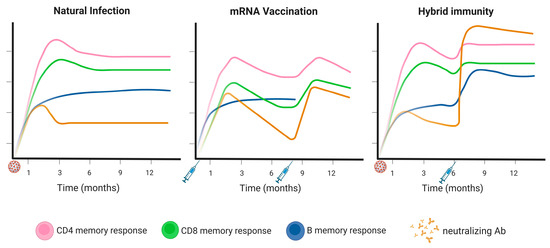
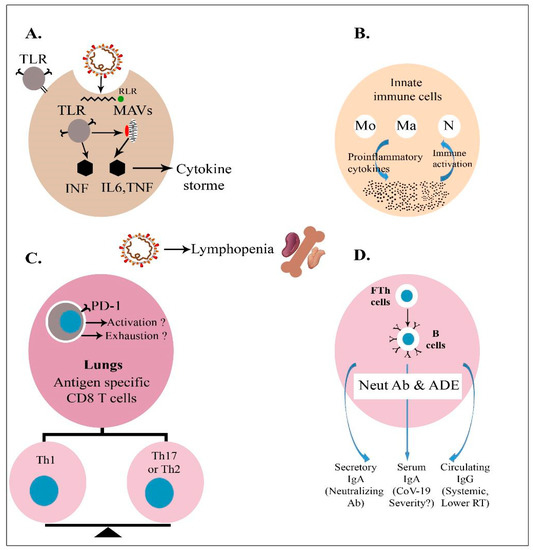
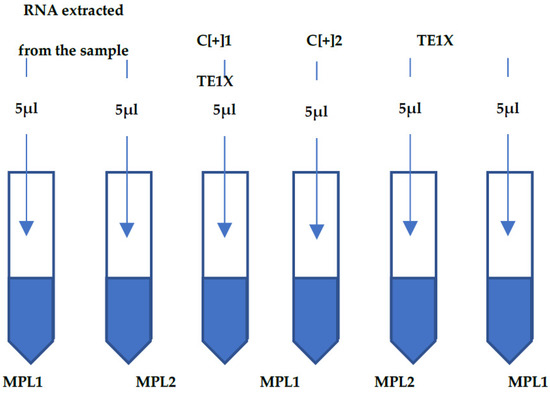
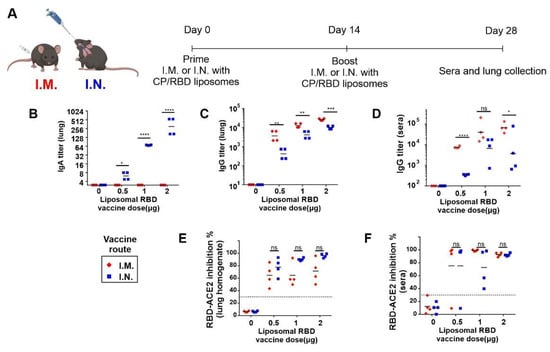
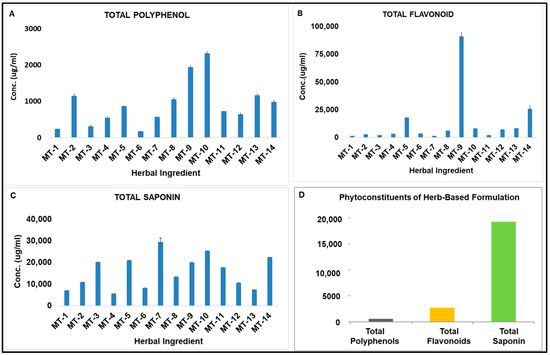
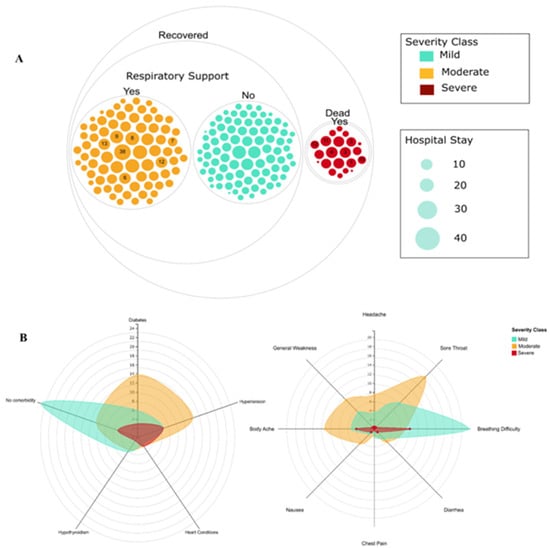
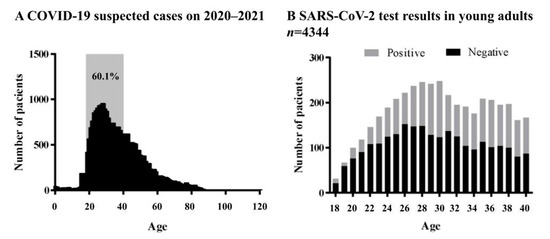
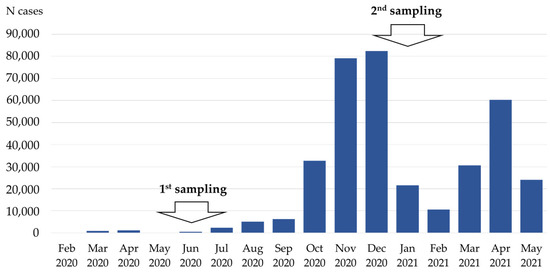
SARS-CoV-2 Infection and COVID-19 Disease (Closed)
A topical collection in Pathogens (ISSN 2076-0817). This collection belongs to the section "Immunological Responses and Immune Defense Mechanisms".
