Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety
Abstract
1. Introduction
2. Methods
3. Function of Selected Carriers Involved in Transport of Anticancer Drugs
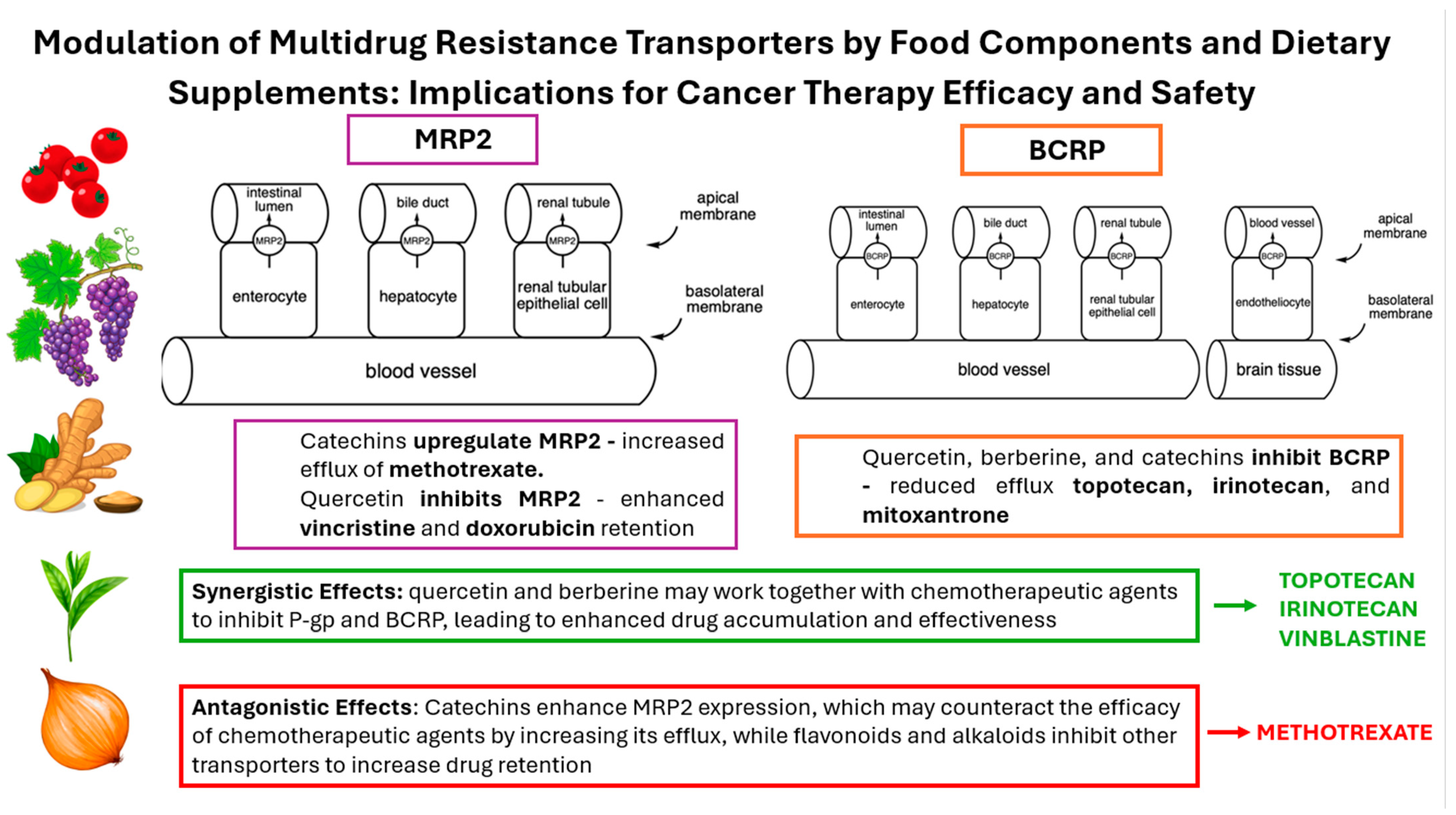
3.1. Multidrug Resistance-Associated Protein 2—MRP2
3.2. Breast Cancer Resistance Protein—BCRP
3.3. P-Glycoprotein—P-gp
4. Influence of Polyphenolic Compounds on MDR Transporters
4.1. Catechins
4.2. Quercetin and Its Derivatives
4.3. Apigenin
4.4. Licochalcone A
4.5. Miscellaneous Flavonoids
4.6. Sinapic Acid
4.7. Resveratrol
4.8. Curcumin
5. Effect of Terpenoids and Sterols on MDR Transporters
5.1. Menthol
5.2. Geraniol
5.3. β-Caryophyllene Oxide
5.4. Carnosic Acid and Carnosol
5.5. Beta-Sitosterol
