Thermography as a Method for Bedside Monitoring of Infantile Hemangiomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
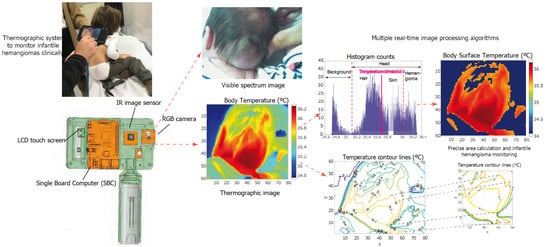
- A single board computer (SBC) is the core of the system. We chose a Raspberry Pi IV board to build the prototype. It controls all of the peripherals and sensors that make up the system. It has a Linux-based operating system and can run real-time image processing algorithms. A custom user interface was programed to acquire, display, and process the images. SBC also has Wi-Fi and Ethernet connectivity, and an expansion bus is available to add extra peripherals or sensors if necessary.
- A visible spectrum camera based on a Sony IMX219 8-megapixel sensor.
- A LEPTON thermal camera operating the long wave infrared (LWIR) band. After calibration, it can take thermal images and measure absolute temperature values within the visual scene.
- A Melexis MLX90614D IR thermometer to calibrate the thermal camera.
- A 5-INCH HDMI LCD Waveshare touch screen to display the images captured by the two image sensors and to control the user interface. The interface allows for the simultaneous acquisition of visible and IR images.
- A standard Poweradd 5 V battery to make the system autonomous and portable.
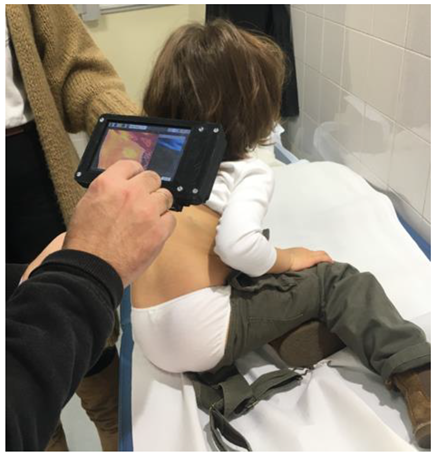
2.2. Methods
- Participants had to have at least one IH diagnosed simultaneously and independently by the two specialists. Patients with vascular anomalies that were not identified as hemangiomas were excluded from the study.
- The patients’ IHs must not have been treated previously.
- All variants of IHs were accepted for the study, independently of their proliferation phase or the patient’s medical history, gender, or ethnicity.
- There was no age limit for patients eligible to participate in the study.
- Patients’ guardians had to agree for their charges to be subjected to thermographic analysis in all upcoming clinical sessions. The scheduled periodicity between control sessions was three months.
2.2.1. Image Processing User Interface
2.2.2. Image Segmentation
- Histogram counts are computed for the thermal image.
- Otsu’s method is applied using the histogram counts to calculate the optimum threshold temperature value, TH, to segment the image.
- The image is thresholded: pixel values lower than TH are set to zero.
- The resulting pixel matrix is stored in the memory and displayed on the system screen.
2.2.3. Temperature Contour Lines and Area Calculations
- Compute and plot N temperature contour lines within the [35.5,38]°C range, that is to say, the range in which the temperature usually varies with the presence of vascular anomalies. This was done by selecting the findContours() predefined contour extraction routine from the OpenCV library [20].
- Select a temperature value to delimit the malformation boundary. The same value will be used to compare the area of the affected region in the different clinical sessions. The findContours() function provides coordinate pairs of the k vertices that delimited the contour line. Note that the vertices have to create a closed contour. The start and end point vertex coordinates are the same and must therefore be considered twice in the computation, i.e., .
- The corresponding contour line is plotted separately for a detailed analysis.