Viewed by 89941Editors
2. Texas Biomedical Research Institute, San Antonio, TX 78245, USA
Interests: virology; vaccines; antivirals; influenza viruses; arenaviruses; Zika virus; coronavirus; SARS-CoV-2; COVID-19; innate immunity; adaptive immunity; interferon; virus-host interactions
Special Issues, Collections and Topics in MDPI journals
Interests: virology; influenza; coronavirus; innate immunity; virus-host interactions; interferons; inflammation; vaccines; antivirals
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
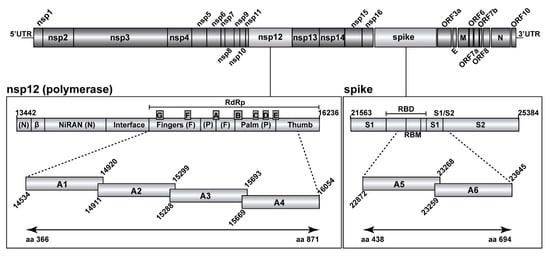
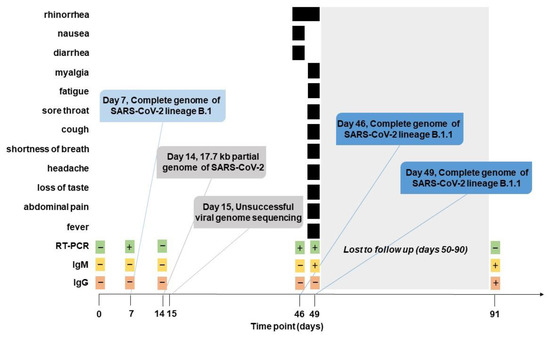
In December 2019, a previously unknown coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in Wuhan, China. SARS-CoV-2 is the causative agent of coronavirus disease 2019 (COVID-19), a world-wide pandemic that has dramatically impacted the global human public health and socioeconomic activities across the world with a magnitude not seen since the “Spanish flu” pandemic in 1918/1919.
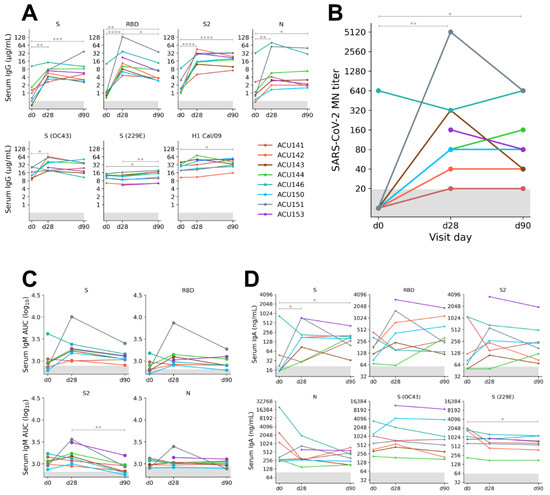
Global efforts to develop a vaccine have resulted in several promising COVID-19 vaccine candidates with excellent safety and efficacy profiles. Currently, three vaccines have been approved by the Food and Drug Administration (FDA) for emergency use for the treatment of SARS-CoV-2 infection.
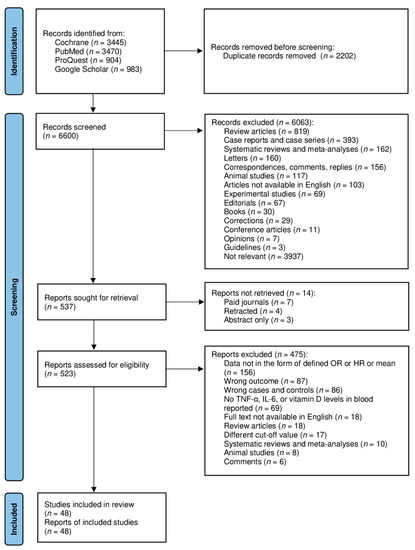
The goal of this Topical Collection “SARS-CoV-2 Infection and COVID-19 Disease” is to cover aspects related to viral infection and pathogenesis, epidemiology and evolution, virus–host interactions, prophylactic vaccine development, therapeutic antivirals, neutralizing antibodies, innate and adaptive immune responses, reverse genetics approaches, recombinant viruses, reporter-expressing viruses, animal models of viral infection, pathogenesis and transmission, and COVID-19 disease.
We hope this Topical Collection will provide researchers with new insights on the biology of SARS-CoV-2 infection and its associated COVID-19 disease with the goal of unifying efforts to develop effective countermeasures to protect against SARS-CoV-2 infection and COVID-19 disease.
Prof. Dr. Luis Martinez-Sobrido
Dr. Marta L. DeDiego
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Pathogens is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- SARS-CoV-2
- COVID-19
- viral infection
- viral pathogenesis
- viral transmission
- epidemiology and evolution
- virus-host interactions
- prophylactics
- vaccines
- therapeutics
- antivirals
- neutralizing antibodies
- innate immunity
- adaptive immunity
- reverse genetics systems
- animal models