defects affecting the heart, nervous system and kidney, aging-related diseases, and cancer. This review summarizes the most recent discoveries about the involvement of formin alterations in monogenic disorders and other human pathological conditions, especially cancer, with which they have been associated. In vitro results and experiments in modified animal models are discussed. Finally, we outline the directions for future research in this field.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Alpha-Synuclein Preformed Fibrils Induce Cellular Senescence in Parkinson’s Disease Models
by
Dinesh Kumar Verma, Bo Am Seo, Anurupa Ghosh, Shi-Xun Ma, Karina Hernandez-Quijada, Julie K. Andersen, Han Seok Ko and Yong-Hwan Kim
Cited by 40 | Viewed by 7587
Abstract
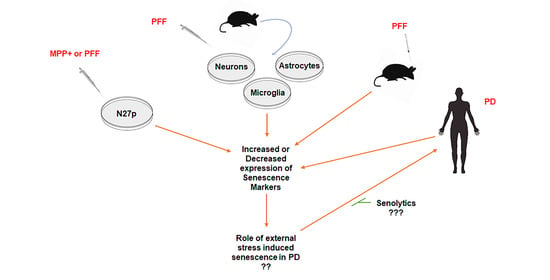
Emerging evidence indicates that cellular senescence could be a critical inducing factor for aging-associated neurodegenerative disorders. However, the involvement of cellular senescence remains unclear in Parkinson’s disease (PD). To determine this, we assessed the effects of α-synuclein preformed fibrils (α-syn PFF) or 1-methyl-4-phenylpyridinium
[...] Read more.
Emerging evidence indicates that cellular senescence could be a critical inducing factor for aging-associated neurodegenerative disorders. However, the involvement of cellular senescence remains unclear in Parkinson’s disease (PD). To determine this, we assessed the effects of α-synuclein preformed fibrils (α-syn PFF) or 1-methyl-4-phenylpyridinium (MPP
+) on changes in cellular senescence markers, employing α-syn PFF treated-dopaminergic N27 cells, primary cortical neurons, astrocytes and microglia and α-syn PFF-injected mouse brain tissues, as well as human PD patient brains. Our results demonstrate that α-syn PFF-induced toxicity reduces the levels of Lamin B1 and HMGB1, both established markers of cellular senescence, in correlation with an increase in the levels of p21, a cell cycle-arrester and senescence marker, in both reactive astrocytes and microglia in mouse brains. Using Western blot and immunohistochemistry, we found these cellular senescence markers in reactive astrocytes as indicated by enlarged cell bodies within GFAP-positive cells and Iba1-positive activated microglia in α-syn PFF injected mouse brains. These results indicate that PFF-induced pathology could lead to astrocyte and/or microglia senescence in PD brains, which may contribute to neuropathology in this model. Targeting senescent cells using senolytics could therefore constitute a viable therapeutic option for the treatment of PD.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Precision Oncology Beyond Genomics: The Future Is Here—It Is Just Not Evenly Distributed
by
Ulrike Pfohl, Alina Pflaume, Manuela Regenbrecht, Sabine Finkler, Quirin Graf Adelmann, Christoph Reinhard, Christian R. A. Regenbrecht and Lena Wedeken
Cited by 11 | Viewed by 4748
Abstract
Cancer is a multifactorial disease with increasing incidence. There are more than 100 different cancer types, defined by location, cell of origin, and genomic alterations that influence oncogenesis and therapeutic response. This heterogeneity between tumors of different patients and also the heterogeneity within
[...] Read more.
Cancer is a multifactorial disease with increasing incidence. There are more than 100 different cancer types, defined by location, cell of origin, and genomic alterations that influence oncogenesis and therapeutic response. This heterogeneity between tumors of different patients and also the heterogeneity within the same patient’s tumor pose an enormous challenge to cancer treatment. In this review, we explore tumor heterogeneity on the longitudinal and the latitudinal axis, reviewing current and future approaches to study this heterogeneity and their potential to support oncologists in tailoring a patient’s treatment regimen. We highlight how the ideal of precision oncology is reaching far beyond the knowledge of genetic variants to inform clinical practice and discuss the technologies and strategies already available to improve our understanding and management of heterogeneity in cancer treatment. We will focus on integrating multi-omics technologies with suitable in vitro models and their proficiency in mimicking endogenous tumor heterogeneity.
