Effect of Surface Structure Complexity on Interfacial Droplet Behavior of Superhydrophobic Titanium Surfaces for Robust Dropwise Condensation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Preparation and Characterization
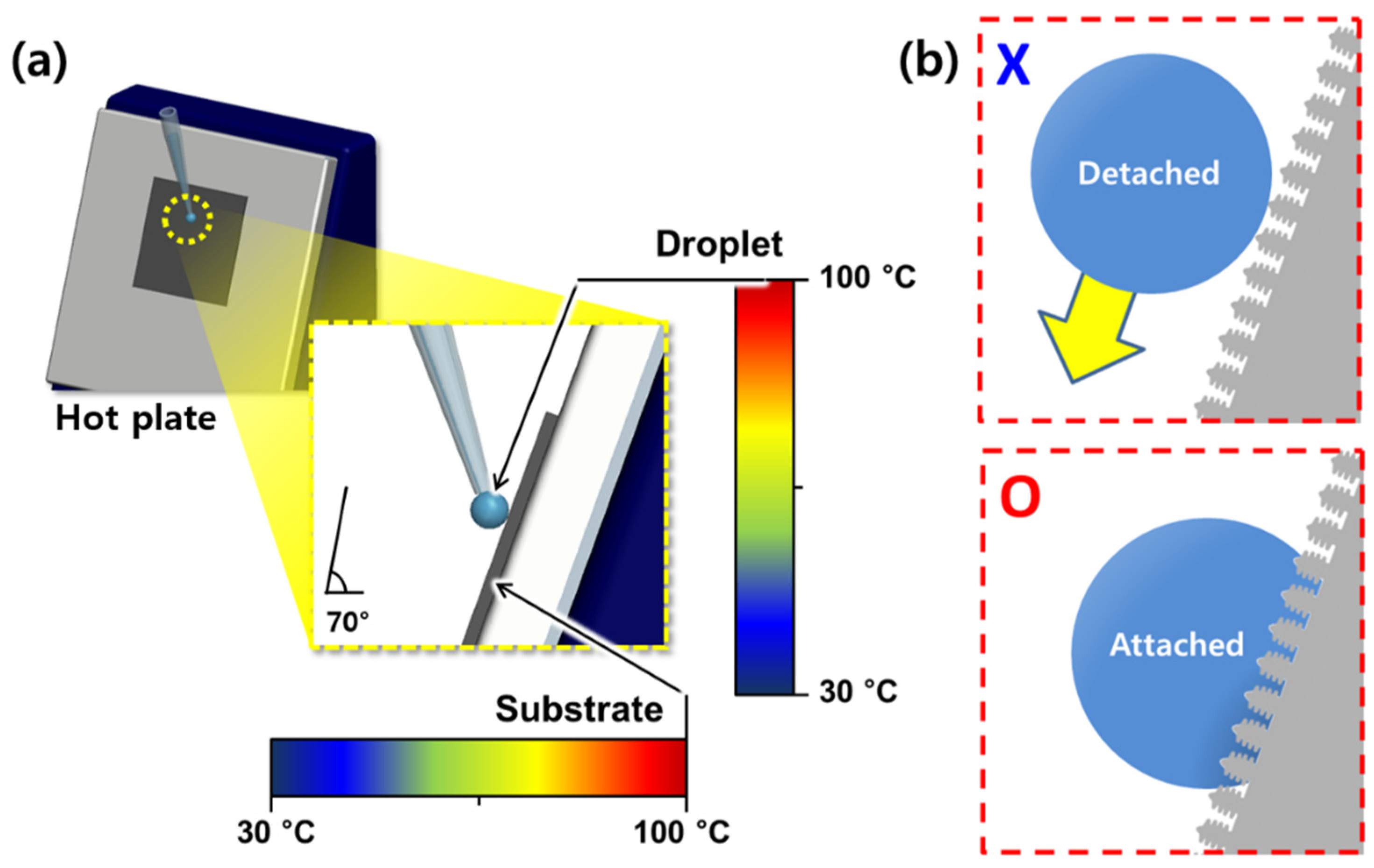
2.3. Droplet-Behavior Experiments
3. Results and Discussion
3.1. Fabrication of Superhydrophobic Titanium Surfaces
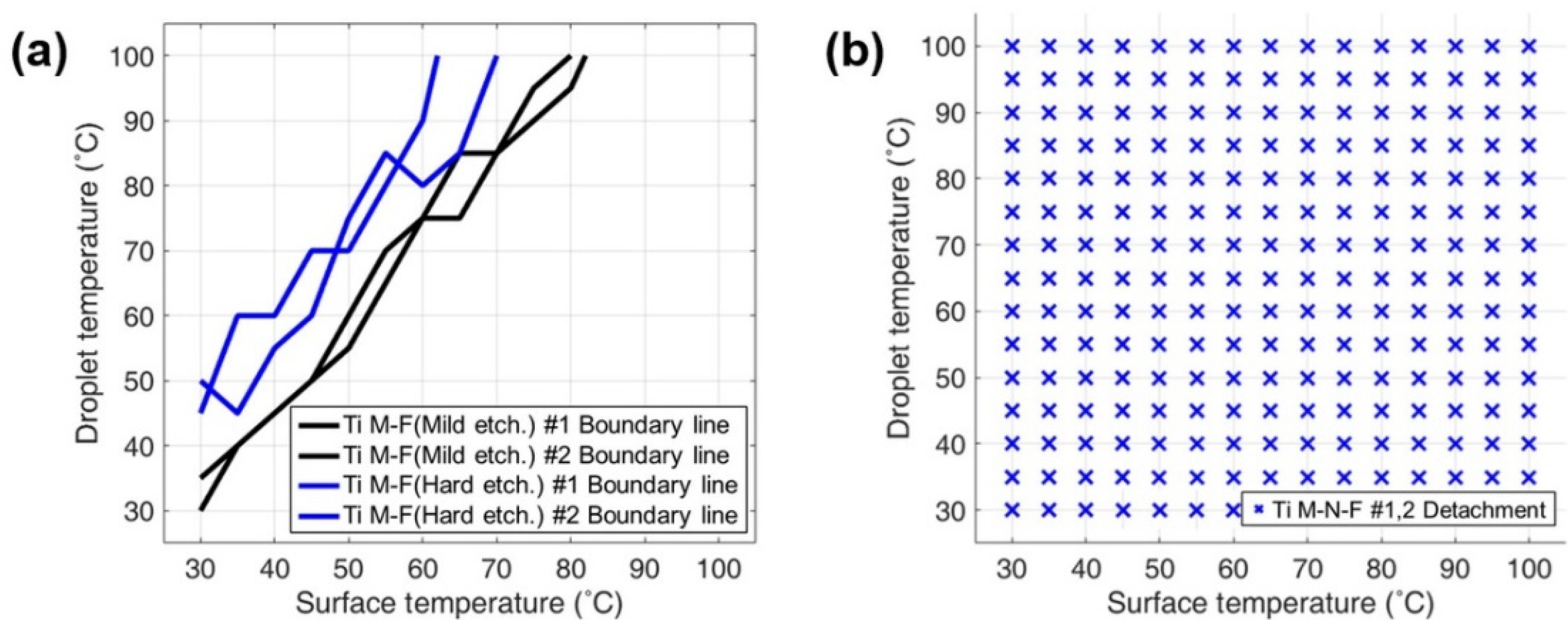
3.2. Repellency Performance Evaluation via DB Mapping
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, L.; Tian, Y.; Jiang, L. Bioinspired super-wettability from fundamental research to practical applications. Angew. Chem. Int. Ed. Engl. 2015, 54, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Song, Y.; Jiang, L. Applications of bio-inspired special wettable surfaces. Adv. Mater. 2011, 23, 719–734. [Google Scholar] [CrossRef]
- Li, X.M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef] [Green Version]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F.; Wetterwald, M.; Lasagni, A.F.; Bonaccurso, E.; Kunze, T. Self-Limited Ice Formation and Efficient De-Icing on Superhydrophobic Micro-Structured Airfoils through Direct Laser Interference Patterning. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Li, S.-Y.; Li, Y.; Wang, J.; Nan, Y.-G.; Ma, B.-H.; Liu, Z.-L.; Gu, J.-X. Fabrication of pinecone-like structure superhydrophobic surface on titanium substrate and its self-cleaning property. Chem. Eng. J. 2016, 290, 82–90. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, F.Y. A review of the recent advances in superhydrophobic surfaces and the emerging energy-related applications. Energy 2015, 82, 1068–1087. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Cho, H.; Kim, D.; Lee, C.; Hwang, W. A simple fabrication method for mechanically robust superhydrophobic surface by hierarchical aluminum hydroxide structures. Curr. Appl. Phys. 2013, 13, 762–767. [Google Scholar] [CrossRef]
- Lee, J.-W.; Hwang, W. Simple fabrication of superoleophobic titanium surfaces via hierarchical microhorn/nanoporous structure growth by chemical acid etching and anodization. J. Alloys Compd. 2017, 728, 966–970. [Google Scholar] [CrossRef]