6. Effect of Alkaloids on MDR Transporters
6.1. Berberine
6.2. Capsaicin
6.3. Piperine
7. Effect of Plant Juices on MDR Transporters
7.1. Rocket Juice
7.2. Cranberry Juice
8. Structure–Activity Relationship
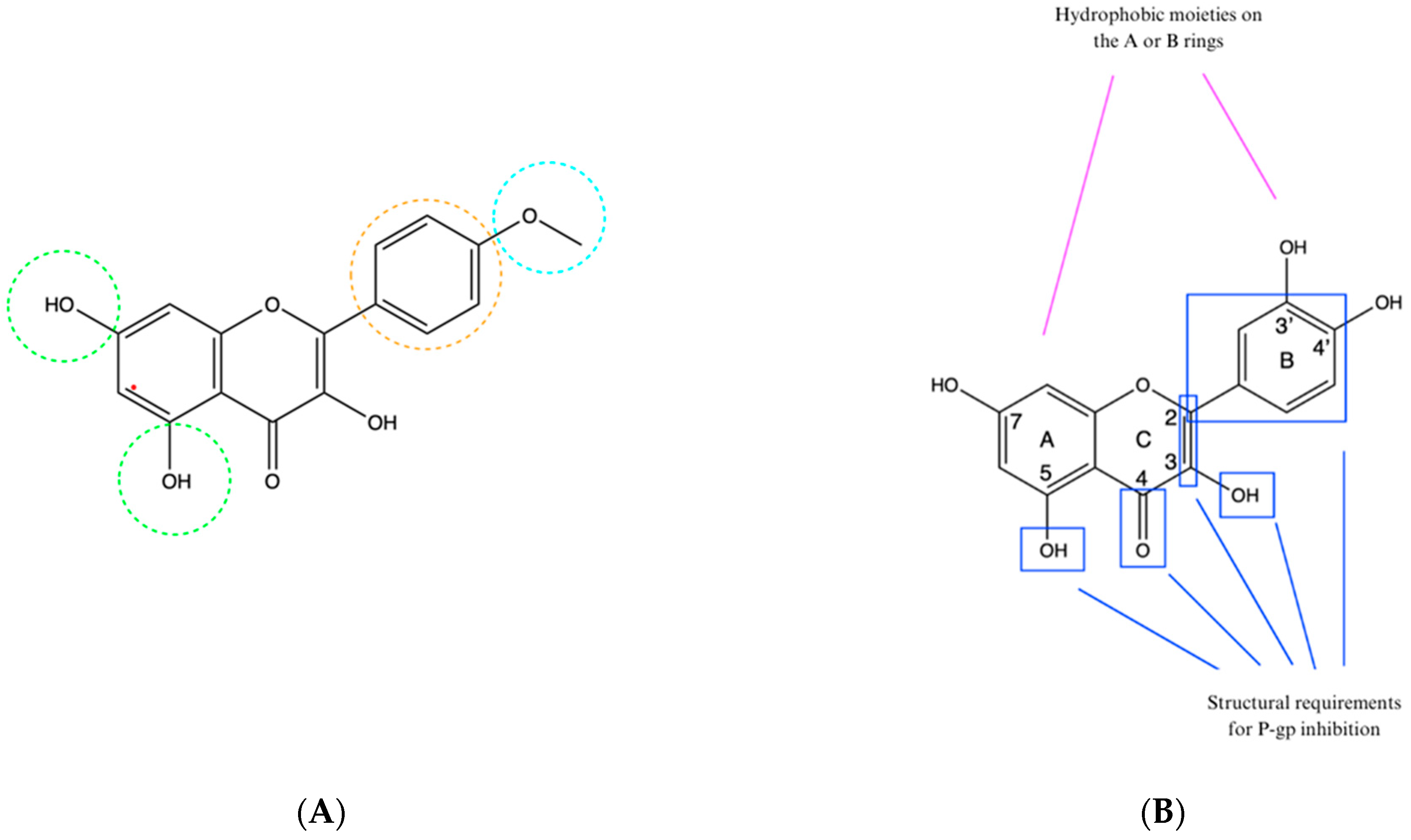

9. The Study Limitations
10. Conclusions
- Enhanced efficacy through targeted nutrients: Incorporating a diet rich in specific nutrients, e.g., antioxidants, has been shown to improve specific aspects of chemotherapy efficacy, e.g., tumor response in in vitro or animal studies. However, patients undergoing chemotherapy should be careful in increasing the intake of these nutrients, especially when taken as dietary supplements, and consult healthcare professionals.