3. Results
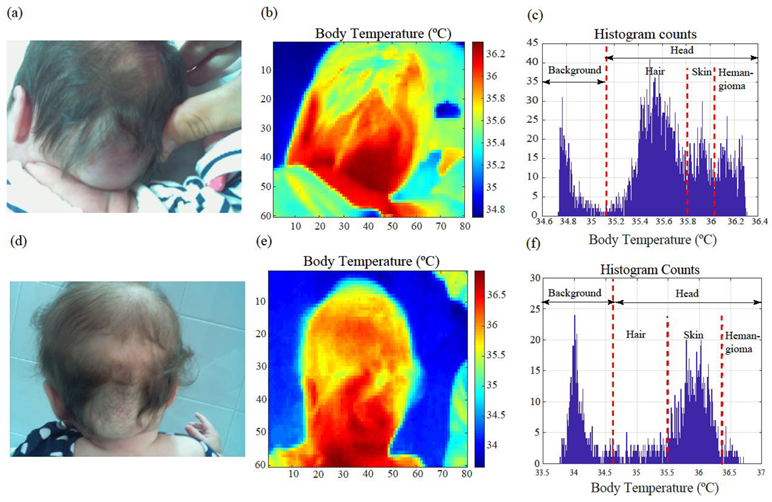
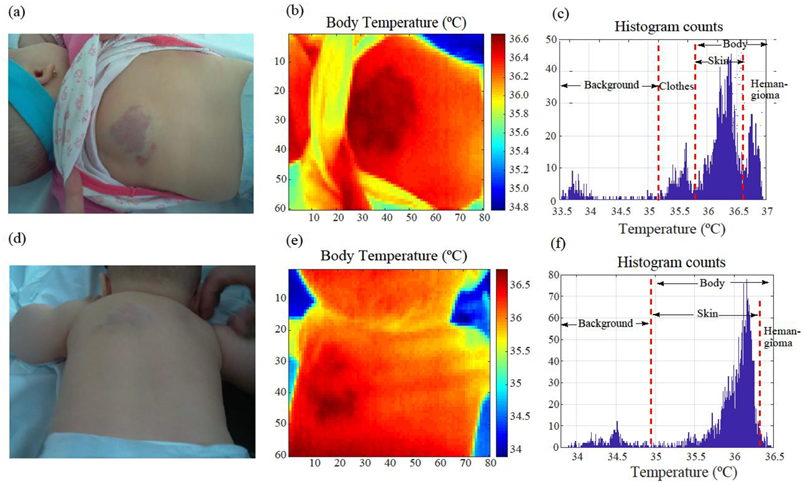
3.1. Medical Image Analysis and Processing
3.2. Image Segmentation, Area Calculation, and Contour Extraction Results
3.3. Pilot Study
- (i)
- There was a relative temperature increment in the hemangioma over time and the dermatologists diagnosed a negative response to treatment.
- (ii)
- There was a relative temperature decrement in the hemangioma, and the dermatologists diagnosed a positive response to treatment.
- (iii)
- There was a null temperature increment in the hemangioma, but the IH area estimation provided by the system was substantially lower than in the previous session.
4. Discussion
Method Limitations and Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kilcline, C.; Frieden, I.J. Infantile Hemangiomas: How Common Are They? A Systematic Review of the Medical Literature. Pediatr. Dermatol. 2008, 25, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, M.J.; Smeulders, M.J.; van der Horst, C.M. Prevalence and characteristics of hemangiomas in young children. Ned. Tijdschr. Geneeskd. 2005, 149, 2455–2458. (In Dutch) [Google Scholar] [PubMed]
- Munden, A.; Butschek, R.; Tom, W.; Marshall, J.S.; Poeltler, D.M.; Krohne, S.E.; Alió, A.B.; Ritter, M.; Friedlander, D.; Catanzarite, V.; et al. Prospective study of infantile haemangiomas: Incidence, clinical characteristics and association with placental anomalies. Br. J. Dermatol. 2014, 170, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Frieden, I.J.; Eichenfield, L.F.; Esterly, N.B.; Geronemus, R.; Mallory, S.B. Guidelines of care for hemangiomas of infancy: American Academy of Dermatology Guidelines/Outcomes Committee. J. Am. Acad. Dermatol. 1997, 37, 631–637. [Google Scholar] [CrossRef]
- Léauté-Labrèze, C.; Hoeger, P.; Mazereeuw-Hautier, J.; Guibaud, L.; Baselga, E.; Posiunas, G.; Phillips, R.J.; Caceres, H.; Gutierrez, J.C.L.; Ballona, R.; et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015, 372, 735–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gade, R.; Moeslund, T.B. Thermal cameras and applications: A survey. Mach. Vis. Appl. 2013, 25, 245–262. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Jimenez, M.A.; Loza-Gonzalez, V.M.; Kolosovas-Machuca, E.S.; Yanes-Lane, M.E.; Ramirez-GarciaLuna, A.S.; Ramirez-GarciaLuna, J.L. Diagnostic accuracy of infrared thermal imaging for detecting COVID-19 infection in minimally symptomatic patients. Eur. J. Clin. Investig. 2020, 51, e13474. [Google Scholar] [CrossRef]
- Saxena, A.K.; Willital, G.H. Infrared thermography: Experience from a decade of pediatric imaging. Eur. J. Pediatr. 2007, 167, 757–764. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An Overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef] [Green Version]
- Purohit, R.C.; McCoy, M.D. Thermography in the diagnosis of inflammatory processes in the horse. Am. J. Vet. Res. 1980, 41, 1167–1174. [Google Scholar]
- Burkes, S.A.; Patel, M.; Adams, D.M.; Hammill, A.M.; Eaton, K.P.; Wickett, R.R.; Visscher, M.O. Infantile hemangioma status by dynamic infrared thermography: A preliminary study. Int. J. Dermatol. 2016, 55, e522–e532. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, J.A.; Balma-Mena, A.; Chakkittakandiyil, A.; Matea, F.; Pope, E. Infrared Thermography to Assess Proliferation and Involution of Infantile Hemangiomas: A prospective cohort study. JAMA Dermatol. 2014, 150, 964–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzezinski, R.Y.; Rabin, N.; Lewis, N.; Peled, R.; Kerpel, A.; Tsur, A.M.; Gendelman, O.; Naftali-Shani, N.; Gringauz, I.; Amital, H.; et al. Automated processing of thermal imaging to detect COVID-19. Sci. Rep. 2021, 11, 17489. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke JT, Titley OG. Thermographic assessment of a vascular malformation of the hand: A new imaging modality. J. Clin. Imaging Sci. 2016, 6, 6–9. [Google Scholar] [CrossRef]