- Lee, J.-W.; Hwang, W. Exploiting the silicon content of aluminum alloys to create a superhydrophobic surface using the sol–gel process. Mater. Lett. 2016, 168, 83–85. [Google Scholar] [CrossRef]
- Cho, H.; Lee, J.; Lee, S.; Hwang, W. Durable superhydrophilic/phobic surfaces based on green patina with corrosion resistance. Phys. Chem. Chem. Phys. 2015, 17, 6786–6793. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Hwang, H.J.; Cho, H.; Choi, D.; Hwang, W. Repeatable replication method with liquid infiltration to fabricate robust, flexible, and transparent, anti-reflective superhydrophobic polymer films on a large scale. Chem. Eng. J. 2018, 350, 225–232. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.W.; Hwang, W. One-Step Eco-Friendly Superhydrophobic Coating Method Using Polydimethylsiloxane and Ammonium Bicarbonate. ACS Appl. Mater. Interfaces 2020, 12, 28869–28875. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Hwang, W. Fabrication of a superhydrophobic surface with fungus-cleaning properties on brazed aluminum for industrial application in heat exchangers. Appl. Surf. Sci. 2018, 442, 461–466. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.Y.; Liu, Q.; Zhu, S.P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Li, L.; Huang, T.; Lei, J.; He, J.; Qu, L.; Huang, P.; Zhou, W.; Li, N.; Pan, F. Robust biomimetic-structural superhydrophobic surface on aluminum alloy. ACS Appl. Mater. Interfaces 2015, 7, 1449–1457. [Google Scholar] [CrossRef]
- Wang, F.; Liang, C.; Zhang, Y.; Zhang, X. Defrosting performance of superhydrophobic fin-tube heat exchanger. Appl. Therm. Eng. 2017, 113, 229–237. [Google Scholar] [CrossRef]
- Ge, C.; Yuan, G.; Guo, C.; Ngo, C.-V.; Li, W. Femtosecond laser fabrication of square pillars integrated Siberian-Cocklebur-like microstructures surface for anti-icing. Mater. Des. 2021, 204. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, D.; Li, J.; Liu, T.; Pu, J.; Zhao, H.; Wang, L. One-step synthesis of superhydrophobic polyhedral oligomeric silsesquioxane-graphene oxide and its application in anti-corrosion and anti-wear fields. Corros. Sci. 2019, 147, 9–21. [Google Scholar] [CrossRef]