- Potential dietary modifications: Based on our findings, we recommend that patients should discuss the possibility of dietary modifications with their oncologists with the help of a clinical pharmacist and clinical dietician. These changes could potentially optimize the effectiveness of chemotherapy and improve overall patient well-being.
- Personalized dietary plans: It is essential for dietary recommendations to be tailored to the individual patient, considering factors such as specific type of cancer, treatment regimen, and personal health conditions. Personalized dietary plans should be developed in collaboration with nutritionists and healthcare providers to ensure the best possible outcomes.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escribano-Ferrer, E.; Reglero, G.; Santoyo, S. Polyphenols as Modulators of Multidrug Resistance Proteins: Potential Clinical Applications. Nutrients 2020, 12, 559. [Google Scholar]
- Noguchi, K.; Katayama, K.; Mitsuhashi, J.; Sugimoto, Y. Dietary Flavonoids and Multidrug Resistance in Cancer Chemotherapy: A Comprehensive Review. Front. Pharmacol. 2021, 12, 741116. [Google Scholar]
- Li, W.; Lu, Y.; Chen, M.; Zeng, G. Natural Products Modulating P-gp and BCRP in Cancer Therapy: Potential Applications in Chemotherapy. Curr. Med. Chem. 2021, 28, 3170–3185. [Google Scholar]
- Tan, H.-Y.; Wang, N.; Tsao, S.-W.; Che, C.-M.; Yuen, M.-F.; Feng, Y. Dietary Supplements and Chemotherapy: Potential Roles and Mechanisms in Modulating Drug Resistance. J. Clin. Med. 2021, 10, 1936. [Google Scholar]
- Goh, D.-L.; Li, J.; Zhu, Y.; Feng, Y. The Influence of Diet on Drug Transporter Expression and Function in Cancer Therapy. Pharmacol. Rev. 2020, 72, 759–796. [Google Scholar]
- Fang, Y.; Cao, W.; Liang, F.; Xia, M.; Pan, S.; Xu, X. Structure Affinity Relationship and Docking Studies of Flavonoids as Substrates of Multidrug-Resistant Associated Protein 2 (MRP2) in MDCK/MRP2 Cells. Food Chem. 2019, 291, 101–109. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, C.; Xu, P.; Liu, M.; Xu, F.; Wang, X. Characterization of In Vitro Mrp2 Transporter Model Based on Intestinal Organoids. Regul. Toxicol. Pharmacol. 2019, 108, 104449. [Google Scholar] [CrossRef]
- Banik, A.; Ghosh, K.; Patil, U.K.; Gayen, S. Identification of Molecular Fingerprints of Natural Products for the Inhibition of Breast Cancer Resistance Protein (BCRP). Phytomedicine 2021, 85, 102375. [Google Scholar] [CrossRef]
- Safar, Z.; Kis, E.; Erdo, F.; Zolnerciks, J.K.; Krajcsi, P. ABCG2/BCRP: Variants, Transporter Interaction Profile of Substrates and Inhibitors. Expert Opin. Drug Metab. Toxicol. 2019, 15, 313–328. [Google Scholar] [CrossRef]
- Yakusheva, E.N.; Titov, D.S. Structure and Function of Multidrug Resistance Protein 1. Biochemistry 2018, 83, 907–929. [Google Scholar] [CrossRef]
- Smolik, M.; Suraj, J.; Kurpinska, A.; Walczak, M. ABC Membrane Transporters and Their Multifunctional Nature. Postepy Hig. Med. Dosw. 2018, 72, 606–622. [Google Scholar] [CrossRef]
- Silva, V.; Gil-Martins, E.; Silva, B.; Rocha-Pereira, C.; Sousa, M.E.; Remião, F.; Silva, R. Xanthones as P-glycoprotein Modulators and Their Impact on Drug Bioavailability. Expert Opin. Drug Metab. Toxicol. 2021, 17, 441–482. [Google Scholar] [CrossRef]
- Drug Development and Drug Interactions|Table of Substrates, Inhibitors and Inducers|FDA [Internet]. Available online: https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers (accessed on 18 May 2024).