- Lenero-Bardallo, J.A.; de la Rosa-Vidal, R.; Padial-Allue, R.; Ceballos-Caceres, J.; Rodriguez-Vazquez, A.; Bernabeu-Wittel, J. A Customizable Thermographic Imaging System for Medical Image Acquisition and Processing. IEEE Sens. J. 2021, 22, 16730–16741. [Google Scholar] [CrossRef]
- Wassef, M.; Blei, F.; Adams, D.; Alomari, A.; Baselga, E.; Berenstein, A.; Burrows, P.; Frieden, I.J.; Garzon, M.C.; Lopez-Gutierrez, J.-C.; et al. Vascular Anomalies Classification: Recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 2015, 136, e203–e214. [Google Scholar] [CrossRef] [Green Version]
- Merrow, A.C.; Gupta, A.; Patel, M.N.; Adams, D.M. 2014 Revised Classification of Vascular Lesions from the International Society for the Study of Vascular Anomalies: Radiologic-Pathologic Update. Radiographics 2016, 36, 1494–1516. [Google Scholar] [CrossRef] [Green Version]
- Strumila, A.; Kazlauskas, V.; Pošiūnas, G.; Verkauskas, G.; Beiša, V. Infantile hemangioma: Predicting proliferation by infrared thermography. Medicina 2017, 53, 85–89. [Google Scholar] [CrossRef]
- Leñero-Bardallo, J.A.; Serrano, C.; Acha, B.; Pérez-Carrasco, J.A.; Bernabeu-Wittel, J. Thermography for the differential diagnosis of vascular malformations. Clin. Exp. Dermatol. 2020, 46, 314–318. [Google Scholar] [CrossRef]
- Itseez Open Source Computer Vision. Available online: https://opencv.org/ (accessed on 5 September 2022).
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing; Pearson: London, UK, 2007. [Google Scholar]
- Solomon, C.; Breckon, T. Fundamentals of Digital Image Processing; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Nixon, M.; Aguado, A. Feature Extraction and Image Processing for Computer Vision; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128149775. [Google Scholar]
- Krowchuk, D.P.; Frieden, I.J.; Mancini, A.J.; Darrow, D.H.; Blei, F.; Greene, A.K.; Annam, A.; Baker, C.N.; Frommelt, P.C.; Hodak, A.; et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019, 143, e20183475. [Google Scholar] [CrossRef]
- Garcia-Romero, M.T.; Chakkittakandiyil, A.; Pope, E. The role of infrared thermography in evaluation of proliferative infantile hemangiomas. Results of a pilot study. Int. J. Dermatol. 2013, 53, e216–e217. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Mani, V.; Noufal, A. Update on the classification of hemangioma. J. Oral Maxillofac. Pathol. 2014, 18 (Suppl. 1), S117–S120. [Google Scholar] [CrossRef] [PubMed]
- Odile, E.; Wassef, M.; Chapot., R. Color Atlas of Vascular Tumors and Vascular Malformations; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Mulliken, J.B.; Glowacki, J. Hemangiomas and Vascular Malformations in Infants and Children: A classification based on endothelial characteristics. Plast. Reconstr. Surg. 1982, 69, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging imaging technologies in dermatology: Part I: Basic principles. J. Am. Acad. Dermatol. 2018, 80, 1114–1120. [Google Scholar] [CrossRef]
- Parapatt, G.K.; Oranges, T.; Paolantonio, G.; Ravà, L.; Giancristoforo, S.; Diociaiuti, A.; Hachem M el Rollo, M. Color Doppler Evaluation of Arterial Resistive Index in Infantile Hemangioma: A Useful Parameter to Monitor the Response to Oral Pro-pranolol? Front. Pediatr. 2021, 9, 1369. [Google Scholar] [CrossRef]




| No. of Patients | No. of Hemangiomas Studied (First Visit Only) | No. of Images withTemperature Variation | Average Temperature Increment (∆°C) | 90% Confidence Interval for Temperature Variation (∆°C) [Min, Max] | Percentage of Hemangiomas with Temperature Variations |
|---|---|---|---|---|---|