- Das, A.K.; Kilty, H.P.; Marto, P.J.; Andeen, G.B.; Kumar, A. The Use of an Organic Self-Assembled Monolayer Coating to Promote Dropwise Condensation of Steam on Horizontal Tubes. J. Heat Transf. 2000, 122, 278–286. [Google Scholar] [CrossRef]
- Paxson, A.T.; Yague, J.L.; Gleason, K.K.; Varanasi, K.K. Stable dropwise condensation for enhancing heat transfer via the initiated chemical vapor deposition (iCVD) of grafted polymer films. Adv. Mater. 2014, 26, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, J.W. Dropwise condensation theory and experiment: A review. Proc. Inst. Mech. Eng. Part A J. Power Energy 2005, 216, 115–128. [Google Scholar] [CrossRef]
- Vemuri, S.; Kim, K.J.; Wood, B.D.; Govindaraju, S.; Bell, T.W. Long term testing for dropwise condensation using self-assembled monolayer coatings of n-octadecyl mercaptan. Appl. Therm. Eng. 2006, 26, 421–429. [Google Scholar] [CrossRef]
- Chen, L.; Liang, S.; Yan, R.; Cheng, Y.; Huai, X.; Chen, S. n-Octadecanethiol self-assembled monolayer coating with microscopic roughness for dropwise condensation of steam. J. Therm. Sci. 2009, 18, 160–165. [Google Scholar] [CrossRef]
- Zhong, L.; Xuehu, M.; Sifang, W.; Mingzhe, W.; Xiaonan, L. Effects of surface free energy and nanostructures on dropwise condensation. Chem. Eng. J. 2010, 156, 546–552. [Google Scholar] [CrossRef]
- Lu, M.-C.; Lin, C.-C.; Lo, C.-W.; Huang, C.-W.; Wang, C.-C. Superhydrophobic Si nanowires for enhanced condensation heat transfer. Int. J. Heat Mass Transf. 2017, 111, 614–623. [Google Scholar] [CrossRef]
- Wang, S.; Yu, X.; Liang, C.; Zhang, Y. Enhanced condensation heat transfer in air-conditioner heat exchanger using superhydrophobic foils. Appl. Therm. Eng. 2018, 137, 758–766. [Google Scholar] [CrossRef]
- Cheng, J.; Vandadi, A.; Chen, C.-L. Condensation heat transfer on two-tier superhydrophobic surfaces. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Del Col, D.; Parin, R.; Bisetto, A.; Bortolin, S.; Martucci, A. Film condensation of steam flowing on a hydrophobic surface. Int. J. Heat Mass Transf. 2017, 107, 307–318. [Google Scholar] [CrossRef]