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial Effects of Green Tea—A Review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Keen, C.L.; Holt, R.R.; Oteiza, P.I.; Fraga, C.G.; Schmitz, H.H. Cocoa Antioxidants and Cardiovascular Health. Am. J. Clin. Nutr. 2005, 81, 298S–303S. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Liu, S.; Qu, F.; Zhang, H.; Chen, Y.; Ni, D. Effect of Stereochemical Configuration on the Transport and Metabolism of Catechins from Green Tea Across Caco-2 Monolayers. Molecules 2019, 24, 1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zuo, Y.; Deng, S.; Zhu, F.; Liu, Q.; Wang, R.; Li, T.; Cai, H.; Wan, X.; Xie, Z.; et al. Using Caffeine and Free Amino Acids to Enhance the Transepithelial Transport of Catechins in Caco-2 Cells. J. Agric. Food Chem. 2019, 67, 5477–5485. [Google Scholar] [CrossRef]
- Tuntiteerawit, P.; Jarukamjorn, K.; Porasuphatana, S. The Effect of Green Tea Catechins on Breast Cancer Resistance Protein Activity and Intestinal Efflux of Aflatoxin B1 via Breast Cancer Resistance Protein in Caco-2 Cells. Toxicol. Res. 2020, 36, 293–300. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, R.; Yang, T.; Wei, Y.; Yang, C.; Zhou, J.; Liu, Y.; Shi, S. Inhibition Effect of Epigallocatechin-3-gallate on the Pharmacokinetics of Calcineurin Inhibitors, Tacrolimus, and Cyclosporine A, in Rats. Expert Opin. Drug Metab. Toxicol. 2021, 17, 121–134. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Lee, J.H.; Lee, Y.J. Evaluation of the Mrp2-mediated Flavonoid-Drug Interaction Potential of Quercetin in Rats and In Vitro Models. Asian J. Pharm. Sci. 2019, 14, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Liu, Y.; Huang, X.; Zhang, R.; Yang, C.; Zhou, J.; Zhang, Y.; Wan, J.; Shi, S. Quercetin-3-O-β-D-glucoside Decreases the Bioavailability of Cyclosporin A Through Regulation of Drug Metabolizing Enzymes, Transporters and Nuclear Receptors in Rats. Mol. Med. Rep. 2018, 18, 2599–2612. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef] [PubMed]
- Raish, M.; Ahmad, A.; Shahid, M.; Jardan, Y.A.B.; Ahad, A.; Kalam, M.A.; Ansari, M.A.; Iqbal, M.; Ali, N.; Alkharfy, K.M.; et al. Effects of Apigenin on Pharmacokinetics of Dasatinib and Probable Interaction Mechanism. Molecules 2023, 28, 1602. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and Anti-inflammatory Activities of Six Flavonoids Separated from Licorice. Food Chem. 2005, 96, 669–674. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Mizutani, K.; Tamura, Y.; Kinoshita, T. Antioxidative and Superoxide Scavenging Activities of Retrochalcones in Glycyrrhiza inflata. Bioorg. Med. Chem. 1998, 6, 339–347. [Google Scholar] [CrossRef]
- Wu, C.P.; Lusvarghi, S.; Hsiao, S.H.; Liu, T.C.; Li, Y.Q.; Huang, Y.H.; Ambudkar, S.V. Licochalcone A Selectively Resensitizes ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Chemotherapeutic Drugs. J. Nat. Prod. 2020, 83, 1461–1472. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Ho, C.T. Hydroxylated Polymethoxyflavones and Methylated Flavonoids in Sweet Orange (Citrus sinensis) Peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bai, J.; Zhao, S.; Hu, M.; Sun, Y.; Wang, B.; Ji, M.; Jin, J.; Wang, X.; Hu, J.; et al. Evaluation of inhibitory effects of flavonoids on breast cancer resistance protein (BCRP): From library screening to biological evaluation to structure-activity relationship. Toxicol. In Vitro 2019, 61, 104642. [Google Scholar] [CrossRef]