Full article
►▼
Show Figures
Open AccessFeature PaperReview
Interaction between Parkin and α-Synuclein in PARK2-Mediated Parkinson’s Disease
by
Daniel Aghaie Madsen, Sissel Ida Schmidt, Morten Blaabjerg and Morten Meyer
Cited by 43 | Viewed by 8109
Abstract
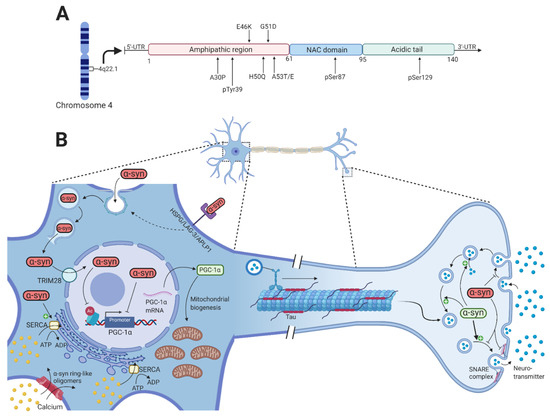
Parkin and
α-synuclein are two key proteins involved in the pathophysiology of Parkinson’s disease (PD). Neurotoxic alterations of
α-synuclein that lead to the formation of toxic oligomers and fibrils contribute to PD through synaptic dysfunction, mitochondrial impairment, defective endoplasmic reticulum and
[...] Read more.
Parkin and
α-synuclein are two key proteins involved in the pathophysiology of Parkinson’s disease (PD). Neurotoxic alterations of
α-synuclein that lead to the formation of toxic oligomers and fibrils contribute to PD through synaptic dysfunction, mitochondrial impairment, defective endoplasmic reticulum and Golgi function, and nuclear dysfunction. In half of the cases, the recessively inherited early-onset PD is caused by loss of function mutations in the
PARK2 gene that encodes the E3-ubiquitin ligase, parkin. Parkin is involved in the clearance of misfolded and aggregated proteins by the ubiquitin-proteasome system and regulates mitophagy and mitochondrial biogenesis.
PARK2-related PD is generally thought not to be associated with Lewy body formation although it is a neuropathological hallmark of PD. In this review article, we provide an overview of post-mortem neuropathological examinations of
PARK2 patients and present the current knowledge of a functional interaction between parkin and
α-synuclein in the regulation of protein aggregates including Lewy bodies. Furthermore, we describe prevailing hypotheses about the formation of intracellular micro-aggregates (synuclein inclusions) that might be more likely than Lewy bodies to occur in
PARK2-related PD. This information may inform future studies aiming to unveil primary signaling processes involved in PD and related neurodegenerative disorders.
Full article
►▼
Show Figures
Open AccessReview
Aberrant Splicing Events and Epigenetics in Viral Oncogenomics: Current Therapeutic Strategies
by
Flavia Zita Francies and Zodwa Dlamini
Cited by 15 | Viewed by 3984
Abstract
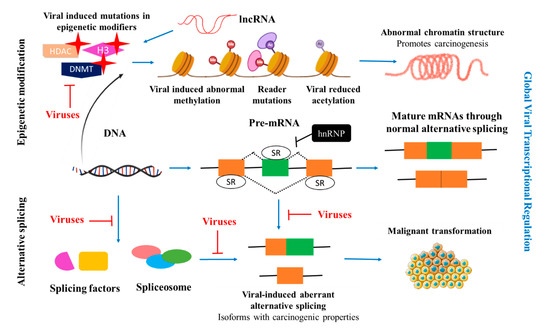
Global cancer incidence and mortality are on the rise. Although cancer is fundamentally a non-communicable disease, a large number of cancers are known to have a viral aetiology. A high burden of infectious agents (Human immunodeficiency virus (HIV), human papillomavirus (HPV), hepatitis B
[...] Read more.
Global cancer incidence and mortality are on the rise. Although cancer is fundamentally a non-communicable disease, a large number of cancers are known to have a viral aetiology. A high burden of infectious agents (Human immunodeficiency virus (HIV), human papillomavirus (HPV), hepatitis B virus (HBV)) in certain Sub-Saharan African countries drives the rates of certain cancers. About one-third of all cancers in Africa are attributed to infection. Seven viruses have been identified with carcinogenic characteristics, namely the HPV, HBV, Hepatitis C virus (HCV), Epstein–Barr virus (EBV), Human T cell leukaemia virus 1 (HTLV-1), Kaposi’s Sarcoma Herpesvirus (KSHV), and HIV-1. The cellular splicing machinery is compromised upon infection, and the virus generates splicing variants that promote cell proliferation, suppress signalling pathways, inhibition of tumour suppressors, alter gene expression through epigenetic modification, and mechanisms to evade an immune response, promoting carcinogenesis. A number of these splice variants are specific to virally-induced cancers. Elucidating mechanisms underlying how the virus utilises these splice variants to maintain its latent and lytic phase will provide insights into novel targets for drug discovery. This review will focus on the splicing genomics, epigenetic modifications induced by and current therapeutic strategies against HPV, HBV, HCV, EBV, HTLV-1, KSHV and HIV-1.
Full article
►▼
Show Figures