- Wier, K.A.; McCarthy, T.J. Condensation on Ultrahydrophobic Surfaces and Its Effect on Droplet Mobility: Ultrahydrophobic Surfaces Are Not Always Water Repellant. Langmuir 2006, 22, 2433–2436. [Google Scholar] [CrossRef]
- Feng, J.; Qin, Z.; Yao, S. Factors affecting the spontaneous motion of condensate drops on superhydrophobic copper surfaces. Langmuir 2012, 28, 6067–6075. [Google Scholar] [CrossRef]
- Sbragaglia, M.; Peters, A.M.; Pirat, C.; Borkent, B.M.; Lammertink, R.G.; Wessling, M.; Lohse, D. Spontaneous breakdown of superhydrophobicity. Phys. Rev. Lett. 2007, 99, 156001. [Google Scholar] [CrossRef] [Green Version]
- Enright, R.; Miljkovic, N.; Al-Obeidi, A.; Thompson, C.V.; Wang, E.N. Condensation on superhydrophobic surfaces: The role of local energy barriers and structure length scale. Langmuir 2012, 28, 14424–14432. [Google Scholar] [CrossRef]
- Seo, D.; Shim, J.; Moon, B.; Lee, K.; Lee, J.; Lee, C.; Nam, Y. Passive Anti-Flooding Superhydrophobic Surfaces. ACS Appl. Mater. Interfaces 2020, 12, 4068–4080. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Enright, R.; Nam, Y.; Lopez, K.; Dou, N.; Sack, J.; Wang, E.N. Jumping-droplet-enhanced condensation on scalable superhydrophobic nanostructured surfaces. Nano Lett. 2013, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Ahn, H.S.; Kwon, T.-S. Experimental investigation of filmwise and dropwise condensation inside transparent circular tubes. Appl. Therm. Eng. 2017, 110, 412–423. [Google Scholar] [CrossRef]
- Jo, H.; Hwang, K.W.; Kim, D.; Kiyofumi, M.; Park, H.S.; Kim, M.H.; Ahn, H.S. Loss of superhydrophobicity of hydrophobic micro/nano structures during condensation. Sci. Rep. 2015, 5, 9901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, D.-Y.; Lee, J.-W.; Hwang, W.; Lee, K.-Y. Experimental study of condensation heat transfer on a horizontal aluminum tube with superhydrophobic characteristic. Int. J. Heat Mass Transf. 2019, 134, 286–295. [Google Scholar] [CrossRef]
