Spinocerebellar Ataxia (SCA): Molecular Mechanisms and Novel Treatment Strategies
A topical collection in Cells (ISSN 2073-4409). This collection belongs to the section "Cells of the Nervous System".
Viewed by 29506Editor
Interests: MJD/SCA3; polyglutamine diseases; function of polyglutamine proteins; therapeutic approaches for polyglutamine diseases; molecular mechanisms and biomarkers of neurodegeneration; genetics of dynamic repeat disorders
Topical Collection Information
Dear Colleagues,
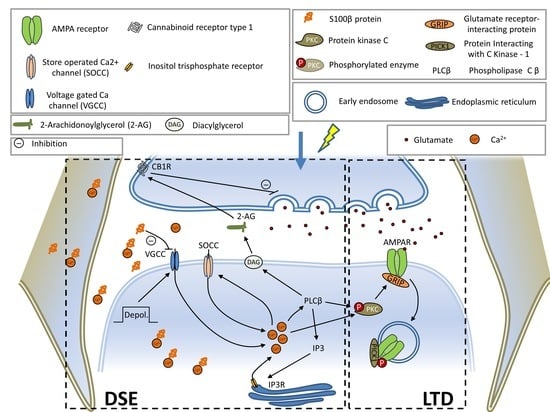
Spinocerebellar ataxias (SCAs) are a group of autosomal dominant inherited diseases of usual onset in adult life. SCAs are phenotypically and genetically heterogeneous disorders with no currently available disease-modifying treatment.
While the major clinical signs of SCAs are progressive gait ataxia, typically accompanied by dysarthria and visual problems, several other symptoms can appear such as limb and trunk ataxia, tremor, rigidity, dystonia, bradykinesia, and cognitive impairment.
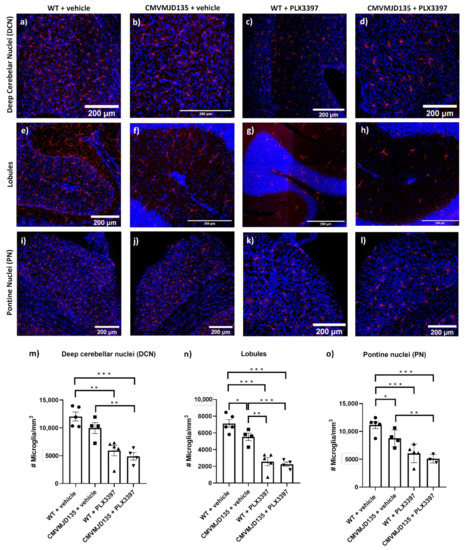
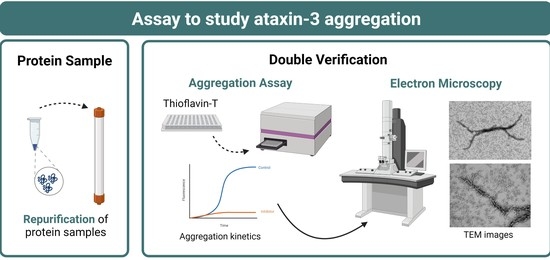
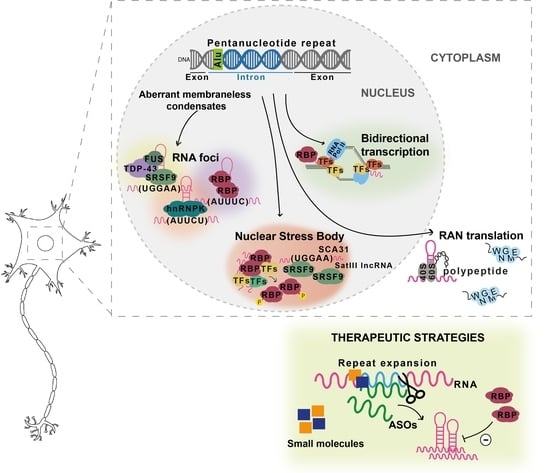
Presently, 48 SCAs can be discerned by the identified mutation or the chromosomal location of their associated disease genes. The most prevalent group of SCAs, accounting for more than 50% of the known SCAs, are caused by expansions of CAG repeats, encoding polyglutamine (polyQ) tracts in the disease proteins, and are therefore designated as polyQ SCAs. Other less-common SCAs are caused by the expansion of other nucleotide repeats, point mutations, deletions, insertions, and duplications in the disease genes.
Overall, research efforts have been showing that whereas SCAs may share disease mechanisms that can be therapeutically targeted for all SCAs, given the heterogenous genetic causes, specific therapeutic strategies for each SCA or subgroup of SCAs may be comparatively more successful. Preclinical research—mostly for the polyQ SCAs—has indeed generated some therapeutic agents that are about to approaching testing in patients.
In this Topical Collection we invite all scientists working on spinocerebellar ataxias to contribute original research articles, reviews, communications, and short perspective articles on all aspects related to molecular mechanisms and novel treatment strategies for SCAs. We particularly welcome articles describing mechanistic insights at the molecular, cellular, or organismal level, as well as those providing translational value.
Dr. Maria do Carmo Costa
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- spinocerebellar ataxia
- polyglutamine
- therapeutic approaches
- disease mechanisms