| 55 | 55 | 48 | 0.20 | [0.16, 0.25] | 87.3% |
| No. of Patients | No. of Hemangiomas Studied (Several Visits) | Positive ∆°C | Negative ∆°C | Null ∆°C | Agreement with Dermatologists’ Decision | Average Initial/Final Temperature Variation Induced by Hemangioma in the Surrounding Skin | Average Hemangioma Temperature Variation during Treatment (∆°C) |
|---|---|---|---|---|---|---|---|
| 9 | 17 | 2 | 14 | 1 | 17/17 | 0.38/0.27 | −0.19 |
| Case | Sex | Age (Years) | IH Type | Localization and Dimensions (mm) | Age at First Consultation (Months) | Treatment Duration (Months) | Administered Treatments |
|---|---|---|---|---|---|---|---|
| 1 | F | 2 | Mixed focal | Dorsal 40 × 40 | 1.5 | Ongoing | Oral propranolol, oral nadolol |
| 2 | F | 2 | Superficial focal | Scalp 30 × 30 | 2 | 10 | Topical timolol |
| 3 | F | 5 | Mixed focal | Right eye inner canthus- | 1.5 | 24 | Oral propranolol, oral nadolol |
| 4 | M | 1 | Segmental | Perianal Ø150 | 1 | 11 | Watch and wait, oral propranolol |
| 5 | M | 2 | Deep focal | Pectoral 30 × 30 | 6 | Ongoing | Topical timolol |
| Mixed focal | Lumbar 60 × 40 | ||||||
| 6 | F | 2 | Multifocal cutaneous hemangiomas | Scalp (2), left mandibular ramus (1), dorsal (2), right elbow (1), left temple (1), 12 × 2 (max. size) | 3 | Ongoing | Topical timolol, oral propranolol |
| 7 | F | 2 | Superficial focal | Occipital 55 × 24 | 0.5 | 6 | Watch-and-wait |
| 8 | F | 1 | Superficial focal | Left forearm 10 × 7 | 0.5 | 12 | Topical timolol |
| 9 | F | 1 | Superficial focal | Vertex 8 × 10 | 1 | Ongoing | Topical timolol, oral propranolol |
| Superficial focal | Left scapula 11 × 9 |
| Case | Hemangioma Temperature Variation | Clinical Response | Incidences and Side Effects |
|---|---|---|---|
| 1 | Overall negative increment. Positive increment after treatment discontinuation (2nd month) | Flattening, disappearance of erythema | Growth (3rd month) upon early treatment discontinuation (2nd month) |
| 2 | Negative increment | Total disappearance | No |
| 3 | Overall negative increment. Positive increment detected after treatment discontinuation (1st month) | Total disappearance | Growth (2nd month) upon early treatment discontinuation (1st month) |
| 4 | Overall negative increment. Positive increment detected after treatment discontinuation (1st month) | Flattening, decrease in erythema | Ulceration and growth (2nd month) upon early treatment discontinuation (1st month) |
| 5 | No temperature variation | Flattening, decrease in erythema | No |
| Negative increment | |||
| 6 | Negative increment | Flattening, total disappearance | No |
| 7 | Negative increment | Total disappearance | No |
| 8 | Negative increment | Decrease in erythema | No |
| 9 | Overall negative increment. Local increment detected upon transitory ulceration (5th month–7th month) | Flattening, decrease in erythema | Ulceration (5th month–7th month) |
| Negative increment | Total disappearance | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leñero-Bardallo, J.A.; Acha, B.; Serrano, C.; Pérez-Carrasco, J.A.; Ortiz-Álvarez, J.; Bernabéu-Wittel, J. Thermography as a Method for Bedside Monitoring of Infantile Hemangiomas. Cancers 2022, 14, 5392. https://doi.org/10.3390/cancers14215392
Leñero-Bardallo JA, Acha B, Serrano C, Pérez-Carrasco JA, Ortiz-Álvarez J, Bernabéu-Wittel J. Thermography as a Method for Bedside Monitoring of Infantile Hemangiomas. Cancers. 2022; 14(21):5392. https://doi.org/10.3390/cancers14215392
Chicago/Turabian StyleLeñero-Bardallo, Juan Antonio, Begoña Acha, Carmen Serrano, José Antonio Pérez-Carrasco, Juan Ortiz-Álvarez, and José Bernabéu-Wittel. 2022. "Thermography as a Method for Bedside Monitoring of Infantile Hemangiomas" Cancers 14, no. 21: 5392. https://doi.org/10.3390/cancers14215392