- Raish, M.; Ahmad, A.; Karim, B.A.; Jardan, Y.A.B.; Ahad, A.; Iqbal, M.; Alkharfy, K.M.; Al-Jenoobi, F.I.; Mohammed, O.M. Pharmacokinetics of Dasatinib in Rats: A Potential Food–Drug Interaction with Naringenin. Eur. J. Drug Metab. Pharmacokinet. 2024, 49, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhao, S.; Fan, X.; Chen, Y.; Zou, X.; Hu, M.; Wang, B.; Jin, J.; Wang, X.; Hu, J.; et al. Inhibitory effects of flavonoids on P-glycoprotein in vitro and in vivo: Food/herb-drug interactions and structure–activity relationships. Toxicol. Appl. Pharmacol. 2019, 369, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Tarapcsák, S.; Gyöngy, Z.; Ritter, Z.; Batta, G.; Bosire, R.; Remenyik, J.; Goda, K. Effects of polyphenols on p-glycoprotein (Abcb1) activity. Pharmaceutics 2021, 13, 2062. [Google Scholar] [CrossRef]
- Nićiforović, N.; Abramovič, H. Sinapic acid and its derivatives: Natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Shahid, M.; Ahmad, A.; Raish, M.; Bin Jardan, Y.A.; Alkharfy, K.M.; Ahad, A.; Kalam, M.A.; Ansari, M.A.; Iqbal, M.; Ali, N.; et al. Herb-drug interaction: Effect of sinapic acid on the pharmacokinetics of dasatinib in rats. Saudi Pharm. J. 2023, 31, 101819. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Mieszala, K.; Rudewicz, M.; Gomulkiewicz, A.; Ratajczak-Wielgomas, K.; Grzegrzolka, J.; Dziegiel, P.; Borska, S. Expression of genes and proteins of multidrug resistance in gastric cancer cells treated with resveratrol. Oncol. Lett. 2018, 15, 5825–5832. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.Y.; Abdelghany, A.A.; Al-Amoudi, H.S.; Efferth, T.; Wink, M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2019, 55, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Yang, H.; Wei, Y.C.; Li, W.C.; Chen, H.Y.; Lin, H.Y.; Chiang, C.P.; Chen, H.M. Natural compounds modulate drug transporter mediated oral cancer treatment. Biomolecules 2020, 10, 1335. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.H.; Xu, X. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J. Pharm. Biomed. Anal. 2021, 201, 114129. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.; Alshawsh, M.A. Therapeutic potential of certain terpenoids as anticancer agents: A scoping review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Nagai, K.; Tamura, M.; Murayama, R.; Fukuno, S.; Ito, T.; Konishi, H. Development of multi-drug resistance to anticancer drugs in HepG2 cells due to MRP2 upregulation on exposure to menthol. PLoS ONE 2023, 18, e0291822. [Google Scholar] [CrossRef]
- Zecchinati, F.; Barranco, M.M.; Arana, M.R.; Tocchetti, G.N.; Domínguez, C.J.; Perdomo, V.G.; Ruiz, M.L.; Mottino, A.D.; García, F.; Villanueva, S.S.M. Reversion of down-regulation of intestinal multidrug resistance-associated protein 2 in fructose-fed rats by geraniol and vitamin C: Potential role of inflammatory response and oxidative stress. J. Nutr. Biochem. 2019, 68, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef]