- Dorrer, C.; Ruhe, J. Condensation and Wetting Transitions on Microstructured Ultrahydrophobic Surfaces. Langmuir 2007, 23, 3820–3824. [Google Scholar] [CrossRef]
- Olceroglu, E.; McCarthy, M. Self-Organization of Microscale Condensate for Delayed Flooding of Nanostructured Superhydrophobic Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 5729–5736. [Google Scholar] [CrossRef] [PubMed]
- Miljkovic, N.; Wang, E.N. Condensation heat transfer on superhydrophobic surfaces. MRS Bull. 2013, 38, 397–406. [Google Scholar] [CrossRef]
- Singh, N.S.; Zhang, J.; Stafford, J.; Anthony, C.; Gao, N. Implementing Superhydrophobic Surfaces within Various Condensation Environments: A Review. Adv. Mater. Interfaces 2020, 8. [Google Scholar] [CrossRef]
- Lee, J.W.; Ji, D.Y.; Lee, K.Y.; Hwang, W. A Study of Droplet-Behavior Transition on Superhydrophobic Surfaces for Efficiency Enhancement of Condensation Heat Transfer. ACS Omega 2020, 5, 27880–27885. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-U.; Ji, D.-Y.; Lee, K.-Y.; Hwang, W.; Lee, C.-H.; Kim, S.-J.; Lee, J.-W. Effect of Surface Structure Complexity on Interfacial Droplet Behavior of Superhydrophobic Titanium Surfaces for Robust Dropwise Condensation. Materials 2021, 14, 4107. https://doi.org/10.3390/ma14154107
Jeong J-U, Ji D-Y, Lee K-Y, Hwang W, Lee C-H, Kim S-J, Lee J-W. Effect of Surface Structure Complexity on Interfacial Droplet Behavior of Superhydrophobic Titanium Surfaces for Robust Dropwise Condensation. Materials. 2021; 14(15):4107. https://doi.org/10.3390/ma14154107
Chicago/Turabian StyleJeong, Je-Un, Dae-Yun Ji, Kwon-Yeong Lee, Woonbong Hwang, Chang-Hun Lee, Sung-Jae Kim, and Jeong-Won Lee. 2021. "Effect of Surface Structure Complexity on Interfacial Droplet Behavior of Superhydrophobic Titanium Surfaces for Robust Dropwise Condensation" Materials 14, no. 15: 4107. https://doi.org/10.3390/ma14154107
APA StyleJeong, J.-U., Ji, D.-Y., Lee, K.-Y., Hwang, W., Lee, C.-H., Kim, S.-J., & Lee, J.-W. (2021). Effect of Surface Structure Complexity on Interfacial Droplet Behavior of Superhydrophobic Titanium Surfaces for Robust Dropwise Condensation. Materials, 14(15), 4107. https://doi.org/10.3390/ma14154107