- Ortiz-Rivero, S.; Peleteiro-Vigil, A.; Abete, L.; Lozano, E.; Hammer, H.S.; Di Giacomo, S.; Abad, M.; Boix, L.; Forner, A.; Reig, M.; et al. Sensitization of cholangiocarcinoma cells to chemotherapy through BCRP inhibition with β-caryophyllene oxide. Biomed. Pharmacother. 2024, 170, 116038. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.P.; Ma, Y.X.; Quan, D.N.; Zhang, L.; Yan, M.; Fan, X.R. Rosemary Extracts Upregulate Nrf2, Sestrin2, and MRP2 Protein Level in Human Hepatoma HepG2 Cells. Evid.-Based Complement. Alternat. Med. 2017, 207, 7359806. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-sitosterol: A promising but orphan nutraceutical to fight against cancer. Nutr. Cancer 2015, 67, 1216–1222. [Google Scholar] [CrossRef]
- Wang, Z.; Zhan, Y.; Xu, J.; Wang, Y.; Sun, M.; Chen, J.; Liang, T.; Wu, L.; Xu, K. β-Sitosterol Reverses Multidrug Resistance via BCRP Suppression by Inhibiting the p53-MDM2 Interaction in Colorectal Cancer. J. Agric. Food Chem. 2020, 68, 3850–3858. [Google Scholar] [CrossRef]
- Gómez-Garduño, J.; León-Rodríguez, R.; Alemón-Medina, R.; Pérez-Guillé, B.E.; Soriano-Rosales, R.E.; González-Ortiz, A.; Chávez-Pacheco, J.L.; Solorio-López, E.; Fernandez-Pérez, P.; Rivera-Espinosa, L. Phytochemicals that interfere with drug metabolism and transport, modifying plasma concentration in humans and animals. Dose-Response 2022, 20, 15593258221120485. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Tang, C.Y.; Chen, L.Y.; Zheng, S.; Zhao, Y.; Ma, L.S.; Xu, L.; Fan, L.H.; Yu, J.D.; Tan, H.S.; et al. Berberine Reverses Breast Cancer Multidrug Resistance Based on Fluorescence Pharmacokinetics in Vitro and in Vivo. ACS Omega 2021, 6, 10645–10654. [Google Scholar] [CrossRef]
- Yu, C.P.; Huang, C.Y.; Lin, S.P.; Hou, Y.C. Activation of P-glycoprotein and CYP 3A by Coptidis Rhizoma in vivo: Using cyclosporine as a probe substrate in rats. J. Food Drug Anal. 2018, 26, S125–S132. [Google Scholar] [CrossRef]
- Whiting, S.; Derbyshire, E.; Tiwari, B.K. Capsaicinoids and capsinoids. A potential role for weight management? A systematic review of the evidence. Appetite 2012, 59, 341–348. [Google Scholar] [CrossRef]
- Zhai, X.; Feng, Y.; Liu, J.; Li, J.; Zong, Y.; Tuo, Z.; Gao, S.; Lv, Y. Pharmacokinetic effects of capsaicin on vinblastine in rats mediated by CYP3A and Mrp2. Fundam. Clin. Pharmacol. 2019, 33, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef]
- Bi, X.; Yuan, Z.; Qu, B.; Zhou, H.; Liu, Z.; Xie, Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine 2019, 54, 98–108. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Yoo, S.D.; Shin, B.S. Effects of phytochemical P-glycoprotein modulators on the pharmacokinetics and tissue distribution of doxorubicin in mice. Molecules 2018, 23, 349. [Google Scholar] [CrossRef] [PubMed]
- Roma, M.I.; Schiariti Lampropulos, V.E.; Ayllón-Cabrera, I.; Salazar Sanabria, A.N.; López Nigro, M.M.; Peroni, R.N.; Carballo, M.A. Modulation of hepatic ABC transporters by Eruca vesicaria intake: Potential diet-drug interactions. Food Chem. Toxicol. 2019, 133, 110797. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Yang, M.S.; Hsu, P.W.; Lin, S.P.; Hou, Y.C. Bidirectional influences of cranberry on the pharmacokinetics and pharmacodynamics of warfarin with mechanism elucidation. Nutrients 2021, 13, 3219. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.P.; Tsai, P.L.; Li, P.Y.; Hsu, P.W.; Lin, S.P.; Lee, C.P.D.; Hou, Y.C. Cranberry Ingestion Modulated Drug Transporters and Metabolizing Enzymes: Gefitinib Used as a Probe Substrate in Rats. Molecules 2022, 27, 5772. [Google Scholar] [CrossRef]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef]
- Ferreira, A.; Pousinho, S.; Fortuna, A.; Falcão, A.; Alves, G. Flavonoid compounds as reversal agents of the P-glycoprotein-mediated multidrug resistance: Biology, chemistry, and pharmacology. Phytochem. Rev. 2015, 14, 233–272. [Google Scholar] [CrossRef]
- Sagnou, M.; Novikov, F.N.; Ivanova, E.S.; Alexiou, P.; Stroylov, V.S.; Titov, I.Y.; Tatarskiy, V.V.; Vagida, M.S.; Pelecanou, M.; Shtil, A.A.; et al. Novel curcumin derivatives as P-glycoprotein inhibitors: Molecular modeling, synthesis, and sensitization of multidrug-resistant cells to doxorubicin. Eur. J. Med. Chem. 2020, 198, 112331. [Google Scholar] [CrossRef]
- El-Araby, M.E.; Omar, A.M.; Khayat, M.T.; Assiri, H.A.; Al-Abd, A.M. Molecular mimics of classic P-glycoprotein inhibitors as multidrug resistance suppressors and their synergistic effect on paclitaxel. PLoS ONE 2017, 12, e0170186. [Google Scholar] [CrossRef]
- Syed, S.B.; Arya, H.; Fu, I.H.; Yeh, T.K.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Targeting P-glycoprotein: Investigation of piperine analogs for overcoming drug resistance in cancer. Sci. Rep. 2017, 7, 7972. [Google Scholar] [CrossRef] [PubMed]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; El-Sheikh, A.A.K.; Ibrahim, A.R.N.; Khedr, M.A.; Al-Taher, A.Y. In silico comparisons between natural inhibitors of ABCB1/P-glycoprotein to overcome doxorubicin-resistance in the NCI/ADR-RES cell line. Eur. J. Pharm. Sci. 2018, 112, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.B.; Lin, S.Y.; Arya, H.; Fu, I.H.; Yeh, T.K.; Charles, M.R.C.; Periyasamy, L.; Hsieh, H.P.; Coumar, M.S. Overcoming vincristine resistance in cancer: Computational design and discovery of piperine-inspired P-glycoprotein inhibitors. Chem. Biol. Drug Des. 2021, 97, 51–66. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Yu, Y.Q.; Yan, X.X.; Wang, W.J.; Tian, X.T.; Wang, L.; Zhu, W.L.; Gong, L.K.; Pan, G.Y. Different structures of berberine and five other protoberberine alkaloids that affect P-glycoprotein-mediated efflux capacity. Acta Pharmacol. Sin. 2019, 40, 133–142. [Google Scholar] [CrossRef]
- Chu, M.; Chen, X.; Wang, J.; Guo, L.; Wang, Q.; Gao, Z.; Kang, J.; Zhang, M.; Feng, J.; Guo, Q.; et al. Polypharmacology of berberine based on multi-target binding motifs. Front. Pharmacol. 2018, 9, 801. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodzicka, A.; Galanty, A.; Paśko, P. Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety. Curr. Issues Mol. Biol. 2024, 46, 9686-9706. https://doi.org/10.3390/cimb46090576
Brodzicka A, Galanty A, Paśko P. Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety. Current Issues in Molecular Biology. 2024; 46(9):9686-9706. https://doi.org/10.3390/cimb46090576
Chicago/Turabian StyleBrodzicka, Agnieszka, Agnieszka Galanty, and Paweł Paśko. 2024. "Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety" Current Issues in Molecular Biology 46, no. 9: 9686-9706. https://doi.org/10.3390/cimb46090576
APA StyleBrodzicka, A., Galanty, A., & Paśko, P. (2024). Modulation of Multidrug Resistance Transporters by Food Components and Dietary Supplements: Implications for Cancer Therapy Efficacy and Safety. Current Issues in Molecular Biology, 46(9), 9686-9706. https://doi.org/10.3390/cimb46090576





